Abstract
Camamu Bay is a shallow estuarine system, and its circulation pattern is governed by tidal forcing. The system is formed by four sectors including the main channel and three hydrodynamic regions, delimited by the influence of the five tributaries. Water samples were collected in two different pluviometric periods (dry and rainy), at nine sampling points over the three hydrodynamic regions, and at a mooring (13°52′27.42″S, 38°57′46.19″W), in the main channel, where samples were collected every 3 h over cycles of spring tides. At each sampling station, physicochemical variables were measured and water samples were collected for analysis of dissolved inorganic nutrients and chlorophyll a, composition and cell density studies of microphytoplankton. A total of 201 taxa were identified, and the great majority of the taxa were from the marine environment. The taxonomic composition varied between the hydrodynamic regions, with greater chain-forming diatom richness, in the two study periods. Although the highest concentration of dissolved inorganic nutrients was observed in the rainy period, microphytoplankton cell density did not increase in this period. The patterns of the estuarine phytoplankton community in tropical oligotrophic systems are still little known when compared to the temperate regions. Camamu Bay is one of the last known areas in the tropical South Atlantic, and this study confirms its oligotrophic characteristics, based on abiotic and biotic conditions. We highlighted the importance of knowledge of pristine coastal systems as a tool for the evaluation of anthropogenic changes in these areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The phytoplankton is a highly diverse polyphyletic group, formed by photosynthesizing microalgae and cyanobacteria, (Margalef 1978; Reynolds 2006) and accounts for about half the primary productivity of the Earth (Cloern and Dufford 2005; Falkowski and Raven 2007; Tilstone et al. 2017). Between 4000 and 5000 marine species are recognized (Sournia et al. 1991; Vaulot 2001; Granéli and Turner 2006; Vargas et al. 2015), although there is a general consensus that this number is underestimated for the true phytoplankton diversity (Vaulot 2001).
The phytoplankton organisms colonize the upper part of the water column, up to the limit of light penetration (Vaulot 2001). The taxonomic composition, population abundance and community structure and their distribution patterns are strongly influenced by physical processes (e.g., currents and turbulence) and chemical variations (e.g., nutrient influx) of the water (Ghosal et al. 2000; Rabalais 2002), in addition to the between-species interactions (e.g., competition for resources and grazing pressure) (Margalef 1978; Litchman and Klausmeier 2008). Primary productivity in aquatic systems thus responds to the action of bottom-up (e.g., light and nutrient availability) and top-down factors (e.g., herbivores) that control the phytoplankton biomass, composition and diversity (Metaxas and Scheibling 1996).
The phytoplankton is the most important group of primary marine producers and makes important contributions, especially in coastal environments such as estuaries (Boney 1975). In function of the terrestrial influx and organic discharges of anthropogenic origin, these environments have high support capacity for the phytoplankton community. This permits the maintenance of high primary productivity rates (Kjerfve 1990; Attrill and Rundle 2002; Cloern et al. 2014) and consequently of other important ecosystemic services, because it represents the first transference link of primary energy in the aquatic food chains sustaining the biomass of the upper trophic levels (Cloern and Dufford 2005).
Estuaries are environments with particular characteristics determined by the entry of river water and a strong addition of mechanical energy, the tides (Simpson et al. 1990; Cloern 1991). Due to pulses of nutrients into the water column (e.g., nutrient re-suspension to more illuminated regions), the tides can cause temporal variations in the phytoplankton community composition, structure and distribution, especially in shallow coastal waters, where generally this fluctuation periodicity is more notable (Blauw et al. 2012). The patterns of the estuarine phytoplankton community, especially in tropical oligotrophic systems, are still little known when compared to the extensive number of studies carried out on estuarine systems in temperate regions (Rochelle-Newall et al. 2011; Manna et al. 2012; Pan et al. 2016).
Camamu Bay is a shallow estuarine system, with a circulation pattern governed mainly by tidal forcing (Amorim et al. 2011), with a predominance of marine influence, most notably in periods with less rainfall when the fluvial inputs are even less pronounced (Amorim et al. 2015). Surrounded by small towns, the bay is inserted in a hydrographic basin which presents one of the best states of conservation on the Brazilian coast (Hatje et al. 2008), where effects from anthropogenic pressure that are commonly reported in other tropical estuaries have not yet been observed (e.g., Figueiredo et al. 2007; Veronez Júnior et al. 2009; Thrush et al. 2013; Van Chu et al. 2014).
Camamu Bay is a system formed by four sectors including the main channel, from the entrance of the Bay with direct communication with the adjacent coastal area, and three ramifications, delimited by the influxes of the five tributaries that flow in from the north, south and central sector (Menezes 2011; Amorim et al. 2015). Considering this hydrodynamic configuration, and starting from the hypothesis that the microphytoplankton structure and composition vary between the regions of the bay and over the tidal cycles, the objectives of the present study were to (1) characterize the microphytoplankton community composition and structure and (2) quantify the relative importance of the environmental variables for the community patterns, related to the hydrodynamic characteristics and rainfall. Although it is the third largest bay in Brazil (Souza-Lima et al. 2003), it is still one of the least-known regions of the coast (Leão et al. 2003), and because it is a pristine tropical estuarine system (Carreira et al. 2016), it can serve as a model for studies of potential environmental changes, and therefore, it is important to know about its natural characteristics.
2 Materials and methods
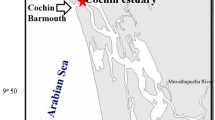
Study area
– Camamu Bay (Fig. 1) is situated on the central coast of the state of Bahia, Brazil (13°40.2′S to 14°12.6′S and 38°55.8′W to 39°9.6′W), a region with a hot and wet climate, 25 °C mean annual temperature, high rainfall, between the isoietas 2400 and 2600 mm year−1 (CRA 2007).
The hydrodynamic circulation inside the bay is forced by tides, with a maximum range of 2.7 m during the high tides and speeds that range from 0.6 to 1.2 m s−1 (Amorim et al. 2015). The main river components that make the bay an estuarine system are: Sirinhaém River is located in the northern part, a shallow channel with 7.3 m average depth. The rivers Igrapiúna, Pinaré and Sorojó are in the central part, a shallow zone, with 3.0 m average depth and maximum depth of 7.0 m, inside the river channels, and the Maraú River is located in the southern part, with 6.2 m average depth (Oliveira et al. 2002; Hatje et al. 2008; Amorim et al. 2011). The Maraú channel is a partially mixed system, the Sirinhaém channel is well mixed during the spring tides and partially mixed during the neap tides, and they are very mixed from the bay entrance to the central region. The system has a seasonally controlled cleaning/purifying capacity, and the water is renewed every 90 days in the dry periods and every 30 days in the rainfall periods (Amorim et al. 2015).
Sampling design
– Sampling was conducted during spring tides, under two different pluviometric periods. The first was in October 2014, after an accumulated rainfall of 198.2 mm (rainfall period), and the second was in January 2015 after a total rainfall of 93.2 mm (dry period), considering the 30-day period prior to each sampling.
The spatial samplings were made at nine sites, distributed three by three (Fig. 1), in each one of the hydrodynamic regions (Serinhaém, Central and Maraú) of the Camamu Bay. Time-series sampling were made at a mooring (13°52′27.42″S 38°57′46.19″W), at 3-h intervals, covering a total variation of 12 h over two tidal cycles (spring tides), in October 2014 and January 2015. The mooring, at the bay entrance (Fig. 1), was chosen because it is representative of all the water exchange of the system, following hydrodynamic modeling of the area, according to Menezes (2011).
Environmental sampling
– The rainfall data were obtained from the National Meteorological Institute (INMET 2001) from records of the automatic meteorological station (13°54′S, 38°58′W) in the municipality of Maraú—Bahia. The fluvial discharges of the main tributaries were calculated using the equation by Smith et al. (1999).
At each sampling station and at each hour of the tidal cycle, the temperature, salinity, pH and water dissolved oxygen were measured in situ using a multiparameter meter (Hanna HI 9829, São Paulo, Brazil). Water transparence was estimated by Secchi disk disappearance depth measurements.
Water samples (5 L) were collected (42 samples/period), using a Van Dorn bottle, for nutrient and chlorophyll a analyses. The samples were stored in polyethylene flasks, previously washed with HCl and distilled water, and samples were immediately filtered after each collection using a vacuum pump with fiberglass filters (Whatman GF/F—0.7 μm pore, Sigma-Aldrich, Missouri, USA) until they were clogged. Aliquots of 250 mL of the filtrated volume of each sample and the filters, wrapped in aluminum paper, were kept frozen for, at most, two weeks until the respective analyses were made.
The dissolved inorganic nutrients (nitrite, nitrate, ammonium, phosphate and silicate) were analyzed by the spectrophotometric method according to Grasshoff et al. (1983). To analyze the chlorophyll a, the trichromatic method was performed in acetone extracts and the concentrations were calculated according to Jeffrey and Humphrey (1975).
Microphytoplankton sampling
– At each sampling station and at each hour of the tidal cycles (time-series sampling), 250 mL water samples (14 samples/period) were collected by a horizontal tows using a plankton net (20 µm mesh opening) to study the microphytoplankton community composition, and 1 L water samples (42 samples/period) were collected from the subsurface (~ half a meter deep) with Van Dorn bottle, for quantitative analyses. All samples were stored in dark polyethylene flasks and fixed with 1% lugol.
The qualitative analyses were carried out by observations on slides, under a light microscope (Olympus CX31, Tokyo, Japan). The taxa were identified using the following references: Cupp 1943; Cleve-Euler 1955; Wood 1968; Dodge 1985; Balech 1988; Hernández-Becerril 1996; Tomas 1997; Tiffany and Hernández-Becerril 2005; Tenenbaum 2006; Throndsen et al. 2007.
Quantitative analyses to determine microphytoplankton cell densities (cell L−1) were made according to the Utermöhl (1958), using 50- or 100-mL sedimentation chambers, depending on the sample, with counting of the bottom of the chamber using an inverted microscope (Motic AE 2000, Hong Kong, China) at 200× to 400× magnification.
Statistical analyses
– The environmental variables were analyzed with the Kruskal–Wallis analysis of variance, after checking the assumptions for parametric analyses (normality and homoscedasticity) using the Shapiro–Wilk and Levene test followed by the value multiple comparison test to assess the occurrence of significant differences (P < 0.05) in the physiochemical variables, dissolved inorganic nutrients and chlorophyll a (1) between the hydrodynamic regions of the bay and (2) between the tidal cycles.
The microphytoplankton community of Camamu Bay was characterized from measurements of species richness (S), the Shannon diversity index (H′) and the Pielou species evenness (J′).
To observe the ranking of the samples as functions of the dissimilarity in species composition and abundance (1) between the hydrodynamic regions of the bay and (2) over the tidal cycles, non-metric multidimensional scaling (NMDS) was carried out based on distance matrixes, calculated from the Bray–Curtis index. The occurrence of significant differences in community composition (1) between the hydrodynamic regions of the bay and (2) the times of the tidal cycle, in the two sampling periods, was tested by permutational multivariate analysis of variance (PERMANOVA, 999 randomizations, P < 0.05) based on the Bray–Curtis dissimilarity index.
Analyses of variance of the microphytoplankton density were made using two-way ANOVA, verifying the prerequisites for parametric analysis (normality and homoscedasticity), with the Shapiro–Wilk and Levene tests, respectively, and the value multiple comparison test P (P < 0.05) a posteriori to assess the occurrence of significant differences (1) between the hydrodynamic regions of the bay and (2) during the tidal cycles.
To quantify the relative contributions of the environmental variables in explaining the patterns of species composition and cell density in function (1) of the spatial distribution in the bay and (2) over the tidal cycles, a variation partitioning analysis was performed (Borcard et al. 1992; Peres-Neto et al. 2006), using the analyzed environmental data, separated into four groups: (1) tide height; (2) riverine discharges; (3) dissolved nutrients (i.e., nitrite, nitrate, phosphate, silicate); and (4) physicochemical (i.e., temperature, salinity, transparency, pH and dissolved oxygen). The environmental data were standardized because of their different measurement units, and the Hellinger transformation was used for the cell density data (Legendre and Gallagher 2001). All the statistical analyses were carried out in an R environment (R Core Team 2016).
3 Results
Environmental variables
– The fluvial discharges ranged from 0.70 to 5.56 m3 s−1 during the two sampling periods, higher in the rainy season (Table 1). The Camamu Bay estuarine system was characterized by mean transparency values in the water column of 1.3 (± 0.6) m to 2 (± 1) m, water temperature ranging from 26 (± 0.2) °C to 30 (± 0.5) °C and salinity of 32 (± 1.6) in the two periods. The mean pH values of the water were 8.2 (± 0.1) in the two periods. The water physicochemical variables did not vary significantly between the hydrodynamic regions, nor at the mooring, in tidal cycles.
Dissolved oxygen and temperature were the only variables that presented significant differences between the sampling periods. Dissolved oxygen saturation rates were above 100% in the first (rainy) sampling period (106.6 ± 31%) and below 50% (42 ± 3%) in the second (dry) sampling period, significantly higher (P = 0.004) in the rainy sampling period. The temperature showed significantly higher values in the second (dry) sampling period (P = 0.0003).
Ammonium concentrations were lower than the detection level of the method (< 0.01 µM) throughout the study, and there were low concentrations, both of the other nitrogen forms (nitrite and nitrate) and also of phosphate and silicate (Table 1). The dissolved inorganic nutrient concentrations did not differ significantly between the hydrodynamic regions, nor at the mooring, in tidal cycles. But nitrite (P = 0.0002), nitrate (P = 0.0003) and silicate (P = 0.0003) exhibited significantly larger concentrations in the first (rainy) sampling period, as did chlorophyll a (P = 0.0004).
Microphytoplankton
– A total of 201 taxa were identified during the study (Table S1). Diatoms (ca. 70%) and dinoflagellates (ca. 25%) were the most abundant groups in the specific composition and marine species predominated. Only 15 taxa identified (ca. 7%) are of freshwater habitat.
The species diversity index in the bay was always around 3 bits ind−1, and there was no dominance of any taxon (J′ = 0.7 a 0.8) under the conditions of the study (Fig. 2a, b)
Variation the species richness (S), Shannon–Weaver Index (H′) and Pielou equability (J′) a in the three hydrodynamic regions of Camamu Bay: SER (Serinhaém), CEN (Central) and MAR (Maraú), and b in the mooring (tidal cycles) in the first (I—October 2014) and second (dry) sampling period (II—January 2015)
Organisms with tycoplanktonic habit represented about 13% of the taxa, and benthic species were also identified: Ostreopsis cf. ovata J. Schmidt, Prorocentrum cf. emarginatum Y. Fukuyo, Prorocentrum cf. rhathymum A.R. Loebl. et al. and Lyngbya majuscula Harvey ex Gomont.
Chain-forming diatoms, including Bacillaria paxillifera (Müller) Hendey, Paralia sulcata (Ehrenberg) Cleve and Odontella aurita (Lyngbye) C. Agardh, were the most abundant taxa in the community.
The mean cell density in the bay, considering the three hydrodynamic regions and the mooring, was 1.0 × 104 (± 3.8 × 103) cells L−1 in the first (rainy) sampling and 1.3 × 104 (± 3.8 × 103) cells L−1 in the second (dry) sampling period, and there were no significant differences between these periods (P = 0.5).
Spatial variations
– A total of 144 taxa were identified in the northern part of the bay (Serinhaém—Ser), with 16 taxa occurring exclusively in this region, while 139 taxa were identified in both the central (Cen) and southern (Maraú—Mar) portions; the central region had 12, and Maraú had 13 exclusive taxa (Table S1).
Based on the abundance of the identified taxa, the pattern observed in the NMDS (stress = 0.16), indicated an ordination of the samples as a function of the significant differences of the composition (PERMANOVA r2 = 0.27, P = 0.001) between the hydrodynamic regions of the bay and the significant species substitution (PERMANOVA r2 = 0.71, P = 0.001) in each region between the two sampling periods (Fig. 3a).
Non-metric multidimensional scaling (NMDS) analysis of the taxa abundance a in the three hydrodynamic regions of Camamu Bay. Up pointing triangle = Serinhaém, circle = Central and rectangle = Maraú, and b in the mooring (tidal cycles). Down pointing triangle = 7:00 a.m., Circle = 10:00 a.m., square = 1:00 p.m., diamond = 4:00 p.m., up pointing triangle = 7:00 p.m. Black symbols = first (rainy) sampling period (October 2014); gray symbols = second (dry) sampling period (January 2015)
The larger richness and taxonomic diversities were recorded in the Serinhaém region (Fig. 2a) in both the sampling periods. The most abundant species in this region were Paralia sulcata and Bacillaria paxillifera in the first (rainy) sampling period and P. sulcata and Guinardia striata (Stolterfoth) Hasle in the second (dry) sampling period. In the central region, the lowest species richness in the study was recorded in the first sampling period (Fig. 2a), with larger abundances of the species B. paxillifera and Odontella aurita, while in the second sampling period B. paxillifera and Navicula sp. were the most abundant species in the region. The Maraú region exhibited the lowest variation in species richness and diversity between the sampling periods (Fig. 2a), with the species O. aurita and Rhabdonema adriaticum Kützing occurring in larger abundances in the first sampling period, and O. aurita and Navicula sp. in the second sampling period.
The microphytoplankton cell density (Table 2) between the hydrodynamic regions ranged from 3.2 × 103 cells L−1 in Maraú (rainfall period) to 2 × 104 cells L−1 in Serinhaém (dry period) and did not differ significantly between the three regions (P = 0.19), or between the sampling periods (P = 0.58).
The variance partition indicated that the environmental variables explained about 76% of the specific composition of the microphytoplankton community. The isolated tidal variation fraction explained 19% of the variation, followed by the isolated fractions of the nutrient and physicochemical variables that each explained 14% (Fig. 4a).
For cell density, the variance partition explained 83% of the total variance, represented mainly by the nutrients fraction (41%), followed by the water physicochemical factors fraction that explained 16% (Fig. 4b).
Tidal cycles variations
– A total of 118 taxa were identified at the mooring (Table 2). Considering their abundance during the tidal cycles, the samples could be ranked as a function of the differences in the specific composition between the two cycles (Fig. 3b). The pattern observed in the NMDS ranking (stress = 0.15) was supported by the significant difference between the two sampling periods (PERMANOVA r2 = 0.42, P = 0.001), and there were no significant differences in composition between the periods of the same tidal cycle (PERMANOVA r2 = 0.10, P = 0.078).
Paralia sulcata and Thalassionema nitzschioides (Grunow) Mereschkowsky were the most abundant species during the tidal cycle in the rainy period, between 07:00 h and 16:00 h, and in terms of greater abundance, P. sulcata was replaced by Melosira moniliformes (O.F. Müller) C. Agardh at 19:00 h. Chaetoceros decipiens Cleve and Pseudo-nitzschia sp. were the most abundant species during the tidal cycle in the dry period, between 07:00 h and 16:00 h, while at 19:00 h P. sulcata was the most abundant species.
Species richness varied more in the rainy period tidal cycle (44–66) than in the dry period (57–67). The species diversity ranged from 2.68 to 3.18 bits ind−1 in the rainy period tidal cycle and from 2.85 to 3.28 bits ind−1 in the dry period, but no taxon dominated (J′) in any tidal cycle (Fig. 2b).
The microphytoplankton cell density variation over the tidal cycles was from 6.0 × 103 to 1.2 × 104 cells L−1 in the rainy period and 8.3 × 103 to 2.0 × 104 cells L−1 (Table 3) in the dry period, but they were not significantly different (P = 0.3) each other. During the rainy period tidal cycle, at 13:00 h, the microphytoplankton density was significantly lower (P < 0.001) than at the other times of the cycle. During the dry period tidal cycle, the microphytoplankton density varied more, differing significantly (P < 0.001) between all the sampling times, except between 10:00 h and 13:00 h.
The variance partition showed that the variation in species composition during the tidal cycles was explained by environmental conditions at 78%, mainly by the isolated fraction of the physical–chemical variables (37%), followed by the shared fractions of the physical–chemical variables and nutrients, that explained 30% (Fig. 5a).
For the microphytoplankton density variation in the tidal cycles, the variance partition exhibited a total explanation of 84.5%, and the physiochemical variables fraction accounted for 63% of the explanation (Fig. 5b).
4 Discussion
Environmental variables
– In tropical (and subtropical) environments, the rainfall is a fundamental modulator of the availability of dissolved nutrients and optical qualities of water (Bastos et al. 2005), and consequently, the larger determinant of chlorophyll a concentrations in the aquatic systems (Losada et al. 2003). In the Camamu Bay, region with an annual mean rainfall of approximately 1480 mm and monthly mean of 123 mm, it was observed that the water physicochemical variables were influenced by the larger riverine discharges in the rainy period (198.2 mm), causing increased in the nutrient concentrations in the system. This entry of fresh water allowed an increase in the chlorophyll a biomass, keeping the waters in the bay well oxygenated and saturated (> 100%) in dissolved oxygen.
In contrast, in the dry period (93.2 mm), the smaller river influxes were reflected in lower nutrient and chlorophyll a concentrations and the establishment of heterotrophic conditions reflected the subsaturated dissolved oxygen (~ 50%). The dissolved oxygen saturation may indicate an increase in primary productivity rates in the systems (Campelo et al. 1999), so it, in future studies, is important to include measures of primary productivity in the Camamu Bay in order to better characterize this system condition.
The increase in the nutrient concentrations during rainy periods is a condition commonly observed in a variety of tropical estuaries (e.g., Dittmar et al. 2001; Sarma et al. 2010; Bastos et al. 2011). However, in spite of the enrichment of the system in the period with more rain, the waters in Camamu Bay remained characteristically oligotrophic (Niencheski et al. 1999; Knoppers et al. 2002). Similar results were observed in oyster cropping areas in the bay by Affe and Santana (2016), due to small riverine discharges because of very small discharges from the tributaries that did not change significantly between the rainy and dry seasons (Amorim et al. 2011). The low anthropogenic impact was shown, for example, by the absence of influences from domestic sewage in the area (Carreira et al. 2016).
Despite the three subsystems (hydrodynamic regions) with different hydrodynamic characteristics (i.e., riverine discharges, residence time and water renewal) (Menezes 2011), significant variations were not observed in the environmental characteristics between the three hydrodynamic regions during the study periods, reflecting the great influence from the marine influx into the bay and the high mixture in the water column (Amorim et al. 2015).
Microphytoplankton
– The microphytoplankton composition, during the two sampling periods, illustrated the hydrodynamic forcing in the estuarine system, and especially, the strong influence from the marine influx, shown by the species predominant in this habitat. The predominance of diatoms (i.e., larger species richness and abundance) in the bay followed a typical coastal water pattern (e.g., Garg and Bhaskar 2000; Gin et al. 2000; Procopiak et al. 2006; Silva et al. 2009; Rochelle-Newall et al. 2011; Rezende et al. 2015; Carvalho et al. 2016) as did the occurrence of tycoplanktonic species that are re-suspended by the action of turbulence in the water column (Tilstone et al. 2000; Odebrecht et al. 2002; Smayda 2002).
The diatoms comprised an important fraction (70%) of the community in the bay, as already observed in other coastal and estuarine environments (e.g., Fernandes and Brandini 2004; Lacerda et al. 2004; Silva et al. 2009). The high number of chain-forming diatoms perhaps is related to (e.g., Paralia sulcata, Bacillaria paxillifera, Guinardia striata, Odontella aurita), greater efficiency in light capture and greater nutrient storage capacity, given the higher surface/volume ratios of cells (Villareal et al. 1993; Hutchings et al. 1995; Sunda and Huntsman 1995; Klausmeier and Litchman 2001), in addition to the greater capacity to regulate fluctuation that favors their predominance in turbulent waters (Round et al. 1990).
Different depths were not compared in the present study, but the conditions of the high mixture in Camamu Bay have been reported previously by Affe and Santana (2016) due to the absence of differences in temperature and salinity in the water column. According to Amorim et al. (2015), there is partial stratification during neap tides, with differences in salinity smaller than two between the surface and the bottom, but during spring tides the water column is very mixed throughout the bay. In this region of Brazil, the semiarid climate of the interior of the state at the heads of the hydrographic regions, and the small discharges (~ 10 m3 s−1) from the tributaries, associated with the tide intervals, result in very mixed water columns, as occurred in Todos os Santos Bay (Cirano and Lessa 2007), situated between 13°S and 22°S on the Brazilian eastern platform (Knoppers et al. 2002).
The marine influx was the main force in the microphytoplankton composition in the Camamu Bay, repeating a pattern found in tidal forced estuarine systems (Bazin et al. 2014), resulting in a high diversity of marine species in the system, during the two sampling periods. In tropical estuaries, there is alternation between periods of high rainfall, with greater riverine discharges and periods when marine influxes dominate and there is less rainfall, as these parameters most influence the species richness distribution of the phytoplankton community (Lacerda et al. 2004; Silva et al. 2009).
During the tidal cycles, the predominance of chain-forming diatoms was also observed in both the study periods. In the rainy period, Paralia sulcata was the most abundant species during the day (07:00–16:00 h), with an inverse pattern in the night sampling (19:00 h), when decreased that species density. The sharp reduction in salinity at night (35.5–24) possibly influenced this change, as in other systems, in which reductions in abundance of P. sulcata were also recorded for low-salinity conditions at the water surface (McQuoida and Nordberg 2003; Gebühr et al. 2009; Guo et al. 2014). In contrast, during the second tidal cycle (dry period) the P. sulcata cell density remained approximately the same throughout the day, increasing at night, when the water salinity remained high (~ 31) throughout the cycle. Because it is a tycoplanktonic species, P. sulcata can be considered as an example of a turbulence indicator species in the bay, and its occurrence is common in brackish and marine environments, with intense vertical mixing (McQuoida and Nordberg 2003; Gebühr et al. 2009).
The microphytoplankton community in the Camamu Bay estuarine system exhibited a similar pattern between the hydrodynamic regions. The indices of phytoplankton diversity characterize the system as of being of high diversity (H′ > 2.5), according to the Margalef (1978) classification, and these indices were similar to those found in other coastal regions in northeastern Brazil, even the most eutrophied (e.g., Silva et al. 2009; Santiago et al. 2010). Maintaining this high diversity, considering the few resources available (i.e., dissolved nutrients) depends on processes that prevent the community from reaching competitive equilibrium, as discussed by Hutchinson (1961). Again, turbulence is indicated as a very important process, also for the phytoplankton community structure, especially in regions with relatively low nutrient concentrations (Barton et al. 2014), with turbulence facilitating nutrient re-suspension to the photic layer and maintaining phytoplankton suspension (Round et al. 1990; Ghosal et al. 2000; Barton et al. 2010; Pan et al. 2016).
Although there was enrichment of dissolved nutrients in the system in the rainy period, a corresponding increase was not observed in the microphytoplankton cell density. This result contradicts that observed in tropical estuaries that have seasonal rainfall variation, where the episodic pulses of fresh water are preponderant in the hydrodynamic and larger nutrient influx in the rainy period tends to lead to higher phytoplankton densities and biomass (Sassi et al. 1991; Eskinazi-Leça et al. 2004; Lancelot and Muylaert 2011), even in the most eutrophied (e.g., Grego et al. 2004; Honorato-da-Silva et al. 2004; Sousa et al. 2008; Costa et al. 2011; Matos et al. 2011, 2012). In Camamu Bay, the greater marine influence in the dry period and increases in the riverine discharges in the inland regions in the rainfall periods (Amorim et al. 2015) bring changes that, although they were shown in the environmental variability (i.e., differences in temperature, salinity) between the two periods analyzed did not determine significant alterations in the community structure and composition. The influence of the Brazil Current that flows all year along the Bahia coast (Signorini et al. 1989), and the predominance of marine influx in the estuarine system, may be the main factors in maintaining the oligotrophic character of the bay, even under different rainfall conditions.
Camamu Bay was shown to be a fairly mixed system in spring tides, with low nutrient concentrations even in conditions of greater riverine discharges in the rainy period. In summary, the data acquired in this study revealed the great tropical shelf water intrusions, which confers oligotrophic characteristics and marine species predominance in the three hydrodynamic regions of the system. The sampling in tidal cycles evidenced the importance of this forcing to the maintenance of the dynamic and high diversity observed in the microphytoplankton community. Microphytoplankton cell density did not vary significantly, but there were changes in species composition between the pluviometric periods, as a function of the variation of the abiotic (although discrete) conditions.
We highlighted the importance of this knowledge given the conservation conditions of the system in the face of the global climate change scenario and growing anthropogenic pressure that alters the dynamic and quality of the water in tropical estuarine systems. Camamu Bay is suggested as a potential model for studies of the ecology of the phytoplankton community, since observing community patterns in preserved oligotrophic environments serve as a basis for studies of the potential environmental changes on many systems.
References
Affe HMJ, Santana RMC (2016) Fitoplâncton em área de cultivo de ostras na Baía de Camamu: Investigação da ocorrência de microalgas potencialmente nocivas. Novas Edições Acadêmicas, NEA–A Ed, Saarbrücken, p 81
Amorim FN, Cirano M, Soares ID, Lentini CAD (2011) Coastal and shelf circulation in the vicinity of Camamu Bay (14°S), Eastern Brazilian Shelf. Cont Shelf Res 31:108–119
Amorim FN, Rezende LF, Cirano M, Lessa GC, Hajte V, Silva PMCA (2015) Oceanographic characteristics of Camamu Bay (14°S, Brazil) during dry and wet conditions. Rev Bras Geof 33:1–16
Attrill MJ, Rundle SD (2002) Ecotone or ecocline: ecological boundaries in estuaries. Estuar Coast Shelf Sci 55:929–936
Balech E (1988) Los dinoflagelados dell atlántico sudoccidental. Instituto Español de Oceanografia, Plublicaciones especiales, Madri, p 219
Barton AD, Dutkiewicz S, Flierl G, Bragg J, Follows MJ (2010) Patterns of diversity in marine phytoplankton. Science 327:1509–1511
Barton AD, Ward BA, Williams RG, Follows MJ (2014) The impact of fine-scale turbulence on phytoplankton community structure. Limnol Oceanogr Fluids Environ 4:34–49
Bastos RB, Feitosa FAN, Muniz K (2005) Variabilidade espaço-temporal da biomassa fitoplanctônica e hidrologia no estuário do rio Una (Pernambuco-Brasil). Trop Oceanogr 33:1–18
Bastos RB, Feitosa FAN, Koening ML, Machado RCA, Muniz K (2011) Caracterização de uma zona costeira tropical (Ipojuca, Pernambuco-Brasil): Produtividade fitoplanctônica e outras variáveis ambientais. BJAST 16:1–11
Bazin P, Jouenne F, Friedl T, Deton-Cabanillas AF, Le Roy B, Véron B (2014) Phytoplankton diversity and community composition along the estuarine gradient of a temperate macrotidal ecosystem: combined morphological and molecular approaches. PLoS ONE 4:1–18
Blauw AN, Beninca E, Laane RWPM, Greenwood N, Huisman J (2012) Dancing with the tides: fluctuations of coastal phytoplankton orchestrated by different oscillatory modes of the tidal cycle. PLoS ONE 11:1–14
Boney AD (1975) Phytoplankton. In: Edward Arnold (Ed) Ltd, Studies in biology, London, p 116
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Campelo MJA, Passavante JZO, Koening ML (1999) Biomassa fitoplanctônica (clorofi la a) e parâmetros ambientais na praia de Carne de Vaca, Goiana, Pernambuco, Brasil. Trab Oceanog Univ Fed PE Recife 27:27–41
Carreira RS, Cordeiro LGMS, Bernardes MC, Hatje V (2016) Distribution and characterization of organic matter using lipid biomarkers: a case study in a pristine tropical bay in NE Brazil. Estuar Coast Shelf Sci 168:1–9
Carvalho RCQ, Cutrim MVJ, Eschrique SA, Azevedo-Cutrim ACG, Moreira EG, Silveira PCA, Coêlho JM (2016) Microphytoplankton composition, chlorophyll-a concentration and environmental variables of the Maranhão Continental Shelf, Northern Brazil. Lat Am J Aquat Res 44:256–266
Cirano M, Lessa GC (2007) Oceanographic characteristics of Baía de Todos os Santos, Brazil. Rev Bras Geof 25:363–387
Cleve-Euler A (1955) Diatomeen von Schweswn und Finland. Alquimist e Wiksells Boktryckeri, Stockholm, p 232
Cloern JE (1991) Tidal stirring and phytoplankton bloom dynamics in an estuary. J Mar Res 49:203–221
Cloern JE, Dufford R (2005) Phytoplankton community ecology: principles applied in San Francisco Bay. Mar Ecol Prog Ser 285:11–28
Cloern JE, Foster SQ, Kleckner AE (2014) Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11:2477–2501
Costa VB, Sousa EB, Pinheiro SCC, Pereira LCC, Costa RM (2011) Effects of a high energy coastal environment on the structure and dynamics of phytoplankton communities (Brazilian Amazon littoral). J Coastal Res 64:354–358
CRA—Centro de Recursos Ambientais (2007) Assessoria de Comunicação Social/ASCOM, Sistema Estadual de Informações Ambientais da Bahia. http://www.seia.ba.gov. Accessed 21 Mar 2017
Cupp EE (1943) Marine plankton diatoms of the west coast of North America. University of California Press, London, p 237
Dittmar T, Lara RJ, Kattner G (2001) River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Mar Chem 73:253–271
Dodge JD (1985) Atlas of dinoflagellates. A scanning electron microscope survey. Ferrand Press, London, p 303
Eskinazi-Leça E, Koening ML, Silva-Cunha MGG (2004) Estrutura e dinâmica da comunidade fitoplanctônica. In: Eskinazi-Leça, E, Newmann-Leitão S, Costa MF (org.) Oceanografia um cenário tropical. Edições Bagaço Recife, pp 353–373
Falkowski PG, Raven JA (2007) Aquatic photosynthesis. University Press, Princeton, p 458
Fernandes LF, Brandini FP (2004) Diatom associations in the shelf waters off Paraná State, Southern Brazil: annual variations in relation to environmental factors. Braz J Oceanogr 52:19–34
Figueiredo JA, Menor EA, Noriega CED, Branco ES (2007) Evolução físico-química de águas do estuário do rio Timbó, Pernambuco: um caso de reavaliação ambiental (1984 e 2003). Estudos Geológicos 17:85–104
Garg A, Bhaskar PV (2000) Fluxes of diatoms in the Dona Paula Bay, west coast of India. J Plankton Res 22:2125–2136
Gebühr C, Wiltshire KH, Aberle N, Van Beusekom JE, Gerdts G (2009) Influence of nutrients, temperature, light and salinity on the occurrence of Paralia sulcata at Helgoland Roads, North Sea. Aquat Biol 7:185–197
Ghosal S, Rogers M, Wray A (2000) The turbulent life of phytoplankton. In: Proceedings of the summer program, Center for Turbulence Research, Stanford, pp 31–45
Gin KYH, Lin X, Zhang S (2000) Dynamics and size structure of phytoplankton in the coastal waters of Singapore. J Plankton Res 22:1465–1484
Granéli E, Turner JT (2006) An introduction to harmful algae. In: Granéli E et al (eds) Ecology of harmful algae. Ecological Studies. Springer, Heidelberg, pp 3–7
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis. Verlag Chemie Weinhein, New York, p 419
Grego CKS, Feitosa FAN, Honorato-da-Silva M, Flores Montes MJ (2004) Distribuição espacial e sazonal da clorofila a fitoplanctônica e hidrologia do estuário do rio Timbó (Paulista - PE). Trop Oceanogr 32:135–236
Guo PY, Shen HT, Wang JH (2014) Species diversity, community structure and distribution of phytoplankton in the Changjiang estuary during dry and flood periods. JMBA 94:459–472
Hatje V, Barros F, Magalhães W, Riato VB, Amorim FN, Figueiredo MB, Spanó S, Cirano M (2008) Trace metals and benthic macrofauna distributions in Camamu Bay, Brazil: sediment quality prior oil and gas exploration. Mar Pollut Bull 56:348–379
Hernández-Becerril DU (1996) A morphological study of Chaetoceros species (Bacillariophyta) from the plankton of the Pacific Ocean of Mexico. Bull Nat Hist Mus Lond (Bot) 26:1–73
Honorato-da-Silva M, Passavante JZO, Silva-Cunha MGG, Nascimento-Vieira DA, Grego CKS, Muniz K (2004) Distribuição espacial e sazonal da biomassa fitoplanctônica e dos parâmetros hidrológicos no estuário do rio Formoso (Rio Formoso, Pernambuco, Brasil). Trop Oceanogr 32:89–106
Hutchings L, Pitcher G, Probyn T, Bailey G (1995) The chemical and biological consequences of coastal upwelling. In: Summerhayes CP, Emers KC, Angel MV, Smith RL, Zeitzchel B (eds) Upwelling in the ocean: modern processes and ancient records. John Wiley and Sons, Berlin, pp 65–83
Hutchinson GE (1961) The paradox of the plankton. Am Nat 95:137–145
INMET- Instituto Nacional de Meteorologia, Centro de Previsão de Tempo e Estudos Climáticos – Instituto Nacional de Pesquisas Espaciais/INMET/CPETEC-INPE (2001) http://proclima.cptec.inpe.br/balanco_hidrico/balancohidrico.shtml Accessed 18 Mai 2017
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae, and natural phytoplankton. Biochem Physiol Pflanz 167:194–204
Kjerfve B (1990) Manual for investigation of hydrological processes in mangrove ecosystems. UNESCO/UNDP, New Delhi, p 79
Klausmeier CA, Litchman E (2001) Algal games: the vertical distribution of phytoplankton in poorly mixed water columns. Limnol Oceanogr 46:1998–2007
Knoppers BA, Ekau W, Figueiredo JAG, Soares GA (2002) Zona costeira e plataforma continental do Brasil. In: Pereira RC, Soares Gomes A (org.) Biologia Marinha. Interciência, Rio de Janeiro, p 631
Lacerda SR, Koening ML, Neumann-Leitão S, Flores-Montes MJ (2004) Phytoplankton nyctemeral variation at a tropical river estuary (Itamaracá-Pernambuco-Brazil). Braz J Biol 64:81–94
Lancelot C, Muylaert K (2011) Trends in estuarine phytoplankton ecology, In: Wolanski E et al (eds) Treatise on estuarine and coastal science: 7. Functioning ecosystems at the land-ocean interface, Den Burg, pp 5–15
Leão ZMAN, Kikuchi RKP, Testa V (2003) Corals and coral reefs of Brazil. In: Latin America coral reefs. Elsevier, Boston, pp 9–52
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Litchman E, Klausmeier C (2008) Trait-based community ecology of phytoplankton. Annu Rev Ecol Evol Syst 39:615–639
Losada APM, Feitosa FAN, Lins IC (2003) Variação sazonal e espacial da biomassa fitoplanctônica nos estuários dos rios Ilhetas e Mamucaba (Tamandaré-PE) relacionada com parâmetros hidrológicos. Trop Oceanogr 31:1–29
Manna S, Chaudhuri K, Sen Sharma K, Naskar P, Bhattacharyya S, Bhattacharyya M (2012) Interplay of physical, chemical and biological components in estuarine ecosystem with special reference to Sundarbans, India. In: Ecological water quality 10. Water treatment and reuse. InTech. Editor, India, pp 205–238
Margalef R (1978) Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 1:493–509
Matos JB, Sodré DKL, Costa KG, Pereira LCC, Costa RM (2011) Spatial and temporal variation in the composition and biomass of phytoplankton in an Amazonian estuary. J Coastal Res 64:1525–1529
Matos JB, Silva NIS, Carneiro LCCP, Costa RM (2012) Caracterização quali-quantitativa do fitoplâncton da zona de arrebentação de uma praia amazônica. Acta Bot Bras 26:979–990
McQuoida NR, Nordberg K (2003) The diatom Paralia sulcata as an environmental indicator species in coastal sediments. Estuar Coast Shelf Sci 56:339–354
Menezes FOS (2011) Modelagem hidrodinâmica da Baía de Camamu. Monograph. Universidade Federal da Bahia, Salvador
Metaxas A, Scheibling RE (1996) Top-down and bottom-up regulation of phytoplankton assemblages in tidepools. Mar Ecol Prog Ser 145:161–177
Niencheski LF, Baumgarten MG, Filmann G, Windom HL (1999) Nutrients and suspended matter behavior in the Patos Lagoon Estuary (Brazil). In: Perillo GME, Piccolo MC, Pizoquivira MP (eds) Estuaries in South America. Springer, Berlin, pp 67–81
Odebrecht C, Azevedo SMF, Garcia VLM, Huszar VLM, Proença LAO, Rörig LR, Tenenbaum DR, Villac MC, Yunes JS (2002) Floraciones de microalgas nocivas en Brasil: estado del arte y proyectos en curso. In: Sar E, Ferrario M, Reguera B (eds) Floraciones algales nocivas en el Cono Sur Americano. Editora del Instituto Espanhol de Oceanografia, Espanha, Espanha, pp 219–233
Oliveira OMC, Queiroz AFS, Argolo JL (2002) Estudo mineralógico do sedimento de manguezal da Baía de Camamu-Ba. Revista Escola de Minas 55:147–151
Pan CH, Chuang YL, Chou LS, Chen MH, Lin HJ (2016) Factors governing phytoplankton biomass and production in tropical estuaries of western Taiwan. Cont Shelf Res 118:88–99
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Procopiak LK, Fernandes LF, Moreira Filho H (2006) Diatomáceas (Bacillariophyta) marinhas do Paraná, Sul do Brasil: lista de espécies com ênfase em espécies nocivas. Biota Neotrop 6:1–28
R development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rabalais NN (2002) Nitrogen in aquatic ecosystems. Ambio 31:102–112
Reynolds CS (2006) The ecology of phytoplankton. Cambridge University Press, Cambridge, p 507
Rezende KRV, Hatherly MMF, Pimenta CMM, Eduardo J, Vianna SC, Mangiavacchi N (2015) Phytoplankton community structure in one sector of Guanabara Bay (RJ, Brazil) during 2011 and 2012. Braz J oceanogr 63:239–254
Rochelle-Newall EJ, Chu VT, Pringault O, Amouroux D, Arfi R, Bettarel Y, Bouvier T, Bouvier C, Got P, Nguyen TMH, Mari X, Navarro P, Doung TN, Cao TTT, Pham TT, Quilom S, Torréton JP (2011) Phytoplankton distribution and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Mar Pollut Bull 62:2317–2329
Round FE, Crawford RM, Mann DG (1990) Diatoms: biology and morphology of the genera. Cambridge University Press, New York, p 747
Santiago MF, Silva-Cunha MGG, Neumann-Leitão S, Costa KMP, Palmeira GCB, Neto FFP, Nunes FSS (2010) Phytoplankton dynamics in a highly eutrophic estuary in tropical Brazil. Braz J Oceanogr 58:189–205
Sarma VVSS, Prasad VR, Kumar BSK, Rajeev K, Devi BMM, Reddy NPC, Sarma VV, Kuma MD (2010) Intraannual variability in nutrients in the Godavari estuary, India. Cont Shelf Res 30:2005–2014
Sassi R, Vêloso TM, Melo GN, Moura GF (1991) Variações diurnas do fitoplâncton e de parâmetros hidrológicos em recifes costeiros do Nordeste do Brasil. In: Encontro Brasileiro de Plâncton. UFPE, Recife pp 61–82
Signorini SR, Miranda LB, Evans DL, Stevenson MR, Imostroza HMV (1989) Corrente do Brasil: estrutura termica entre 19° e 25°S e circulação geostrofica. Bull lnst Oceanogr 37:33–49
Silva MH, Silva-Cunha MGG, Passavante JZO, Grego CKS, Muniz K (2009) Estrutura sazonal e espacial do microfitoplâncton no estuário tropical do rio Formoso, PE, Brasil. Acta Bot Bras 23:355–368
Simpson JH, Brown J, Matthews J, Allen G (1990) Tidal straining, density currents, and stirring in the control of estuarine stratification. Estuaries 13:125–132
Smayda TJ (2002) Turbulence, watermass stratification and harmful algal bloms: an alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 1:95–112
Smith SV, Marshall CJI, Crossland CJ (1999) Mexican and Central American coastal lagoon systems: carbon, nitrogen and phosphorus fluxes. LOICZ Reports and Studies, Germany, p 115
Sournia A, Chrétiennot-Dinet MJ, Ricard M (1991) Marine phytoplankton: how many species in the world ocean? J Plankton Res 11:1093–1099
Sousa EB, Costa VB, Pereira LCC, Costa RM (2008) Microfitoplâncton de águas costeiras amazônicas: Ilha Canela (Bragança-Pará-Brasil). Acta Bot Bras 22:626–636
Souza-Lima W, Manso CLC, Andrade EJ, Grillo JL (2003) Bacias Sedimentares Brasileiras. Bacia de Camamu. Phoenix 54:1–6
Sunda W, Huntsman SA (1995) Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390:389–392
Tenenbaum DR (2006) Dinoflagelados e Tintinídeos da Região Central Econômica Exclusiva Brasileira. Guia de Identificação. Rio de Janeiro Museu Nacional, Série livros, Rio de Janeiro, p 287
Throndsen J, Hasle GR, Tangen K (2007) Dinoflagellates-Dinophyta. In: Throndsen J, Hasle GR, Tangen K (eds) Phytoplankton of Norwegian coastal Waters. Almater Forlag AS, Oslo, Norway, pp 41–110
Thrush SF, Townsend M, Hewitt JE, Davies K, Lohrer AM, Lundquist C, Cartner K (2013) The many uses and values of estuarine ecosystems. In: Dymond JR (ed) Ecosystem services in New Zealand: conditions and trends. Manaaki Whenua Press, New Zealand, pp 226–237
Tiffany MA, Hernández-Becerril DU (2005) Valve development in the diatom family Asterolampraceae HL. Smith 1872. Micropaleontology 51:217–258
Tilstone GH, Míguez BM, Figueiras FG, Fermín EG (2000) Diatom dynamics in a coastal ecosystem affected by upwelling: coupling between species succession, circulation and biogeochemical processes. Mar Ecol Prog Ser 205:23–41
Tilstone GH, Lange PK, Misra A, Brewin RJW, Cain T (2017) Micro-phytoplankton photosynthesis, primary production and potential export production in the Atlantic Ocean. Prog Oceanogr. http://dx.doi.org/10.1016/j.pocean.2017.01.006
Tomas CR (1997) Identifying marine phytoplankton. Florida Academic Press, Miami, p 858
Utermöhl H (1958) Zur Vervolkommung der quantitativen phytoplankton-methodik. Mitt Int Ver Theor Angew Limnol 9:1–38
Van Chu T, Torréton JP, Mari X, Nguyen HMT, Pham KT, Pham TT, Bouvier T, Bettarel Y, Pringault O, Bouvier O, Rochelle-Newall E (2014) Nutrient ratios and the complex structure of phytoplankton communities in a highly turbid estuary of Southeast Asia. Environ Monit Assess 186:8555–8572
Vargas C, Audic S, Henry N et al (2015) Eukaryotic plankton diversity in the sunlit ocean. Science 348:1–12
Vaulot D (2001) Phytoplankton. Encyclopedia of life sciences. Nature Publishing Group, London, pp 1–7
Veronez Júnior P, Bastos AC, Quaresma VS (2009) Morfologia e distribuição sedimentar em um sistema estuarino tropical: Baía de Vitória, ES. Rev Bras Geof 27:609–624
Villareal TA, Altabet MA, Culver-Rymsza K (1993) Nitrogen transport by vertically migrating diatoms mats in the North Pacific Ocean. Nature 363:709–712
Wood EJF (1968) Dinoflagelates of the Caribbean Sea and adjacent areas. University of Miami Press, Florida, p 143
Acknowledgements
This research has been financial supported by the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, No. RED0006/2012). We are grateful to Dr. Diogo S.B. Rocha by the assistance in statistical analysis and map of the study area, to Dr. Marcelo F. Landim for making the laboratory available for the analyses of nutrients and chlorophyll besides the aid in the analysis of environmental data and the oceanographer Lorena Petersen N. Santos for their valuable logistical support during the fieldwork. JMCN acknowledges Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 307368/2015-7) for a Productivity Scholarship in Research.
Author information
Authors and Affiliations
Contributions
HMJA wrote the draft version of the manuscript. HMJA, MM and JMCN conceived the review. HMJA, MM and JMCN contributed to the concept and design of the study. All authors read and approved the final version of the article.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Affe, H.M.J., Menezes, M. & Nunes, J.M.C. Microphytoplankton in a tropical oligotrophic estuarine system: spatial variations and tidal cycles. Braz. J. Bot 41, 337–349 (2018). https://doi.org/10.1007/s40415-018-0447-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-018-0447-y