Abstract
Phytoplankton diversity and abundance in estuarine systems are controlled by many factors. Salinity, turbidity, and inorganic nutrient concentrations and their respective ratios have all been proposed as principal factors that structure phytoplankton diversity and influence the emergence of potentially toxic species. Although much work has been conducted on temperate estuaries, less is known about how phytoplankton diversity is controlled in tropical, monsoonal systems that are subject to large, seasonal shifts in hydrology and to rapidly changing land use. Here, we present the results of an investigation into the factors controlling phytoplankton species composition and distribution in a tropical, monsoonal estuary (Bach Dang estuary, North Vietnam). A total of 245 taxa, 89 genera from six algal divisions were observed. Bacillariophyceae were the most diverse group contributing to 51.4 % of the microalgal assemblage, followed by Dinophyceae (29.8 %), Chlorophyceae (10.2 %), Cyanophyceae (3.7 %), Euglenophyceae (3.7 %) and Dictyochophyceae (1.2 %). The phytoplankton community was structured by inorganic nutrient ratios (DSi:DIP and DIN:DIP) as well as by salinity and turbidity. Evidence of a decrease in phytoplankton diversity concomitant with an increase in abundance and dominance of certain species (e.g., Skeletonema costatum) and the appearance of some potentially toxic species over the last two decades was also found. These changes in phytoplankton diversity are probably due to a combination of land use change resulting in changes in nutrient ratios and concentrations and global change as both rainfall and temperature have increased over the last two decades. It is therefore probable in the future that phytoplankton diversity will continue to change, potentially favoring the emergence of toxic species in this system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities are responsible for the discharge of large amounts of nutrient-rich organic and inorganic waste into estuaries and coastal areas. Already a problem in many areas, eutrophication is now causing serious environmental impacts in developing countries. Faced with accelerating industrial and economic development, many estuarine and coastal ecosystems in these regions are rapidly becoming degraded. Increased urban, agricultural, and industrial inputs have led to alterations in the ecological and biogeochemical functioning of freshwater, marine and coastal ecosystems, leading to eutrophication and toxic algal blooms events, which in turn are causing huge economic losses to coastal communities (Anderson et al. 2002). Although many studies have highlighted the factors controlling phytoplankton diversity and community composition in temperate ecosystems, less work has focused on tropical systems, particularly those in Southeast Asia. This is despite the ecological and economic importance of these coastal ecosystems (Borges 2005; Halpern et al. 2008).
Phytoplankton species are sensitive to changes in environmental factors (Chapman 1996; Fonge et al. 2012). Variations in the structural characteristics of phytoplankton communities (i.e., diversity, richness, and dominant species groups) in estuaries and coastal waters can be due to changes in the environment such as eutrophication or turbidity shifts (Paerl 2009; Tas et al. 2009; Jaanus et al. 2009). Changes in nutrient concentration (e.g., N, P, Si) and the relative ratios between these nutrients play a significant role in controlling phytoplankton species diversity (Justić et al. 1995a, b). Redfield stated that for balanced Bacillariophyceae growth, the ratio between dissolved silicate (DSi), dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP) should be 16:16:1 (Redfield et al. 1963). Moreover, given that DSi is an essential element for Bacillariophyceae growth, decreases in this ratio will mean that other, non-siliceous species such as the Dinophyceae or the Chlorophyceae may well become dominant (Officer and Ryther 1980; Justić et al. 1995b).
The Red River system in North Vietnam is characteristic of Southeast Asian estuaries in that it has high temperatures, a rapidly growing population, an intensification of agriculture accompanied by large changes in land use, combined with increasing urbanization and industrialization. All of which have considerably altered the biogeochemistry and phytoplankton diversity of the ecosystem, particularly in the upstream waters (Trinh et al. 2012; Duong et al. 2006). The intensification of agriculture and industry in a drainage basin often results in an increase in nutrient concentrations and in shifts in their relative ratios (Howarth et al. 2012; Xu et al. 2010). Both of these are known to be important in structuring phytoplankton diversity in several East and Southeast Asian estuaries (Xu et al. 2010; Lui and Chen 2011; Lie et al. 2011; Huang et al. 2013). Finally, the monsoonal climate of Southeast Asia also means that large seasonal changes in hydrology are observed resulting in large changes in salinity, turbidity, and colored and non-colored dissolved organic matter over the length of the estuary (Lefebvre et al. 2012; Rochelle-Newall et al. 2011, 2014), all of which are important factors in structuring planktonic communities (Nayar et al. 2005b; Karlsson et al. 2009; Muylaert et al. 2009; Canini et al. 2013).
The aim of this study was to identify the main factors controlling phytoplankton community composition and abundance along the salinity gradient of the Bach Dang estuary during a complete annual cycle. We then compared this data to previous datasets from the last two decades in order to determine if phytoplankton community composition is changing and to propose what might be the causes of this change.
Methods
Description of study area
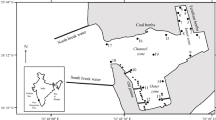
The Bach Dang estuary, located to the northeast of the Red River delta is a semi-closed, well mixed system characterized by a typical funnel-shaped estuary (Fig. 1). This area is strongly influenced by the tropical monsoon with a profound seasonal differentiation due to the northeast monsoon from November to April, and the South-West monsoon from May to October (Vu 1994). Average solar radiation in the Haiphong area is 10.5 kcal cm−2 year−1 (range 6 kcal cm−2 year−1 in February to 12 kcal cm−2 year−1 in May) and average annual rainfall in Haiphong area is 1,600–1,800 mm year−1 with the wet season from May to October accounting for 80–90 % of annual rainfall. Lowest rainfall is observed in December and January (Nguyen et al. 2014).
Location of sampling stations in the Bach Dang estuary, North Vietnam. The five stations are shown extending from the freshwater regions (Stn. 4) out to the offshore stations (Stn. 28). Latitude and longitude of each station as well as the maximum depths are provided in Table 1
Sampling
Samples were collected monthly at 1.5-m depth from October 2009 to October 2010 using a 5-L Niskin bottle at five fixed stations (Stns. 4, 10, 15, 23 and 28) along the salinity gradient (Fig. 1; Table 1). The sampling depth was selected to avoid the surface slick during periods when wave-height was elevated while still ensuring that the samples were collected in the upper surface mixed layer. All sampling was performed during neap tide to reduce the effect of the tide which has a range of 4 m in this area (Lefebvre et al. 2012). The distance between the most seaward station (Stn. 28) and the furthest inland station (Stn. 4) is approximately 20 km and the stations were spaced approximately 5.0 km apart. These stations form part of the grid of stations used in a series of programs on the Bach Dang estuary (e.g., Lefebvre et al. 2012; Rochelle-Newall et al. 2011) and so to ensure comparability with the other work, the same station identifiers are used.
Physical and chemical measurements
At each sampling station, temperature, salinity and fluorescence were measured in situ with a CTD profiler (SBE19+, Sea-Bird Electronics, Inc., Bellevue, WA, USA). Turbidity (in Formazin Turbidity Units, FTU) was measured with a Seapoint turbidity meter attached to the CTD package.
Sub-samples for inorganic nutrient (nitrate, nitrite, and phosphate) analyses were stored in 30-mL Nalgene vials after filtration (Whatman GF/F), and then pasteurized at 85 °C during 5 h (Aminot and Kerouel 1998). The samples for analysis of DSi followed the same treatment except that Whatman GF/F filters were replaced by cellulose ester membranes of 0.2 μm porosity (Nuclepore membrane). Samples for analyses of ammonium were immediately fixed and analyzed using the indophenol method of Eaton et al. (1995). Nitrites were measured following the method of Benschneider and Robinson (1952). Nitrates were reduced using the cadmium-copper method (Wood et al. 1967) and then measured as nitrites. DIPs were measured following the method of Murphy and Riley (1962) and silicates (dissolved and colloidal) were determined according to Mullin and Riley (1955) as adapted by Fanning and Pilson (1973). The detection limits were 0.005, 0.05, 0.005, and 0.05 μM for nitrite, nitrate, phosphate, and silicate, respectively. DIN was determined from the sum of nitrate, nitrite, and ammonium concentrations. Chlorophyll a (Chl a) was measured fluorometrically on methanol-extracted samples collected on GF/F filters using the method of Holm-Hansen et al. (1965).
Phytoplankton diversity and abundance
Samples for phytoplankton identification and abundance were collected at 1.5-m depth and fixed on boat with acidified Lugol’s solution (3 mL L−1; 0.3 % final concentration) and stored in the dark until return to the laboratory. Samples were allowed to settle for 48 h in 1-L glass cylinders in the dark. The supernatant was then gently siphoned off the top with a narrow plastic tube until about 50–100 mL remained. The remaining 50–100 ml was then transferred to 100 mL glass cylinders. After a further 24 h of settling, the upper volume was siphoned off leaving a volume of 20–30 mL. Samples were then stored in small vials until identification and counting, and three replicate samples were counted.
Phytoplankton species were identified based on their morphology under epifluorescence microscopy (Olympus BX51, Japan) using standard references (Balech 1995; Cronberg and Annadotter 2006; Duong and Vo 1997; Fukuyo et al. 1990; Ricard 1987; Taylor 1976; Tomas 1997; Truong 1993; Yamagishi 1992). Cell density was determined using an inverted microscope (Leica DMIL) and a Sedgwick-Rafter counting slide (1 mL volume).
Statistical analysis
The species diversity and dominance were investigated according to Shannon and Pielou (Shannon and Weaver 1963) and as discussed by Pielou (1975), Berger and Parker (1970) and Chao et al. (2005). The biological diversity (H′) and the dominance (d) indices were calculated according to the following equations:
where P i = n i /N, n i is the number of cells of the i species, N is the total number of cells of all the species in a sample.
where N max is the number of cells of the most abundant species, N is the total number of cells of all the species in a sample.
The correlation between phytoplankton diversity and the environmental factors was assessed by Canonical Correspondence Analysis (CCA). CCA was performed with MVSP v3 software (Kovach Computing Service, Anglesey Wales). Relative abundances of phytoplankton species were transformed with arcsin (x 0.5) according to Legendre and Legendre (1998) to normalize the distribution of the data as it is a condition required before applying multivariate statistical analysis (Dollhopf et al. 2001). Relationships between environmental factors and the abundance of the most dominant species in the estuary were estimated by Spearman correlation analysis with Systat.Sigma Plot v11 software (Systat Software Inc.).
Results
Environmental parameters
Monthly rainfall from October to April (dry season) was less than 100 mm. Maximum rainfall occurred during August (532 mm; Fig. 2) in the middle of the wet season. The lowest average temperatures over the whole transect were recorded in January (19.6 °C) whereas the highest were observed in July (30.1 °C; Table 1) and tended to decrease slightly (∼1 °C) from the freshwater stations seaward. Salinity varied between 0.1 and 31.8, with the lowest values observed at Stn. 4 and the highest at Stn. 28 (Fig. 3a; Table 1). Average salinities were highest in April and lowest in August (Fig. 3a). In general, turbidity decreased from riverine to seaward stations with the maximum of 182.9 FTU observed in Stn. 4 and minimum 0.1 FTU in Stn. 28 (Fig. 3b; Table 1). The lowest turbidity occurred during the dry season with average values of less than 20 FTU in March and April. Turbidity then peaked during the wet season with the average values higher than 90 FTU observed in July and August (Fig. 3b).
Contour plots of the temporal variation in salinity (ppt; panel a), turbidity (FTU; panel b) and Chl a (μg L−1; panel c) for each station over the sampling period. The intensity of the hot colors (red, orange) indicate higher concentrations and then darkness of the cooler colors (blue) indicate lower temperatures
Chlorophyll a and nutrients
Chl a concentration ranged from 0.4 μg L−1 at Stn. 28 in September to 24.6 μg L−1 at Stn. 23 in July (Fig. 3c) with no clear seasonal pattern. Concentrations of nitrate + nitrite and ammonium varied between station and season (Fig. 4a, b). The lowest concentrations of these nutrients were observed in the dry season and the highest were found in the wet season with a peak during July and August coincident with higher rainfall. The only exception was in early spring when a secondary peak of nutrients was observed (Fig. 4a–d). The proportion of nitrate to total DIN decreased from the riverine station to the most seaward station, varying between 75 (Stn. 4) and 21 % (Stn. 28) whereas the proportion of ammonium varied in the opposite direction, reaching 22 and 77 % in Stn. 4 and Stn. 28, respectively. The concentrations of nitrite were always low and represented only 4.9 % (±4.3) of the total DIN for all stations and all seasons combined (data not shown).
Contour plots of the temporal variation in dissolved nutrients at each station during the sampling period. Panel a nitrate + nitrite (μM), panel b ammonium (μM), panel c phosphate (μM) and panel d silicate (μM). The same color scheme is used as for Fig. 3
In general, phosphate concentrations were highest at Stn. 4 and lowest at Stn. 28 and followed the same pattern as for DIN with maximum values observed during July and August (Fig. 4c). Silicate concentrations also followed a similar pattern with peaks during the wet season and lower values observed during the dry season (December and January; Fig. 4d). This pattern of higher silicate concentrations during the wet season when flow is highest is common in estuaries where silicate is transported in river water from its terrestrial source.
In general, the DIN:DIP decreased seaward (Table 2) and varied between 7.2 in January at Stn. 28 to over 338 in July at Stn. 23 and, apart from a few exceptions, was always higher than the Redfield ratio of 16 (Redfield et al. 1963). In general, the DIN:DIP ratio was significantly higher during the wet season than during the dry season (Table 2; p < 0.05), the only exception was at Stn 23. The DSi:DIP was always higher than the value for balanced Bacillariophyceae growth of 16 (Justić et al. 1995a) and in one case was over 600 at Stn. 23 (July). Similar to the DSi:DIP, the DSi:DIN ratio was in excess of the values for balanced growth (a value of 1).
Phytoplankton composition and distribution
Phytoplankton abundance
In general, phytoplankton abundance varied along the estuary with a clear seasonal difference (Fig. 5a; Table 3, Supp. Mats Table 2). Two peaks of cell density were observed, the first during July–August concomitant with the highest Chl a concentrations (over 1 × 106 cells L−1 at Stn. 23 on the 1st of August), and the second during November–December (Fig. 5a). In contrast, the lowest cell densities were observed during the dry season with only 2,000 cells L−1 observed at Stn. 4 during March.
Contour plots of the variation in total cell abundance and in the Shannon index of the phytoplankton community. Panel a cell density (cells L−1), a log scale was used to present the contour lines. Panel b H′ index. The same color scheme is used as in Fig. 3
Phytoplankton community composition
A total of 245 species and sub-species (including 48 unidentified species), hereafter referred as taxa, belonging to 89 genera from 6 algal classes were observed (Table 3; Supp. Mats. Table 1). Of the 126 Bacillariophyceae taxa, the main genera were Chaetoceros (23 taxa), Coscinodiscus (10 taxa), Rhizosolenia (8 taxa) and Pleurosigma (7 taxa). The most common genera of the Dinophyceae were Protoperidinium (22 taxa) and Neoceratium (12 taxa). These genera were generally found in the marine influenced area of estuary. The two dominant Chlorophyceae genera (i.e. Pediastrum and Scenedesmus) had 9 taxa each and were mainly found in the inner, lower salinity part of the estuary. Euglena was the most common Euglenophyceae and was observed in the riverine-influenced area of estuary. There were 52 freshwater and brackish water taxa found in the estuary, belonging mainly to the Chlorophyceae and Cyanophyceae (34 taxa; Supp. Mats Table 1).
Phytoplankton species composition showed a typical variation pattern along the salinity gradient. The number of species of Bacillariophyceae, Dinophyceae and Dictyochophyceae increased gradually seaward. In contrast, the number of Chlorophyceae, Cyanophyceae and Euglenophyceae species were highest in the low salinity areas and decreased with increasing salinity (Fig. 6; Table 3, Supp. Mats. Tables 1, 2).
Contour plots of the temporal variation of the abundance of the six algal groups (cells L−1) for the sampling period. The same log scale was used for all of the plots. The same color scheme is used as in Fig. 3
In general, the Bacillariophyceae dominated the phytoplankton assemblage with only sporadic increases in dominance of the other phytoplankton groups (Fig. 6). For example, in Stns. 15, 23 and 28 Skeletonema costatum dominated during the entire annual cycle. Other species such as Thalassionema frauenfeldii, Lauderia annulata, Chaetoceros curvisetus, Pseudo-nitzschia spp., Thalassiosira spp. and Neoceratium spp. were also observed during the dry season (Supp. Mats. Table 2). In contrast, in the upstream stations (Stn. 4 and 10), although S. costatum was present throughout the entire annual cycle, Pediastrum simplex var. simplex (Chlorophyceae) and Microcystis spp. (Cyanophyceae) were also dominant during the wet season. During the dry season at these stations Pseudo-nitzschia spp., C. curvisetus, Thalassiosira spp. (Bacillariophyceae), Neoceratium furca (Dinophyceae) were the dominant species (Supp. Mats. Table 2).
In summary, the lower salinity stations were characterized by a community dominated by freshwater Bacillariophyceae, Cyanophyceae, Chlorophyceae, and some Euglenophyceae whereas the higher salinity stations were characterized by a community dominated by marine Bacillariophyceae and Dinophyceae (Fig. 6, Supp. Mats. Tables 1, 2).
The Cyanophyceae and Chlorophyceae were more abundant during the wet season than the dry season (Figs. 6c–d). The Dictyochophyceae were only observed in the offshore stations whereas the Euglenophyceae were only observed in the freshwater stations during the wet season. Both of these groups had very low abundances as compared to the dominant species (<1,000 cells L−1 and <500 cells L−1, for Dictyochophyceae and the Euglenophyceae, respectively). Diversity index (Shannon or H′ index) varied from less than 1 during the period of high flow (July and August) to over 4 at the end of the dry season (Fig. 5b).
Relationship between phytoplankton abundance, diversity, and environmental factors
In order to further understand the interactions between each environmental parameter and phytoplankton species abundance, a canonical correspondence analysis (CCA) was performed on the entire data set (Fig. 7). The two axes explained almost 40 % of the variations observed. Nutrient ratios, turbidity and temperature and salinity strongly structure the populations as evidenced by the distribution of the points along the first axis and the inflation values (Table 4). Moreover, the spread of the monthly points for each station also reflects the shifting importance of each parameter in structuring the phytoplankton community. For example, during the dry season, Stn. 4 is located in the upper left quadrant, whereas during the wet season, Stn. 4 is located on the right hand side of the first axis highlighting the role of salinity in structuring the populations at this station during periods of high flow. Variance inflation factors (VIF) measure how much the variance of the canonical coefficients is inflated by the presence of correlations among explanatory variables. A value of over 20 indicates multicollinearity whereas a variable with a VIF of 1 is one that only has unique information. The highest inflation factors were observed for the nutrient ratios DSi:DIP and DIN:DIP (8.9 and 11.5, respectively) compared to salinity (1.7) and turbidity (1.6).
Canonical correspondence analysis (CCA) of phytoplankton diversity and environmental parameters (salinity, turbidity, temperature and nutrient ratios) in the Bach Dang estuary for each of the five stations and for each date. The inflation values are provided in Table 4
Spearman correlation analysis was also performed in order to understand the relationship between environmental factors and the abundance of the twelve dominant species in the Bach Dang estuary (Table 5). As with the CCA for the whole community, the abundance of each of the dominant species was significantly correlated (p < 0.05) with salinity, turbidity and with the DIN:DIP ratio, regardless of where in the estuary the species were found. In general, when a species was negatively correlated with salinity (e.g. S. costatum; Table 5), it was positively correlated with turbidity and DIN:DIP ratio, reflecting the lower turbidities and DIN:DIP ratios at higher salinities. The opposite was also the case for species that were positively correlated with salinity (e.g., N. furca; Table 5) and therefore, negatively correlated with turbidity and DIN:DIP.
Discussion
It is widely accepted that salinity and turbidity are the two main structural factors in both temperate (Fisher et al. 1988; Mette et al. 2011; Muylaert et al. 2009) and tropical estuaries (Palleyi et al. 2008; Lueangthuwapranit et al. 2011). Here, we show that, unexpectedly, nutrient ratios and more specifically DSi:DIP and DIN:DIP, along with salinity and turbidity, were also prominent structuring agents of phytoplankton diversity in the Red River estuary. This is in contrast to previously published data from this system that proposed that salinity and turbidity, along with heavy metal concentrations were better determinants of diversity and production (Rochelle-Newall et al. 2011; Bettarel et al. 2011; Combe et al. 2013). However, the sampling effort was much lower in these studies (they only sampled once or twice during the year) and given the large temporal variability in the diversity index (H′; Fig. 5b) observed in our study, these contrasting results obtained in the two studies are not surprising.
Nutrients and chlorophyll a
In estuarine systems, the sources of organic and inorganic nutrients are multiple and include riverine inputs which are strongly influenced by terrestrial point and non-point source inputs, as is the case in this system (Le et al. 2010). The concentrations of nitrite, nitrate, and ammonium observed here are comparable to those found in other eutrophic estuaries (e.g., Delaware, Hudson and Chesapeake Bays) (Fisher et al. 1988) and to what have been previously observed in other tropical systems in Southeast Asia (e.g., Li et al. 2014, who worked in Hainan Island, China). We observed two peaks in nutrient concentration. The first corresponds to the rainy season and is probably due to an increase in runoff of land derived organic and inorganic nutrients. The second, observed towards the end of the dry season is potentially due to a combination of two different factors. The first possible mechanism is from the washout of nutrients from land and from atmospheric deposition as occurs during the first rain event after a prolonged dry period. Indeed, although it is considered as being the “dry season” rainfall does occur during the months prior to the true “rainy season”, as can be seen from the rainfall data (Fig. 2). A second hypothesis is that these nutrients come from benthic resuspension within the estuary and other work from this area has pointed out the importance of benthic nutrient fluxes in nutrient cycling in the Red River system (Trinh et al. 2012). However, given the two layer stratification in the system, it is probable that the upward flux of nutrients from the benthos is limited to the deeper, higher salinity layer at the deeper stations. It is therefore probable that the peak in nutrients observed during February and March is due to washout during small rain events. We also observed some intermediate increases in Chl a during this period that may well be fuelled by the higher inorganic nutrient concentrations observed at the start of spring at the end of the dry season. During July and August, the peaks in Chl a concentration occurred offshore where lower turbidities were observed, similar to the situation observed in other estuaries (e.g., Fisher et al. 1988). Indeed, the combination of the relaxation of light limitation associated with a reduction in turbidity and the presence of high nutrient concentrations from riverine inputs often leads to increased Chl a concentrations in the mid-salinity regions of estuaries (Fisher et al. 1998; Rochelle-Newall and Fisher 2002; Elliott and McLusky 2002).
Structural factors
We observed large changes in the diversity index of the phytoplankton species present (H′; Fig. 5b). This is similar to what has been observed in other tropical estuarine systems subject to shifting nutrient regimes and to a monsoonal climate (Canini et al. 2013; Huang et al. 2004). Our maximum H′ values were very similar to those of Huang et al. (2004; range 1.24-3.55) who worked in the Pearl River estuary, a system subject to the same monsoon as the Bach Dang estuary. Their H′ values were also lowest during the wet season similar to what was observed in this work. This is due to the presence of one or two species that strongly dominated the community during the wet season. In contrast, during the dry season, H′ values were higher because although total abundances were lower, the number of species present was higher. During the dry season, the system tends to be more stable and flow and turbidity is lower, allowing a larger range of phytoplankton species to develop over the salinity gradient. This is in contrast to the situation during the wet season when there is higher flow, more nutrients and turbidity and a lower range of phytoplankton species, with one or two highly dominant species develop as has also been shown in the Pearl River estuary (Huang et al. 2004).
Nutrient concentrations and the ratios between elements play an important role in structuring phytoplankton abundance and diversity (Justić et al. 1995a, b). Seasonal shifts in discharge can lead to changes in nutrient ratios associated with shifts in the sources of N and P between periods of high and low discharge (Lee et al. 2001). These shifts can then lead to changes in phytoplankton diversity and production (Fisher et al. 1999; Anderson et al. 2002). In the Tolo Harbour system in Hong Kong, Lei et al. (2011) also found that changing nutrient ratios were responsible for shifts in phytoplankton community composition and for the dominance of the Bacillariophyceae. In the work presented here, we found that all of the dominant species (e.g., S. costatum, T. frauenfeldii, Table 5) were correlated with DIN:DIP and most of them were negatively correlated with DIN:DIP. Ou et al. (2008) reported that S. costatum had a relatively higher P demand for growth and that this species rapidly uses up P resources in order to sustain its high growth rates. Hence, the dominance of S. costatum might be one of the reasons for the lower DIP concentrations at stations where this species dominated. The density of S. costatum was always positively correlated (p < 0.05) with DIN:DIP and DSi:DIP ratios (Table 5). Moreover, this species dominated at Stn. 23 in July 2010 with d = 0.97 (the highest d value of the dominant species recorded in the estuary—Suppl. Mat. Table 2) while the concentration of DIP was only 0.07 μM (data not shown) and DIN:DIP and DSi: DIP ratios were highest. The importance of nutrient ratios in structuring phytoplankton community composition is also supported by the results of the CCA (Fig. 7, Table 4) that shows that nutrient ratios, along with salinity and turbidity, are shaping the phytoplankton community.
Justić et al. (1995b) proposed that if Si:P > 22 and DIN:P > 22 then there was potential P limitation, that if DIN:P < 10 and Si:DIN > 1, then there was a potential N limitation and if Si:P < 10 and Si:DIN < 1, then there was potential Si limitation. In our dataset, we failed to observe any situations of DSi:DIP of less than 10 and only observed a DSi:DIN of less than 1 twice during the year: Stn. 10 (in July) and Stn. 28 (in September). In contrast, DSi:DIP was mostly over 22 except for Stn. 28 in January when a value of less than 20 was observed. DIN:DIP was frequently over 22 in the lower salinity stations and never under 22 in the more seaward stations. Considering these values, it is probable that DSi was not a limiting factor. DIP, however, was a potential limiting factor in the lower salinity stations throughout the year and in the higher salinity stations during the wet season. DIN was a potential limiting factor in the offshore station (Stn. 28) in January 2010. This pattern of differential nutrient limitation along the salinity gradient has also been observed before in temperate estuaries (e.g., in Chesapeake Bay; Fisher et al. 1999).
Data from the last few decades in the Red River system and in the offshore Tonkin Gulf, and in the Bach Dang estuary in particular, show that S. costatum frequently dominates the phytoplankton community. This species is a pivotal species for structuring the phytoplankton assemblage (Truong and Do 1983 and this present work; Vu 1994; Chu and Tran 2003) as has been observed in other tropical systems (Nayar et al. 2005a; Patil and Anil 2008; Chu et al. 2012). Over the period 1982–1984, a total of 183 species were recorded in the southern part of the estuary (Vu 1994). In February 1993, Chu (1996) found 185 and 145 species in the Red and Bach Dang rivers, respectively and 125 and 108 species in September of the same year in the Red and Bach Dang rivers, respectively. This is in comparison to 109 and 92 species for February and September, respectively for the period 2009–2010 (Table 3). Thus, phytoplankton composition in the present study appears less diverse than was previously recorded. Interestingly, this lower diversity was concomitant with the appearance of harmful algal bloom events during the last two years (2011–2012; Nguyen et al. 2012). Indeed, these blooms of Gonyaulax polygramma, Phaeocystis globosa, Chattonella sp. were the first to be recorded in the northern coast of Vietnam and probably indicate a shift in the system. It is also worth noting that during the wet season a potentially toxic species, Microcystis sp., formed an important part of the phytoplankton community at the lower salinity stations. This species of Cyanophyceae is known to proliferate in Vietnamese freshwaters and recent work from a reservoir within the Red River system has highlighted the potential toxicity of the strains found in this region (Duong et al. 2013).
Given the importance of nutrient ratios in structuring phytoplankton populations, we can anticipate the effects of the continuing rapid economic development on the estuary. In 1995, concentrations of ammonium measured at a series of stations in the Bach Dang estuary, rarely exceeded 1 μM (HIO 1995; Supp Mats. Table 3). In contrast, in 2001, inorganic nitrogen concentrations were considerably higher with concentrations of ammonium often exceeding 20 μM (Tran and Dinh 2004). Phosphate and silicate concentrations tended to follow the same pattern as for ammonium, with higher values during 2001 than in 2010 (Tran and Dinh 2005). Little data exists on nitrate concentrations and so it is difficult to compare the DSi:DIN or DIN:DIP, however, we can compare how DSi:DIP has changed between the 1990s and the early 2000s. In 2001, DSi:DIP was 43.1 ± 8.2 (mean ± se) at the end of the dry season/beginning of the wet season (May) and 141.5 ± 53.6 in the middle of the wet season (July and August) for a station with similar salinities to our Stn. 4 (Supp Mats, Table 3). This is in comparison to our values (Table 2) of over 154.9 in April 2010 and 153.8 for August 2010 for DSi:DIP. The only data that exist for DIN:DIP in the estuary is from April 2003 when a value of 5.9 (±2.2) was observed (Supp. Mat Table 3; Tran and Dinh 2005). This value is considerably lower than what we found during April 2010 and over a factor of almost 10 lower than the average DIN:DIP over the whole sampling campaign, pointing towards N limitation during this period.
Comparing nutrient concentrations inter-annually is fraught with difficulty as many factors often vary between years such as rainfall and land use, any of which can influence nutrient export from land to aquatic systems. Total annual rainfall was 1,369, 1,907, and 1,438 mm for 1995, 2001, and 2003, respectively. This is in comparison to 1,681 mm for the annual cycle examined in this work and this should be borne in mind when considering the causes of the shifting nutrient ratios in the estuary over the last few decades. Nevertheless, despite the origin of the shifts in nutrient ratios (meteorology, land use, or another factor), it is probable that shifting nutrient concentrations and changing nutrient ratios play an important role in the changing phytoplankton diversity in the system.
In this work, we have only looked at some of the factors potentially controlling phytoplankton diversity during neap tide cycles. It is known that the tidal cycle can influence not only salinity but also dissolved nutrient concentrations and turbidity in estuarine systems (e.g., Vörösmarty and Loder 1994) and it is probable, given the high tidal amplitude in this system (4 m; Lefebvre et al. 2012) that it is also the case here. Obviously, in order to fully understand the myriad of factors controlling phytoplankton diversity in this complex system, we would have had to sample during both neap and spring tides. Nevertheless, the conclusions presented here for the situation during neap tides help to provide some answers towards the shifting situation in the Bach Dang estuary.
Expected future trends and scenarios
Strong seasonal variations in environmental conditions, such as are observed in this and other Southeast Asian estuaries play an important role in determining phytoplankton diversity and succession. However, overlain upon these seasonal forcing factors are the longer term impacts of human activities and of climate change. Rainfall has significantly decreased by about 2 % during the period 1971–2010 in the studied area (Nguyen et al. 2014). This is concomitant with an average temperature increase of 0.27 °C per decade over the same period (Nguyen et al. 2014). The Bach Dang estuary is also strongly affected by anthropogenic activities that have led to high amounts of nutrients, heavy metals, and other contaminants mainly from land-based sources ending up in the estuary (Navarro et al. 2012) all of which can alter phytoplankton diversity (Rochelle-Newall et al. 2008; Howarth and Paerl 2008; Howarth et al. 2012). The increase in abundance of certain species (i.e., S. costatum) and the appearance of some toxic species relative to that of Vu (1994) and Truong and Do (1983) are likely due to the changes in nutrient concentrations and their respective ratios over the last two decades. Turbidity has also changed over the last two decades. He et al. (2007) reported that suspended sediment loads in the upper Red River increased over the period 1960–1990 due to a combination of climate and land use change and recently Vu et al. (2014) showed that this was also the case for the Bach Dang system. This increase in turbidity may well have played a role in altering the phytoplankton community structure through light limitation (Elliott and McLusky 2002). Such shifts in phytoplankton community composition due to increased dredging and suspended sediment have also been observed in other tropical estuaries. For example, Nayar et al. (2005a) also proposed that the dominance of S. costatum in the Ponggol estuary, Singapore was due to increased dredging and the capacity of this species to adapt to the shifting light regimes caused by increased turbidity. It is therefore probable that other tropical estuaries faced with increased anthropogenic pressures and future climate change may well also experience the same shifts in phytoplankton diversity.
While it is difficult to fully compare all of the potential limiting factors over the last few decades because of the lack of data, the few results presented here seem to point towards the role of long term change in temperature, salinity and turbidity accompanied by changes in nutrient concentrations and their respective ratios in the decrease of phytoplankton diversity observed in this estuary. It is therefore probable that in the future, given the intensification of activities in and around the estuary and the expected shifts in the global and local climate, that phytoplankton diversity may well change even more in this system, further favoring the emergence of potentially toxic species.
References
Aminot, A., & Kerouel, R. (1998). Pasteurization as an alternative method for preservation of nitrate and nitrite in seawater samples. Marine Chemistry, 61(3–4), 203–208.
Anderson, D. M., Glibert, P. M., & Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4), 704–726. doi:10.1007/bf02804901.
Balech, E. (1995). The genus Alexandrium Halim (Dinoflagellata) (Vol. 1). Sherkin Island, Co. Cork, Ireland: Sherkin Island Marine Station.
Benschneider, K., & Robinson, R. J. (1952). A new spectrophotometric method for the determination of nitrite in sea water. Journal of Marine Research, 11, 87–96.
Berger, W. H., & Parker, F. L. (1970). Diversity of planktonic Foraminifera in deep-sea sediments. Science, 168, 1345–1347.
Bettarel, Y., Bouvier, T., Agis, M., Bouvier, C., Chu, V. T., Combe, M., et al. (2011). Viral distribution and life strategies in the Bach Dang estuary, Vietnam. Microbial Ecology, 62, 142–154.
Borges, A. V. (2005). Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries, 28(1), 3–27.
Canini, N. D., Metillo, E. B., & Azanza, R. V. (2013). Monsoon-influenced phytoplankton community structure in a Philippine mangrove estuary. Tropical Ecology, 54(3), 331–343.
Chao, A., Chazdon, R. L., Colwell, R. K., & Shen, T.-J. (2005). A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology Letters, 8(2), 148–159. doi:10.1111/j.1461-0248.2004.00707.x.
Chapman, D. (1996). Water Quality Assessments - A Guide to Use of Biota, Sediments and Water in Environmental Monitoring: UNESCO/WHO/UNEP
Chu, V. T. (1996). Phytoplankton in the three rivermouths: Tien Yen, Bach Dang and Red river. In Collection of Marine Environment and Resources (Vol. III, pp. 223–242): Science and Technics Publishing House, Hanoi, Vietnam.
Chu, V. T., & Tran, V. D. (2003). An overview of current activities relating to phytoplankton monitoring in coastal waters of North Vietnam. Paper presented at the Workshop on Red Tide Monitoring in Asian Coastal Waters, March 10–12, 2003, University of Tokyo, Japan
Chu, V. T., Nguyen, T. M. H., Pham, T. T., & Le, T. T. (2012). Some new data on phytoplankton in the western of Tonkin Gulf. Vietnam Journal of Marine Science and Technology, 12, 32–46.
Combe, M., Bouvier, T., Pringault, O., Rochelle-Newall, E., Bouvier, C., Agis, M., et al. (2013). Freshwater prokaryote and virus communities can adapt to a controlled increase in salinity through changes in their structure and interactions. Estuarine, Coastal and Shelf Science, 133, 58–66. doi:10.1016/j.ecss.2013.08.013.
Cronberg, G., & Annadotter, H. (2006). Manual on aquatic cyanobacteria—a photo guide and a synopsis of their toxicology. Denmark: ISSHA.
Dollhopf, S. L., Hashsham, S. A., & Tiedje, J. M. (2001). Interpreting 16S rDNA T-RFLP data: application of self-organizing maps and principal component analysis to describe community dynamics and convergence. Microbial Ecology, 42(4), 495–505.
Duong, D. T., & Vo, H. (1997). Vietnam freshwater algae—taxonomy of order Chlorococcales. Hanoi: Agriculture Publishing House.
Duong, T. T., Coste, M., Feurtet-Mazel, A., Dang, D. K., Gold, C., Park, Y. S., et al. (2006). Impact of urban pollution from the Hanoi area on benthic diatom communities collected from the red, Nhue and Tolich rivers (Vietnam). Hydrobiologia, 563, 201–216.
Duong, T. T., Le, T. P. Q., Dao, T.-S., Pflugmacher, S., Rochelle-Newall, E., Hoang, T., et al. (2013). Seasonal variation of cyanobacteria and microcystins in the Nui Coc Reservoir, Northern Vietnam. Journal of Applied Phycology, 25(4), 1065–1075. doi:10.1007/s10811-012-9919-9.
Eaton, A. D., Clesceri, L. S., & Greenberg, A. E. (1995). Standard methods for the examination of water and wastewater (19 ed.). Washington, DC.
Elliott, M., & McLusky, D. S. (2002). The need for definitions in understanding estuaries. Estuarine, Coastal and Shelf Science, 55(6), 815–827. doi:10.1006/ecss.2002.1031.
Fanning, G. O., & Pilson, M. E. Q. (1973). On the spectrophotometric determination of dissolved silica in natural waters. Analytical Chemistry Symposia Series, 45, 136–140.
Fisher, T. R., Harding, L. W., Stanley, D. W., & Ward, L. G. (1988). Phytoplankton, nutrients and turbidity in the Chesapeake, Delaware and Hudson estuaries. Estuarine and Coastal Shelf Science, 27, 61–93.
Fisher, T. R., Hagy, J. D., & Rochelle-Newall, E. (1998). Dissolved and particulate organic carbon in Chesapeake Bay. Estuaries, 21(2), 215–229.
Fisher, T. R., Gustafson, A. B., Sellner, K., Lacouture, R., Haas, L. W., Wetzel, R. L., et al. (1999). Spatial and temporal variation of resource limitation in Chesapeake Bay. Marine Biology, 133(4), 763–778.
Fonge, B. A., Tening, A. S., Egbe, E. A., Yinda, G. S., Fongod, A. N., & Achu, R. M. (2012). Phytoplankton diversity and abundance in Ndop wetland plain, Cameroon. African Journal of Environmental Science and Technology, 523(6), 247–257.
Fukuyo, Y., Takano, H., Chihara, M., & Matsuoka, K. (1990). Red tide organisms in Japan—an illustrated taxonomic guide. Tokyo, Japan: Uchida Rokakuho.
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D'Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science, 319(5865), 948–952. doi:10.1126/science.1149345.
He, D., Ren, J., Fu, K., & Li, Y. (2007). Sediment change under climate changes and human activities in the Yuanjiang-Red River Basin. Chinese Science Bulletin, 52(2), 164–171. doi:10.1007/s11434-007-7010-8.
HIO. (1995). Assessment of water quality in the Haiphong harbour and the coastal waters of Haiphong city (Project database). Haiphong, Vietnam: Institute of Marine Environment and Resources.
Holm-Hansen, O., Lorenzen, C. J., Holmes, R. W., & Strickland, J. D. H. (1965). Fluorimetric determination of chlorophyll. [Rapp P-V Reun Cons Int Explor Mer]. Journal of Construct International Exploration Mer., 30, 3–15.
Howarth, R., & Paerl, H. W. (2008). Coastal marine eutrophication: control of both nitrogen and phosphorus is necessary. Proceedings of the National Academy of Sciences of the United States of America, 105(49), E103–E103. doi:10.1073/pnas.0807266106.
Howarth, R., Swaney, D., Billen, G., Garnier, J., Hong, B. G., Humborg, C., et al. (2012). Nitrogen fluxes from the landscape are controlled by net anthropogenic nitrogen inputs and by climate. Frontiers in Ecology and the Environment, 10(1), 37–43. doi:10.1890/100178.
Huang, L., Jian, W., Song, X., Huang, X., Liu, S., Qian, P., et al. (2004). Species diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Marine Pollution Bulletin, 49(7–8), 588–596. doi:10.1016/j.marpolbul.2004.03.015.
Huang, W., Zhu, X., Chen, Q., He, Q., Liao, Y., Du, P., et al. (2013). Seasonal variability of phytoplankton community structure in relation to different nitrogen-phosphorus ratios in the southern coastal waters of Zhejiang, China. Journal of Ocean University of China, 12(4), 577–588. doi:10.1007/s11802-013-2192-y.
Jaanus, A., Toming, K., Hällfors, S., Kaljurand, K., & Lips, I. (2009). Potential phytoplankton indicator species for monitoring Baltic coastal waters in the summer period. In J. H. Andersen, & D. J. Conley (Eds.), Eutrophication in Coastal Ecosystems (Vol. 207, pp. 157–168, Developments in Hydrobiology): Springer Netherlands.
Justić, D., Rabalais, N. N., & Turner, R. E. (1995a). Stoichiometric nutrient balance and origin of coastal eutrophication. Marine Pollution Bulletin, 30(1), 41–46. doi:10.1016/0025-326X(94)00105-I.
Justić, D., Rabalais, N. N., Turner, R. E., & Dortch, Q. (1995b). Changes in nutrient structure of river-dominated coastal waters: stoichiometric nutrient balance and its consequences. Estuarine, Coastal and Shelf Science, 40(3), 339–356. doi:10.1016/S0272-7714(05)80014-9.
Karlsson, J., Bystrom, P., Ask, J., Ask, P., Persson, L., & Jansson, M. (2009). Light limitation of nutrient-poor lake ecosystems. Nature, 460(7254), 506–509.
Le, T. P. Q., Billen, G., Garnier, J., Thery, S., Ruelland, D., Nghiem, X. A., et al. (2010). Nutrient (N, P, Si) transfers in the subtropical Red River system (China and Vietnam): Modelling and budget of nutrient sources and sinks. Journal of Asian Earth Sciences, 37(3), 259–274. doi:10.1016/j.jseaes.2009.08.010.
Lee, K. Y., Fisher, T. R., & Rochelle-Newall, E. (2001). Modeling the hydrochemistry of the Choptank river basin using GWLF and Arc/Info: 2. Model validation and application. Biogeochemistry, 56(3), 311–348.
Lefebvre, J.-P., Ouillon, S., Vinh, V., Arfi, R., Panché, J.-Y., Mari, X., et al. (2012). Seasonal variability of cohesive sediment aggregation in the Bach Dang–Cam Estuary, Haiphong (Vietnam). Geo-Marine Letters, 32(2), 103–121. doi:10.1007/s00367-011-0273-8.
Legendre, P., & Legendre, L. (1998). Numerical ecology. Amsterdam: Elsevier.
Li, R. H., Liu, S. M., Li, Y. W., Zhang, G. L., Ren, J. L., & Zhang, J. (2014). Nutrient dynamics in tropical rivers, lagoons, and coastal ecosystems of eastern Hainan Island, South China Sea. Biogeosciences, 11(2), 481–506. doi:10.5194/bg-11-481-2014.
Lie, A. A. Y., Wong, C. K., Lam, J. Y. C., Liu, J. H., & Yung, Y. K. (2011). Changes in the nutrient ratios and phytoplankton community after declines in nutrient concentrations in a semi-enclosed bay in Hong Kong. Marine Environmental Research, 71(3), 178–188. doi:10.1016/j.marenvres.2011.01.001.
Lueangthuwapranit, C., Sampantarak, U., & Wongsai, S. (2011). Distribution and abundance of phytoplankton: influence of salinity and turbidity gradients in the Na Thap River, Songkhla Province, Thailand. Journal of Coastal Research, 27(3), 585–594. doi:10.2112/jcoastres-d-10-00123.1.
Lui, H. K., & Chen, C. T. A. (2011). Shifts in limiting nutrients in an estuary caused by mixing and biological activity. Limnology and Oceanography, 56(3), 989–998. doi:10.4319/lo.2011.56.3.0989.
Mette, E. M., Vanni, M. J., Newell, J. M., & Gonzalez, M. J. (2011). Phytoplankton communities and stoichiometry are interactively affected by light, nutrients, and fish. Limnology and Oceanography, 56(6), 1959–1975. doi:10.4319/lo.2011.56.6.1959.
Mullin, J. B., & Riley, J. P. (1955). The spectrophotometric determination of silicate-silicon in natural waters with special reference to sea water. Analytica Chimica Acta, 12, 162–170.
Murphy, J., & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36. doi:10.1016/S0003-2670(00)88444-5.
Muylaert, K., Sabbe, K., & Vyverman, W. (2009). Changes in phytoplankton diversity and community composition along the salinity gradient of the Schelde estuary (Belgium/The Netherlands). Estuarine, Coastal and Shelf Science, 82(2), 335–340.
Navarro, P., Amouroux, D., Thanh, N. D., Rochelle-Newall, E., Ouillon, S., Arfi, R., et al. (2012). Fate and tidal transport of butyltin and mercury compounds in the waters of the tropical Bach Dang Estuary (Haiphong, Vietnam). Marine Pollution Bulletin, 64(9), 1789–1798. doi:10.1016/j.marpolbul.2012.05.036.
Nayar, S., Goh, B. P. L., & Chou, L. M. (2005a). Dynamics in the size structure of Skeletonema costatum (Greville) Cleve under conditions of reduced photosynthetically available radiation in a dredged tropical estuary. Journal of Experimental Marine Biology and Ecology, 318(2), 163–182. doi:10.1016/j.jembe.2004.12.013.
Nayar, S., Goh, B. P. L., & Chou, L. M. (2005b). Environmental impacts of diesel fuel on bacteria and phytoplankton in a tropical estuary assessed using in situ mesocosms. Ecotoxicology, 14(3), 397–412.
Nguyen, V. N., Le, T. T., Luu, X. H., Nguyen, V. T., Nguyen, C. T., & Fukuyo, Y. (2012). Recent HAB events in Halong Bay: increase in frequency and harmful effects. Paper presented at the XV International Conference on Harmful Algae Changwon, Korea, 28 October—2 November 2012
Nguyen, D.-Q., Renwick, J., & McGregor, J. (2014). Variations of surface temperature and rainfall in Vietnam from 1971 to 2010. International Journal of Climatology, 34(1), 249–264. doi:10.1002/joc.3684.
Officer, C. B., & Ryther, J. H. (1980). The possible importance of silicon in marine eutrophication. Marine Ecology: Progress Series, 3(1), 83–91. doi:10.3354/meps003083.
Ou, L. J., Wang, D., Huang, B. Q., Hong, H. S., Qi, Y. Z., & Lu, S. H. (2008). Comparative study of phosphorus strategies of three typical harmful algae in Chinese coastal waters. Journal of Plankton Research, 30(9), 1007–1017.
Paerl, H. W. (2009). Controlling eutrophication along the freshwater-marine continuum: dual nutrient (N and P) reductions are essential. Estuaries and Coasts, 32(4), 593–601. doi:10.1007/s12237-009-9158-8.
Palleyi, S., Kar, R. N., & Panda, C. R. (2008). Seasonal variability of phytoplankton population in the Brahmani Estuary of Orissa, India. Journal of Applied Science and Environmental Management, 12(3), 19–23.
Patil, J. S., & Anil, A. C. (2008). Temporal variation of diatom benthic propagules in a monsoon-influenced tropical estuary. Continental Shelf Research, 28(17), 2404–2416. doi:10.1016/j.csr.2008.06.001.
Pielou, P. C. (1975). Ecological diversity. New York: Wiley.
Redfield, A. C., Ketchum, B. H., & Richards, F. A. (1963). The influence of organisms on the composition of sea-water. In M. N. Hill (Ed.), The sea (Vol. 2, pp. 26–77). New York: Wiley.
Ricard, M. (1987). Atlas du phytoplankton marin, Diatomophycées (Vol. 2. (1–297, Paris Editions)). Paris: CNRS.
Rochelle-Newall, E. J., & Fisher, T. R. (2002). Chromophoric dissolved organic matter and dissolved organic carbon in Chesapeake Bay. Marine Chemistry, 77, 23–41.
Rochelle-Newall, E. J., Delesalle, B., Mari, X., Rouchon, C., Torréton, J. P., & Pringault, O. (2008). Zinc induces shifts in microbial carbon flux in tropical coastal environments. Aquatic Microbial Ecology, 52(1), 57–68.
Rochelle-Newall, E. J., Chu, V. T., Pringault, O., Amouroux, D., Arfi, R., Bettarel, Y., et al. (2011). Phytoplankton diversity and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Marine Pollution Bulletin, 62, 2317–2329.
Rochelle-Newall, E., Hulot, F. D., Janeau, J. L., & Merroune, A. (2014). CDOM fluorescence as a proxy of DOC concentration in natural waters: a comparison of four contrasting tropical systems. Environmental Monitoring and Assessment, 186, 589–596. doi:10.1007/s10661-013-3401-2.
Shannon, C., & Weaver, W. (1963). The mathematical theory of communication: Univ. of Illinois Press.
Tas, S., Yilmaz, I. N., & Okus, E. (2009). Phytoplankton as an indicator of improving water quality in the Golden Horn Estuary. Estuaries and Coasts, 32(6), 1205–1224. doi:10.1007/s12237-009-9207-3.
Taylor, F. (1976). Dinoflagellates from the International Indian Ocean Expedition—A report on material collected by the R.V."Anton Bruun 1963–1964". Stuttgart: Schweizerbart'sche Verlagsbuchhandlung (Nägele u. Obermiller).
Tomas, C. R. (1997). Identifying marine phytoplankton. San Diego: Academic Press Harcourt Brace & Company.
Tran, D. T., & Dinh, V. H. (2004). Assessment of potentiality, utilization and management of wetland in the coastal area of Haiphong city (Project report). Haiphong, Vietnam: Institute of Marine Environment and Resources.
Tran, D. T., & Dinh, V. H. (2005). Zoning and planning for alluvial soil utilization in the coastal area of Haiphong city (Project database). Haiphong, Vietnam: Institute of Marine Environment and Resources.
Trinh, A. D., Meysman, F., Rochelle-Newall, E. J., & Bonnet, M.-P. (2012). Quantification of sediment water interactions in a polluted tropical river through biogeochemical modeling. Global Biogeochemical Cycles, 26, GB3010. doi:10.1029/2010GB003963.
Truong, N. A. (1993). Taxonomy of marine diatoms in Vietnam. Hanoi, Vietnam: Science and Technics Publishing House.
Truong, N. A., & Do, T. B. L. (1983). The occurrence of diatom Skeletonema costatum (Grev.) Cleve in the coastal waters of Haiphong. (Project Report). Haiphong, Vietnam: Institute of Marine Environment and Resources.
Vörösmarty, C. J., & Loder, T. C. (1994). Spring-neap tidal contrasts and nutrient dynamics in a marsh-dominated estuary. Estuaries, 17(3), 537–551. doi:10.2307/1352402.
Vu, T. T. (1994). Estuarine ecosystems of Vietnam. Hanoi, Vietnam: Science and Technics Publishing House.
Vu, D. V., Ouillon, S., Tran, D. T., & La, V. C. (2014). Impact of the Hoa Binh Dam (Vietnam) on water and sediment budgets in the Red River basin and delta. Hydrology and Earth System Sciences Discussions, 11(1), 333–370. doi:10.5194/hessd-11-333-2014.
Wood, E., Armstrong, F., & Richards, F. (1967). Determination of nitrate in seawater by cadmium copper reduction to nitrite. Journal of the Marine Biological Association of the United Kingdom, 47, 23–31.
Xu, S. S., Song, J. M., Li, X. G., Yuan, H. M., Li, N., Duan, L. Q., et al. (2010). Changes in nitrogen and phosphorus and their effects on phytoplankton in the Bohai Sea. Chinese Journal of Oceanology and Limnology, 28(4), 945–952. doi:10.1007/s00343-010-0005-3.
Yamagishi, T. (1992). Plankton algae in Taiwan (Formosa). Tokyo, Japan.: Uchida Rokakuho.
Acknowledgements
This work was financed by the Vietnam Academy of Science and Technology and the EC2CO project ‘HAIPHONG’, the French Institute of Research for Development (IRD), the Centre National de la Recherche Scientifique (CNRS) and the Ministry of Science and Technology of Vietnam (the MOST project on ‘Black Carbon’). We thank to the colleagues from the IMAGO Service Unit, IRD for their help with the chemical analyses. The National Hydro-Meteorological Service of Vietnam is thanked for the rainfall data. This article is dedicated to the memory of Dr. Do Trong Binh who passed away during the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 468 kb)
Rights and permissions
About this article
Cite this article
Chu, T.V., Torréton, JP., Mari, X. et al. Nutrient ratios and the complex structure of phytoplankton communities in a highly turbid estuary of Southeast Asia. Environ Monit Assess 186, 8555–8572 (2014). https://doi.org/10.1007/s10661-014-4024-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-4024-y