Abstract
Being an important garden plant, Dianthus chinensis flower has a great variety of colors and color patterns. Chalcone synthase (CHS) is the key enzyme in the anthocyanin biosynthetic pathway. Although CHS genes have been isolated and characterized in ornamental plants, the CHS gene is still unknown in D. chinensis. In our study, three CHS genes, DchCHS1 (KX893854), DchCHS2 (MK404175) and DchCHS3 (MK416198) were isolated in D. chinensis. Their deduced amino acid sequences show high homology with the known CHS sequences in Caryophyllaceae. The phylogenetic tree suggests that the DchCHS1 and the DchCHS3 have a close relation with the known CHS sequences in Caryophyllaceae and the DchCHS2 is different from them. The DchCHSs were characterized by the Tobacco Rattle Virus (TRV)-based virus-induced gene silencing (VIGS) system. We obtained white or pale purple flowers in the DchCHS1-silenced flowers and reducing purple flowers in the DchCHS2-silenced and the DchCHS3-silenced flowers. The anthocyanin content and the transcript level of the silenced DchCHS were significantly reduced in accordance with the silencing phenotypes. The DchCHSs showed different expression patterns during floral bud developments, among flower colors and in organs. Their expression levels in the purple flower were significantly higher than those in the white flower. Compared with DchCHS2 and DchCHS3, DchCHS1 was abundantly expressed at each floral bud stage, in each flower color and in the flower organ. In conclusion, the three DchCHSs are all involved in the anthocyanin synthesis and the flower coloration, and DchCHS1 probably plays a major role in D. chinensis flowers.
Key message
At least three DchCHSs are involved in the anthocyanin synthesis and the flower coloration in D. chinensis. It may be the reason for its richness in flower colors and color patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flower color is a commercially important characteristic in ornamental plants. Anthocyanin is the largest class of flavonoids and responsible for pink, red, violet, blue and purple colors of flowers and other tissues. Chalcone synthase (CHS) is the first key enzyme in the anthocyanin biosynthesis pathway, which has been extensively described. The reduction of CHS transcript inhibits flower pigmentation and leads to flower phenotypes ranging from small streaks of white through to completely white flowers (Deroles et al. 1998; Chen et al. 2004; Fukusaki et al. 2004; Koseki et al. 2005; Ohno et al. 2018; Gu et al. 2019; Nabavi et al. 2020). Numerous CHS genes have been isolated and characterized in ornamental plants. Out of the 12 copies of CHSs identified in petunia hybrid, the expressions of CHSA (AF233638) and CHSJ (X14597) are mainly restricted to floral tissues (Koes et al. 1989) and are dramatically reduced in the white sectors of Petunia hybrida ‘Red Star’ flowers (Koseki et al. 2005). The natural bicolor floral phenotype of Petunia hybrida ‘Picotee’ and ‘Star’ is caused by the spatial repression of CHSA (Morita et al. 2012). Three CHS-like genes are active during the corolla development in Gerbera hybrida. Among them, CHS1 (Z38096) and CHS3 (Z38098) are temporally expressed and correlated with the flavanol and anthocyanin synthesis (CHS1) (Helariutta et al. 1995). Dahlias have redundant CHSs derived from their high polyploidy. In pure white flowers of the octoploid dahlia, CHS1 (AB576660) and CHS2 (AB591825) are simultaneously silenced by post-transcriptional gene silencing (Ohno et al. 2011). CHSA (AB058638), CHSB (AB058639) and CHSC (AB058640) in Asiatic hybrid lily are expressed in anthocyanin-pigmented tepals, but the expression pattern of CHSB is different from those of CHSA and CHSC (Nakatsuka et al. 2003; Suzuki et al. 2016). The CHS genes are known to diversify into a super-gene family in plants. The studies suggest that the number of CHS super-gene family members is different in plant species and in controlling flower colors.
Dianthus chinensis is an important ornamental species in the genus Dianthus, Caryophyllaceae. Except for yellow, it exhibits a wide variation of flower colors, such as pink, purple, red, white, ivory white, etc. It is also rich in color patterns of flowers, including the occurrence of several types of marking in the center and bicolor phenotypes, in which two distinct colors occur on individual petals. Gaining and characterizing CHS genes involved in the anthocyanin biosynthesis pathway and the flower coloration is important to illustrate a diversity of flower colors in D. chinensis. Virus-induced gene silencing (VIGS) has been widely used in various plant species to explore the gene functions (Chen et al. 2004; Quadrana et al. 2011; Singh et al. 2012; Manmathan et al. 2013; Zhong et al. 2014; Dobnik et al. 2016). In this paper, three CHS genes were isolated in D. chinensis and their functions were studied using VIGS. Meanwhile, their expression profiles were studied during floral bud developments, among flower colors and in organs. The results demonstrate that the three CHS genes are associated with the anthocyanin synthesis and the flower coloration, and DchCHS1 is a major CHS gene working on the flower color in D. chinensis.
Materials and methods
Plant materials
Dianthus chinensis seeds (www.ebay.co.uk/) were planted in plastic pots with Pindstrup growing media (Pindstrup Substrate, Latvia) and kept in a growth chamber with a cycle of 16 h light with 22 °C and 8 h dark with 20 °C. The light intensity was 150 µmol m−2 s−1. The seedlings were transplanted in the greenhouse at Inner Mongolia Agricultural University, Hohhot, China.
Floral buds were classified into three development stages (Fig. 1a). Buds at stage 1 and stage 2 were about 1 cm and 1.5 cm in length, respectively. Both had no pigment in petals. Buds at stage 3 were about 2.0 cm in length with pigments in petals.
Expressions of DchCHSs at 3 stages of floral buds in D. chinensis. a The floral buds and its own petals in the floral buds; b qRT-PCR analysis of three DchCHSs at 3 stages compared with their respective expressions at stage1; c qRT-PCR analysis of three DchCHSs at 3 stages compared with DchCHS1 expression at stage 1
Flowering of detached floral buds
Floral buds with stems were collected from the greenhouse and placed immediately in water. They were delivered to the laboratory within 20 min of harvest. The stems were recut to 2 cm under water. The buds were inserted into plastic tubes containing nutrition buffers and placed in the growth chamber as mentioned above. The nutrition buffer was prepared according to Shang et al. (2007). The flowering percentage of the detached floral buds was calculated.
Isolation of DchCHSs
Total RNA was extracted with Trizol reagent (Invitrogen) from leaves. The first-strand cDNA was synthesized from 1 μg of total RNA and used as the template. Based on the CHS sequence of D. caryophyllus (Z67982.1), DchCHS1-F (5ʹ-GTCGCTTCATGCTCTACCAAC-3ʹ) and DchCHS1-R (5ʹ-GCTAGGACTGAACGCATCCTC-3ʹ) were designed to amplify a 407 bp fragment of DchCHS1 (KX893854). The PCR product was cloned into the pGEM-T Easy vector (Promega) and sequenced (Takara Biotechnology Co., Ltd). The 3-end cDNA sequence of DchCHS1 was amplified with the gene-specific primer (5ʹ-GGCTCACTTTTCACCTGCTC-3ʹ) and the 3′RACE universal primer by the RACE technique. Based on in-house unpublished RNA-seq data of D. chinensis, two unigenes annotated as chalcone synthases, c22817 and c33072 were selected.
Sequence analysis
Homologous sequences of the DchCHSs were identified using the BLAST program on the website of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Amino acid sequence alignment was generated using DNAMAN 5.0. Phylogenetic analysis was performed using MEGA 6.0 with a neighbor-joining method.
Plasmid construction
The first-strand cDNA was used as the template. For DchCHS1 silencing, a 407 bp fragment (1–407 bp) was amplified with DchCHS1-F and DchCHS1-R. For DchCHS2 silencing, a 446 bp fragment (126–571 bp) was amplified with 5ʹ-ACCCTCCCAACCAAATGACC-3ʹ and 5ʹ-TAGTCGCAAAACCGTACCCC-3ʹ. For DchCHS3 silencing, a 474 bp fragment (884–1357 bp) was amplified with 5ʹ-TCCAGACCTGACAATTGAGC-3ʹ and 5ʹ-TGAAGCACAACGGTCTCAAC-3ʹ. The amplified fragments were inserted into pGEM-T Easy vectors and sequenced. The inserted fragments were excised from the plasmids by EcoRI restriction enzyme and sub-cloned into pTRV2 vectors to generate pTRV2-DchCHS1, pTRV2-DchCHS2 and pTRV2-DchCHS3. The resulting plasmids were sequenced to verify correct insertions of the fragments.
Preparation of Agrobacterium
Electrocompetent cells of Agrobacterium tumefaciens strain GV3101 were transformed with pTRV1, pTRV2, pTRV2-DchCHS1, pTRV2-DchCHS2 and pTRV2-DchCHS3, respectively. The transformed cells were selected on LB plates containing 50 mg L−1 kanamycin with 50 mg L−1 rifampicin. The positive colonies of pTRV1 and pTRV2 were verified using pTRV1 primers (5ʹ-TTACAGGTTATTTGGGCTAG-3ʹ and 5ʹ-CCGGGTTCAATTCCTTATC-3ʹ) and pTRV2 primers (5ʹ-ACGGACGAGTGGACTTAGATTC-3ʹ and 5ʹ-GTTTAATGTCTTCGGGACATGC-3ʹ), respectively. The positive colonies of pTRV2-DchCHS1, pTRV2-DchCHS2 and pTRV2-DchCHS3 were confirmed with pTRV2 primers. The transformed Agrobacterium cells were cultured overnight at 28 °C in LB medium containing appropriated antibiotics. After centrifugation, the Agrobacterium cells were suspended and incubated in the infiltration buffer (10 mM MES, 150 µM acetosyringone and 10 mM MgCl2) to a final OD600 of 3.0 at 25 °C.
Vacuum infiltration of detached floral buds
According to the flowering percentage of detached floral buds in the nutrition buffer, we collected the buds at stage 2 and stage 3. The buds at the same stage from the same plant were divided into two parts. One part was submerged in the infiltration buffer containing a 1:1 ratio mixture of pTRV1 with pTRV2 (control), and the other part in the infiltration buffer containing a 1:1 ratio mixture of pTRV1 with pTRV2 constructs containing different DchCHS fragments. After infiltration in a −100 kPa vacuum chamber for 20 min, the buds were inserted into plastic tubes containing the nutrition buffer and put in the growth chamber as mentioned above.
Analysis of anthocyanin contents and DchCHSs expressions in the silenced flowers
The flowers of the control and the flowers with silencing phenotypes were collected and ground to a fine powder under liquid nitrogen.
Pigments were extracted from 0.1 g of the ground powder. The ground powder was added to 600 μL of 1% HCl in methanol (v/v) and incubated overnight at 4 °C with gentle shaking. The extract was mixed with 400 μL of water and 400 μL of chloroform. After centrifugation, the absorbance of the supernatant was measured at 530 and 657 nm. The anthocyanin content was calculated using A530−0.25A657 (Rabino and Mancinelli 1986).
Total RNA was extracted from 0.1 g of the ground powder and used to synthesize the first-strand cDNA, which was used as the template for qRT-PCR. QRT-PCR was performed using Roche LightCycler 480 II (Switzerland). Reactions contained 10 μL 2 × SYBR Advantage qPCR Premix (Takara), 0.2 μL cDNA template and 0.6 μM of each gene-specific primer in a final volume of 20 μL. The gene-specific primers were 5ʹ-TGTTGAGCGACTTTGGGAAC-3ʹ with 5ʹ-CCCTTCACCTGTTGTGGTTG-3ʹ for DchCHS1, 5ʹ-CCGCAAATAGCATACAAAAGC-3ʹ with 5ʹ-CCTCCTGGGTGAACCACATAAA-3ʹ for DchCHS2 and 5ʹ-CGCCGATTACCAGCTCACC-3ʹ with 5ʹ-CCGTGCCTCCAGCAAAGC-3ʹ for DchCHS3. These primers were designed outside the region used for VIGS to avoid amplification of RNA from the silencing vectors. DchACTIN (KX664102) from in-house unpublished RNA-seq data was used as an internal control and its primers were 5ʹ-ATGCCCCCGCTATGTATGT-3ʹ and 5ʹ-GCCAAATCAAGACGCAAGAT-3ʹ. The thermal program was as follows: 1 cycle of 10 min at 95 °C; 40 cycles of 15 s at 95 °C and 30 s at 60 °C. Three biological replicates were made. The relative expression level of the gene was presented by 2 _∆∆CT (Livak and Schmittgen 2001).
Analysis of expression levels of the DchCHSs
Total RNA was extracted from petals of floral buds at each stage (Fig. 1a), flowers of three kinds of flower colors (Fig. 2a) and organs of purple flowering plants (Fig. 3a), and was used to synthesize the first-strand cDNA. The organs included roots, lower-stems (with pigment in nodes and internodes), upper-stems (with no pigment), leaves and flowers. The procedure of qRT-PCR, the gene-specific primers for amplifying DchCHSs transcripts and the internal control were the same as mentioned above.
Results
Sequence analysis of the DchCHS genes
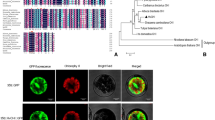
The full-length DchCHS1 cDNA of 898 bp (KX893854) includes a predicted open reading frame (ORF) of 705 bp. Two unigenes encoding putative chalcone synthases were identified in an in-house D. chinensis transcriptome database and re-named as DchCHS2 (MK404175) and DchCHS3 (MK416198). The full-length DchCHS2 cDNA of 1332 bp includes an ORF of 1197 bp and the full-length DchCHS3 cDNA of 1690 bp includes an ORF of 1176 bp. Their deduced amino acid sequences share high homology with the predicted CHSs in Caryophyllaceae, including the DcaCHS (Z67982) in Dianthus caryophyllus, the DmoCHS (AF267173) in Dianthus monspessulanus, the GpaCHS (AY309966) in Gypsophila paniculate and the SliCHS (KT954903) in Silene littorea (Fig. 4).
Alignment of the putative protein sequence of DchCHSs with its homologues from caryophyllus family. The accession numbers of the used amino acid sequences are as follows: DcaCHS (Z67982) in Dianthus caryophyllus, DmoCHS (AF267173) in Dianthus monspessulanus, GpaCHS (AY309966) in Gypsophila paniculate, SliCHS (KT954903) in Silene littorea
The evolutionary relationships of the DchCHSs, the four CHSs in Caryophyllaceae and nine CHSs from other ornamental plants were studied (Fig. 5). The nine CHSs, including two of Petunia hybrida in Solanaceae (Koes et al. 1989; Koseki et al. 2005), two of Dahlia variabilis (Ohno et al. 2011) and two of Gerbera hybrida in Asteraceae (Helariutta et al. 1995), and three of Lily hybrida in Lilium (Nakatsuka et al. 2003; Suzuki et al. 2016), are related with flower pigments. The DchCHS1, the DchCHS3 and the four CHSs belong to the Caryophyllaceae family and cluster together in the phylogenetic tree (Fig. 5). It might be that they diverge from a recent common ancestor. The DchCHS2 in a separate group shows that it has a remote relation not only with the CHSs from other ornamental plants, but also with the CHSs in Caryophyllaceae (Fig. 5). The result suggests that the DchCHSs encoding proteins might be different in enzymatic activities.
Phylogenetic tree based on the amino acid sequences of DchCHSs with its homologues from other species. The tree was constructed using MEGA 6.0 and a neighborjoining method, with 1 000 bootstrap replications. The proteins of used genes (with GenBank accession number) in this analysis were as follows:: DcaCHS (Z67982) in Dianthus caryophyllus, DmoCHS (AF267173) in Dianthus monspessulanus, GpaCHS ( AY309966) in Gypsophila paniculate, SliCHS (KT954903) in Silene littorea, DvaCHS1 (AB576660) and DvaCHS2 (AB591825) in Dahlia variabilis, GhyCHS1 (Z38096) and GhyCHS3 (Z38098) in Gerbera hybrida, LhyCHSA (BAB40786), LhyCHSB (AB058639) and LyhCHSC (AB058640) in Lilium hybrid, PhyCHSA (AF233638) and PhyCHSJ (X14597) in Petunia x hybrida
Effect of silencing DchCHSs in flowers
Fifty percent of the detached flower buds were opened at stage 1, and 100% were opened at stage 2 and stage 3 in the nutrition buffer (Fig. 1a). Therefore, the buds at stage 2 and stage 3 were used as explants for infiltration. Four days after infiltration, only the flowers from the detached floral buds at stage 2 showed silencing phenotypes (Fig. 6Aa, Ba, Ca).
VIGS of DchCHSs using the TRV vector in D. chinensis. A Floral buds were infiltrated with empty vector (left) and with pTRV2-DchCHS1 (right). a Silencing phenotype. b Anthocyanin content. c qRT-PCR analysis; B Floral buds were infiltrated with empty vector (left) and with pTRV2-DchCHS2 (right). a Silencing phenotype. b Anthocyanin content. c qRT-PCR analysis; C Floral buds were infiltrated with empty vector (left) and with pTRV2-DchCHS3 (right). a Silencing phenotype. b Anthocyanin content. c qRT-PCR analysis. The * symbol represents significant difference (P < 0.05) according to Student’s t-test
One hundred detached floral buds were treated with pTRV2-DchCHS1. Nineteen flowers showed white or pale purple colors (Fig. 6Aa). The anthocyanin content in the silenced flower (8.16 ± 1.41 per 100 mg fresh petals) was dramatically lower than that in the TRV control flowers (28.54 ± 0.72 per 100 mg in fresh petals) (Fig. 6Ab). Meanwhile, DchCHS1 expression was significantly down-regulated (Fig. 6Ac).
Eleven flowers appeared pale purple when pTRV2-DchCHS2 was delivered into 100 detached floral buds (Fig. 6Ba). The silencing phenotype was associated with a significant reduction of the anthocyanin content (17.54 ± 4.56 per 100 mg fresh petals versus 27.80 ± 3.06 per 100 mg fresh petals) (Fig. 6Bb) and of DchCHS2 expression (0.45 ± 0.31 versus 1.00 ± 0.55) (Fig. 6Bc).
Fifteen percent of the detached floral buds treated with pTRV2-DchCHS3 showed reducing purple colors in the flowers (Fig. 6Ca). Compared with the TRV control, the anthocyanin content (Fig. 6Cb) and DchCHS3 expression greatly decreased in the silenced flower (Fig. 6Cc).
Expression patterns of the DchCHSs
The floral buds at stage 1 and stage 2 with un-pigmented petals and those at stage 3 with pigmented petals were collected to study expression profiles of the DchCHSs in D. chinensis (Fig. 1). The expressions of DchCHS1 and DchCHS2 were sharply down-regulated from stage 1 to stage 2 and kept stable from stage 2 to stage 3 (Fig. 1b). No obvious difference in DchCHS3 expression was observed during the floral bud development (Fig. 1b). DchCHS1 expression at each stage was significantly higher than those of DchCHS2 and DchCHS3, and DchCHS3 expressions at stage 2 and 3 were significantly higher than the DchCHS2 (Fig. 1c).
We chose three kinds of flower colors to investigate expression patterns of the DchCHSs (Fig. 2). The expressions of DchCHS1 and DchCHS3 in purple flowers were higher than those in white with purple center (W + P) flowers, and those in W + P flowers were dramatically higher than those in white flowers (Fig. 2b). DchCHS2 expression in the purple was 20.93 times higher than that in the white and 40.25 times higher than that in the W + P (Fig. 2b). In each flower color, DchCHS1 expression was substantially higher than the DchCHS3, and the DchCHS3 was substantially higher than the DchCHS2 (Fig. 2c).
We also evaluated expression levels of the DchCHSs in organs (Fig. 3). The expression levels of DchCHS1 in flowers were 2.82 times higher than those in leaves, 4.78 times higher than those in lower-stems, 5.73 times higher than those in roots and 44.08 times higher than those in upper-stems (Fig. 3b). DchCHS2 expressions in leaves were dramatically higher than those in the other organs, and there is no difference among those in the other organs. No obvious difference in DchCHS3 expression was observed among the organs except for upper-stems (Fig. 3b).
Discussion
CHS has been shown to be encoded by a multigene family in ornamental plants. Twelve CHS genes have been isolated in Petunia hybrida (Koes et al. 1989). Three CHS-like genes isolated in Gerbera hybrida are specifically expressed in the corolla (Helariutta et al. 1995). Four and three CHS genes have been obtained in Dahlia variabilis (Ohno et al. 2011) and in Asiatic hybrid lily (Nakatsuka et al. 2003; Suzuki et al. 2016), respectively. D. chinensis exhibits a wide variation in flower colors. In our paper, we got three DchCHSs in D. chinensis. The DchCHS1, the DchCHS3 and the four CHSs from Caryophyllaceae in one cluster show that they might diverge from a recent common ancestor (Fig. 5). The DchCHS2 is different. It is clustered into a separate group from the CHSs in Caryophyllaceae and the CHSs from other plant species (Fig. 5). Low homology at the protein level between expressed CHS genes indicates that these genes encoding proteins probably have slightly different enzymatic activities (Koes et al. 1989).
The Tobacco rattle virus (TRV)-based VIGS system has been developed and used for verifying gene functions in diverse plants (Chen et al. 2004; Singh et al. 2012; Zhong et al. 2014; Dobnik et al. 2016). The infection of virus vector carrying sequences of plant genes is replicated and produces double-stranded RNA molecules in infected plants. These double-stranded RNA molecules will be cut into small oligonucleotides (siRNA). The siRNA acts as a guide to target the degradation of endogenous mRNA homologous and the symptoms would appear in the infected plant as the loss of the function of the target protein (Kalantidis et al. 2008; Demircan and Akkaya 2010; Senthil-Kumar and Mysore 2011). CHS is the first key enzyme in the anthocyanin synthesis. Suppression of the CHS gene will obtain white or light color flowers (Suzuki et al. 2000; Aida et al. 2000). The TRV-VIGS approach was used to silence the endogenous CHS gene in D. chinensis. Silencing DchCHS1 produced white or attenuated purple flowers (Fig. 6Aa). Silencing DchCHS2 (Fig. 6Ba) or DchCHS3 (Fig. 6Ca) resulted in reducing purple flowers, but no white sector was in the flowers. The silencing phenotypes were associated with substantial reductions of anthocyanin contents and transcript levels of the silenced DchCHS (Fig. 6Aa, Ab, Ac, Bb, Bc, Cb, Cc). The silencing rate of DchCHS1 was 19% and higher than those of DchCHS2 and DchCHS3. The results show that the three DchCHSs are involved in the anthocyanin biosynthesis and are related to the flower color in D. chinensis.
The CHS genes, which are expressed in floral tissues and involved in anthocyanin synthesis, have different expression levels and expression patterns in ornamental plants. In Petunia hybrida, the expression level of CHSA is far higher than that of CHSJ in floral tissues (Koes et al. 1989), and probably encodes a major CHS protein working in the anthocyanin biosynthesis (Koseki et al. 2005; Morita et al. 2012). Two of three CHS-like genes are specifically expressed in the corolla and their encoding enzymes have different catalytic properties during the corolla development in Gerbera hybrida (Helariutta et al. 1995). The expressions of CHS1 and CHS2 in the colored areas of bicolor flower petals are stronger than those in the pure white areas and are unrelated with the petal developmental stage except for the early stage in Dahlia variabilis. Simultaneous post-transcriptional gene silencing of them produces pure white parts of petals (Ohno et al. 2011). Recent research shows that CHS2 is the key gene involved in bicolor formation of dahlia (Ohno et al. 2018). Three CHS genes in Asiatic hybrid lily are expressed in anthocyanin-pigmented tepals, but their expression patterns are different (Nakatsuka et al. 2003; Suzuki et al. 2016). Our study showed that the expression patterns of the DchCHSs were different during floral bud developments (Fig. 1b), among flower colors (Fig. 2b) and in organs (Fig. 3b). Their expression levels were inconsistent with the pigment accumulation in the petals of floral buds (Fig. 1a, b) and the size of pigments in the flowers (Fig. 2a, b). DchCHS1 expression remained the highest level and DchCHS2 expression remained the lowest level at each floral bud stage (Fig. 1c) and in each flower color (Fig. 2c). The expression of DchCHS1 in flowers and the expression of DchCHS2 in leaves were far higher than those in other organs (Fig. 3b). DchCHS3 expression had no difference during floral bud development (Fig. 1b), between the purple and the white with purple center (Fig. 2b) and among organs except for upper-stems (Fig. 3b). The expression levels of DchCHS1, DchCHS2 and DchCHS3 increased 9.25-fold, 20.93-fold and 24.92-fold between purple flowers and white flowers, respectively (Fig. 2c). It further shows that their encoding proteins might be involved in the flower color. At least three DchCHSs are anthocyanin-related genes in D. chinensis, which may be the reason that it is rich in flower colors and color patterns of flowers. However, how they function on the anthocyanin biosynthesis and the flower coloration is still unknown.
Conclusion
The three DchCHSs obtained in D. chinensis are involved in the anthocyanin biosynthesis and in flower color, but their functions might be slightly different. DchCHS1 is the major CHS gene expressed at each floral bud stage, in each flower color and in flower organs, and probably encodes a major CHS protein working in the anthocyanin synthesis in D. chinensis flowers.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable for that section.
References
Aida R, Kishimoto S, Tanaka Y, Shibata M (2000) Modification of flower color in torenia (Torenia fournieri Lind.) by genetic transformation. Plant Sci 153:33–42. https://doi.org/10.1016/S0168-9452(99)00239-3
Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS (2004) Chalconesynthaseasareporterinvirus-inducedgenesilencingstudiesof flowersenescence. Plant Mol Biol 55:521–530. https://doi.org/10.1007/s11103-004-0590-7
Demircan T, Akkaya MS (2010) Virus induced gene silencing inBrachypodium distachyon, a model organism for cereals. Plant Cell Tissue and Organ Culture 100(1):91–96. https://doi.org/10.1007/s11240-009-9623-x
Deroles SC, Bradley JM, Schwinn KE, Markham KR, Bloor S, Manson DG, Davie KM (1998) An antisense chalcone synthase cDNA leads to novel colour patterns in lisianthus (Eustoma grandiflorum) flowers. Mol Breeding 4(1):59–66
Dobnik D, Lazar A, Stare T, Gruden K, Vleeshouwers VGAA, Žel J (2016) Solanum venturii, a suitable model system for virus-induced gene silencing studies in potato reveals StMKK6 as an important player in plant immunity. Plant Methods 12:29–40. https://doi.org/10.1186/s13007-016-0129-3
Fukusaki EI, Kawasaki K, Kajiyama S, Ana Ch-II, Suzuki K, Tanaka Y, Kobayashi A (2004) Flower color modulations of Torenia hybridaby downregulation of chalcone synthase genes with RNAinterference. J Biotechnol 111:229–240. https://doi.org/10.1016/j.jbiotec.2004.02.019
Gu ZY, Men SQ, Zhu J, Hao Q, Tong NN, Liu ZA, Zhang HC, Shu Q, Wang LS (2019) Chalcone synthase is ubiquitinated and degraded viainteractions with a RING-H2 protein in petals of Paeonia‘He Xie.’ J Exp Bot 70(18):4749–4762. https://doi.org/10.1093/jxb/erz245
Helariutta Y, Elomaa P, Kotilainen M, Griesbach RJ, SchrÖder J, Teeri TH (1995) Chalcone synthase-like genes active during corolla development are differentially expressed and encode enzymes with different catalytic properties in Gerbera hybrida (Asteraceae). Plant Mol Biol 28(1):47–60. https://doi.org/10.1007/BF00042037
Kalantidis K, Schumacher HT, Alexiadis T, Helm JM (2008) RNA silencingmovement in plants. Biol Cell 100(1):13–26. https://doi.org/10.1042/BC20070079
Koes RE, Spelt CE, Mol JNM (1989) The chalcone synthase multigene family ofPetunia hybrid(V30): differential, light-regulated expression during flower development and UV light induction. Plant Mol Biol 12(2):213–225. https://doi.org/10.1007/BF00020506
Koseki M, Goto K, Masuta C, Kanazawa A (2005) The star-type color pattern in Petunia hybrida “red Star” flowers is induced by the sequence-specific degradation of the chalcone synthase RNA. Plant Cell Physiol 46(11):1879–1883. https://doi.org/10.1093/pcp/pci192
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expressiondata using real-time quantitative PCR and the2-△△CT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Manmathan H, Shaner D, Snelling J, Tisserat N, Lapitan N (2013) Virus-induced gene silencing of Arabidopsis thaliana genehomologues in wheat identifies genes conferring improveddrought tolerance. J Exp Bot 64(5):1381–1392. https://doi.org/10.1093/jxb/ert003
Morita Y, Saito R, Ban Y, Tanikawa N, Kuchitsu K, Ando T, Yoshikawa M, Habu Y, Ozeki Y, Nakayama M (2012) Tandemly arranged chalcone synthase A genes contribute to the spatially regulated expression of siRNA and the natural bicolor floral phenotype inPetunia hybrida. Plant J 70:739–749. https://doi.org/10.1111/j.1365-313X.2012.04908.x
Nabavi SM, Šamec D, Tomczyk M, Milella L, Russo D, Habtemariam S, Suntar I, Rastrelli L, Daglia M, Xiao J, Giampieri F, Battino M, Sobarzo-Sanchez E, Nabavi SF, Yousefi B, Jeandet P, Xu S, Shirooie S (2020) Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2018.11.005
Nakatsuka A, Izumi Y, Yamagishi M (2003) Spatial and temporal expression of chalcone synthase and dihydroflavonol 4-reductase genes in the Asiatic hybrid lily. Plant Sci 165(4):759–767. https://doi.org/10.1016/S0168-9452(03)00254-1
Ohno S, Hori W, Hosokawa M, Tatsuzawa F, Doi M (2018) Post-transcriptional silencing of chalcone synthase is involved in phenotypic labilityin petals and leaves of bicolor dahlia (Dahlia variabilis) ‘Yuino.’ Planta 47(2):413–428. https://doi.org/10.1007/s00425-017-2796-3
Ohno S, Hosokawa M, Kojima M, Kitamura Y, Hoshino A, Tatsuzawa F, Doi M, Yazawa S (2011) Simultaneous post-transcriptional gene silencing of two different chalcone synthase genes resulting in pure white flowers in the octoploid dahlia. Planta 234(5): 945–958. https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/189856/1/s00425–011–1456–2.pdf
Quadrana L, Rodriguez MC, López M, Bermúdez L, Nunes-Nesi A, Fernie AR, Descalzo A, Asis R, Rossi M, Asurmendi S, Carrari F (2011) Coupling virus-induced gene silencing to exogenous green fluorescence protein expression provides a highly efficient system for functional genomics in Arabidopsis and across all stages of tomato fruit development. Plant Physiol 156(3):1278–1291. https://doi.org/10.1104/pp.111.177345
Rabino I, Mancinelli AL (1986) Light, temperature, and anthocyanin production. Plant Physiol 81:922–924
Senthil-Kumar M, Mysore KS (2011) New dimensions for VIGS inplant functional genomics. Trends Plant Sci 16(12):656–665. https://doi.org/10.1016/j.tplants.2011.08.006
Shang YJ, Schwinn KE, Bennett MJ, Hunter DA, Waugh TL, Pathirana NN, Brummell DA, Jameson PE, Davies KM (2007) Methods for transient assay of gene function in floral tissues. Plant Methods 3(1):1–12. https://doi.org/10.1186/1746-4811-3-1
Singh A, Liang YC, Kumar P, Jiang CZ, Reid MS (2012) Co-silencing of the Mirabilis antiviral protein (MAP) permits virusinducedgene silencing (VIGS) of other genes in Four O’Clockplants (Mirabilis jalapa). J Hortic Sci Biotech 87(4):334–340. https://doi.org/10.1080/14620316.2012.11512873
Suzuki KI, Xue HM, Tanaka Y, Fukui Y, Fukuchi-Mizutani M, Murakami Y, Katsumoto Y, Tsuda S, Kusumi T (2000) Flower color modifications of Torenia hybrida by cosuppression of anthocyanin biosynthesis genes. Mol Breed 6:239–246. https://doi.org/10.1023/A:1009678514695
Suzuki K, Suzuki T, Nakatsuka T, Dohra H, Yamagishi M, Matsuyama K, Matsuura H (2016) RNA-seq-based evaluation of bicolor tepal pigmentation in Asiatic hybrid lilies (Lilium spp.). BMC Genom 17(1):611–629. https://doi.org/10.1186/s12864-016-2995-5
Zhong XH, Yuan X, Wu Z, Khan MA, Chen J, Li XX, Gong BH, Zhao Y, Wu J, Wu CY, Yi MF (2014) Virus-induced gene silencing for comparative functional studiesin Gladiolus hybridus. Plant Cell Rep 33(2):301–312. https://doi.org/10.1007/s00299-013-1530-2
Acknowledgments
This study was supported by the National Natural Science Foundation of China ( 31960611).
Funding
This study was supported by the National Natural Science Foundation of China (31960611).
Author information
Authors and Affiliations
Contributions
Author contributions
JL conducted the experiments and acquired the results. XLH carried out the data analysis. XQH designed the research and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Henryk Flachowsky.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Hao, XL. & He, XQ. Characterization of three chalcone synthase-like genes in Dianthus chinensis. Plant Cell Tiss Organ Cult 146, 483–492 (2021). https://doi.org/10.1007/s11240-021-02081-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02081-8