Abstract
Studies of annual successions and inter-annual variations in sub-Arctic and Arctic microplankton assemblages are required in order to understand the structure and function of marine ecosystems. This study depicts the microplankton (>20 μm) structure in a sub-Arctic tidewater glacial fjord system, SW Greenland. The descriptions are based on monthly net hauls collected between January 2006 and December 2010. Two blooms, with distinctive species compositions, were identified across all years: a spring bloom and a summer/autumn bloom. In addition, the winter season—with weak stratification, deep tidal mixing, and dense coastal inflows—was characterised by a separate species composition at much lower abundance. Here, the highest variety of microplankton groups was recorded and represented by diatoms (Chaetoceros spp. and Thalassiosira spp.), silicoflagellates, dinoflagellates, and ciliates. During the spring bloom, species correlated with higher light intensities, i.e. haptophytes and diatoms (Thalassiosira spp. and Fragilariopsis spp.), dominated the microplankton assemblage. Among these, diatoms were also correlated with cooler and fresher waters influenced by springtime melt. During the summer/autumn bloom, the microplankton assemblage was mainly represented by diatoms, such as Chaetoceros spp. ‘Low-saline’ chrysophytes were also present. The latter bloom coincides with elevated temperatures in the fjord and renewal of nutrients due to the onset of glacial meltwater run-off from the Greenland Ice Sheet. Our study shows a yearly recurrent succession of microplankton assemblages and that the annual succession is controlled primarily by ocean–fjord–glacier interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microplankton (mainly phytoplankton) assemblages have been studied in various sub-Arctic and Arctic regions, but these studies are generally limited to the spring season (Quillfeldt 1996, 2001; Wassmann et al. 1999; Degerlund and Eilertsen 2010) and the summer season (Hasle and Heimdal 1998; Wiktor and Wojciechowska 2005; Kraberg et al. 2010). Diatoms and haptophytes are common in these blooms, with dinoflagellates observed later in the summer. Diatoms are mostly represented by Chaetoceros spp., Thalassiosira spp., and Fragilariopsis spp. (Quillfeldt 1996, 2001; Degerlund and Eilertsen 2010). According to Quillfeldt (2005), the pennate chain-forming Fragilariopsis spp. precede the centric genera (Thalassiosira and Chaetoceros) during the bloom. Among the centric forms, Thalassiosira spp. often appear prior to Chaetoceros spp. The commonly studied coastal sub-Arctic waters of southern Greenland and Iceland as well as the Arctic waters of northern Greenland, Svalbard, and the Arctic Ocean are characterised by a high abundance and diversity of diatoms (Grøntved and Seidenfaden 1938; Hasle and Heimdal 1998; Quillfeldt 2001; Jensen 2003; Degerlund and Eilertsen 2010). However, spring and summer bloom diatom assemblages vary depending on the seasonal sea ice cover (e.g. Quillfeldt 2001) and available light.
As one of the main primary producers, diatoms are also important due to their widespread distribution in the sub-Arctic and Arctic regions (Hasle and Syvertsen 1996; Quillfeldt 2001; Katsuki et al. 2009; Poulin et al. 2011). Along the coasts of Greenland, melting sea ice and glacial discharge influence the distribution, structure, and succession of diatoms and other phytoplankton assemblages inhabiting these coastal waters (Grøntved and Seidenfaden 1938; Gradinger and Baumann 1991; Quillfeldt 2001). Degerlund and Eilertsen (2010) stress that microplankton (i.e. phytoplankton) species characteristics should be related to hydrographic conditions, climate zone, distance from the coast, water depth (photic zone), and/or life cycle (e.g. spore formation). In broader high-latitude studies, these species–environment relationships are rather general due to time-limited and seasonally biased sampling. The lack of baseline information on species–environment relationships from time series thus likely prevents us from identifying changes that are more widespread. The ongoing MarineBasic-Nuuk long-term monitoring programme describes hydrography, chemistry, primary production, algal biomasses, and microplankton with larger meso-zooplankton composition in the outer sill region of Godthåbsfjord, SW Greenland, on a monthly basis (Juul-Pedersen et al. 2012). In this monitored sub-Arctic fjord system, light is available all year, including wintertime. The recorded annual time series show high planktonic diversity, particularly among diatoms, which seems to be characteristic of the Arctic and sub-Arctic sectors off West Greenland (Grøntved and Seidenfaden 1938; Smidt 1979; Thomsen 1982; Booth and Smith 1997; Poulsen and Reuss 2002).
This study aims to present the annual and seasonal successions of the microplankton assemblage based on monthly net hauls from the monitoring programme. We present the overall succession patterns, bloom dynamics, and distinct changes in species composition. The microplankton assemblage is described in relation to the studied area’s hydrographic conditions. We hypothesise that physical variables strongly influence seasonal species composition in the sub-Arctic, glacier-influenced fjord environment. A detailed list of microplankton species is presented, providing a reference for future investigations in Greenland and comparative high-latitude studies.
Materials and methods
Study site
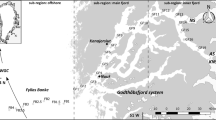
Godthåbsfjord is a 190-km-long sill fjord located in the SW Greenland and in direct contact with three tidewater outlet glaciers from the Greenland Ice Sheet, several river outlets, and (via the West Greenland continental shelf) the West Greenland Current (WGC) (Fig. 1). The fjord system is composed of a number of fjord branches and covers a surface area of ~2013 km2, with a mean depth of ~260 m (maximum depths ~620 m). The main sill of the fjord (depth ~170 m) is located near the mouth of the fjord, where significant tidal forces (tidal range up to 4.6 m; Richter et al. 2011) modify the water masses entering and exiting the fjord. The main fjord has been divided into two regional domains: the outer sill region and the main fjord basin (Fig. 1).
Map of the Godthåbsfjord system and the adjacent continental shelf and slope showing position of GF3 (star), main sill (black polygon), and regional domains (delimitated by dashed line), with an overall scheme of the Greenland region circulation system. Relatively warm waters: IC Irminger Current; WGC West Greenland Current. Cold waters: EGC East Greenland Current. Three tidewater outlet glaciers are marked: NS Narsap Sermia, AS Akullersuup Sermia, KNS Kangiata Nunaata Sermia
Freshwater run-off from outlet glaciers and surrounding land during spring and particularly summer affects surface salinity distribution and creates a salinity gradient from the inner parts of the fjord to the outer shelf. Towards late summer/autumn, the hydrography of Godthåbsfjord is partly driven by a circulation mode triggered by subglacial freshwater discharge from tidewater outlet glaciers sending large volumes of subsurface meltwater into the inner parts of the fjord. During winter, regional dense coastal water and waters sourced in the WGC originating from the outer shelf region affect the deeper parts of the fjord system, setting up a density-driven circulation mode referred to as dense coastal inflow. A detailed hydrographic description of regional domains, water masses, freshwater, and circulation modes in Godthåbsfjord is provided by Mortensen et al. (2011, 2013, 2014).
Sampling
Microplankton samples were collected using net hauls at a station in the outer sill region of Godthåbsfjord (GF3; 64°07′N, 51°53′W; Fig. 1). Monthly pelagic samplings are performed as part of the MarineBasic-Nuuk programme, a component of the Greenland Ecosystem Monitoring Programme (www.g-e-m.dk). Our study uses data obtained in the period of 2006–2010. The monitoring programme included hydrographical data, such as water temperature, salinity, irradiance (PAR), and fluorescence, obtained by a SBE19plus CTD profiler equipped with a Seapoint Chlorophyll a (Chl a) Fluorometer and a Biospherical/Licor sensor. Water samples were collected using a Niskin water sampler at depths of 1, 5, 10, 15, 20, 30, and 50 m. For Chl a, 50–2000 ml of water from each depth was filtered through GF/C filters and extracted in 10 ml 96 % ethanol for a minimum of 18 h. The samples were analysed on a fluorometer (TD-700, Turner Designs, USA) calibrated against a pure Chl a standard (Turner Designs). Water samples for nutrient analyses were pre-filtered through a GF/C filter and kept frozen (−18 °C) prior to analysis. Phosphate (PO4 3−) and silicate (SiO2) concentrations were determined by spectrophotometric approach (Strickland and Parsons 1972; Grasshoff et al. 1983), while nitrate and nitrite (NO2 − + NO3 −) concentrations were measured by vanadium chloride reduction (Braman and Hendrix 1989). Primary production and zooplankton species composition were also considered during this study. Detailed information on water chemistry, nutrient concentration, and primary production is described in Juul-Pedersen et al. (2015) and on meso-zooplankton in Arendt et al. (2013).
Microplankton processing
Triplicate vertical hauls were taken monthly from 60 m to the surface using a 20-μm-mesh net. Each sample was transferred to an amber glass bottle and preserved with Lugol’s iodine to a final concentration of 1 %. 2.5-ml subsamples were studied using plate-counting chambers (similar to Utermöhl chambers) and an inverted microscope (Nikon Eclipse TE 300 with Plan Fluor objectives). Differential illumination contrast was used to permit detailed structural analysis of cells. A two-step examination of each subsample was performed to obtain at least 500 cells: large, less-abundant cells were counted at low magnification (100×), and small, abundant cells were counted at higher magnification (400× or 600× with oil immersion for photographic identification; see Horner 2002). The number of cells (including colonies) in each subsample was calculated by multiplying the number of individuals counted in photomasks (field of view) by the ratio of the whole chamber area to the area of the counted photomasks (for both lower and higher objective magnifications). Monthly cell counts were obtained by averaging the triplicate subsample counts, and percentage values were calculated for graphical illustration. The per cent species composition within the counted subsample represents the species composition within the entire sample.
Standard sample processing consisted of rinsing, then cleaning by hydrogen peroxide treatment, and finally mounting in Naphrax® for more accurate (species-level) identification of critical diatom species using a light microscope (LM). In addition, due to the mass chain formation of diatoms in May 2009, a more detailed study was performed using a scanning electron microscope (SEM).
Identification of microplankton species was based on Hasle and Syvertsen (1996), Hasle and Heimdal (1998), Quillfeldt (2001), Horner (2002), Throndsen et al. (2007), Hoppenrath et al. (2009), and Kraberg et al. (2010).
Data analysis
Multivariate analysis was used to explore the temporal patterns in species composition from net hauls. Relative abundances of all species in pooled replicates were square root-transformed, and Bray–Curtis similarities between samples (dates) were depicted through ordination by non-metric multidimensional scaling (nMDS) using the Primer v.6 software. The Bray–Curtis similarity between two samples, j and k, is defined as:
with y ij and y jk being the square root-transformed relative species abundances of species i in samples j and k, respectively. S ranges from 0 (no shared species) to 100 (identical samples).
Canonical correspondence analysis (CCA) was carried out to analyse the relationships between measured variables, i.e. salinity, temperature, PAR, Chl a, and nutrients (averaged values from 0 to 60 m) and microplankton species data with more than 0.2 % relative abundance. The statistical significance of relationships between species and variables was evaluated using permutation testing (1000 permutations) to calculate P values. This analysis was carried out using the XLSTAT program (www.xlstat.com).
Results
Hydrography
During winter and spring, the water column in the outer sill region of Godthåbsfjord was weakly stratified due to low freshwater run-off from land and deep tidal mixing (Fig. 2a). Low concentrations of Chl a, ranging from 0.05 to 0.2 μg l−1, were recorded in the upper 60 m (averaged values from 0 to 60 m water depth; Fig. 2c). The first phytoplankton bloom was typically observed from April to May, resulting in a peak in concentrations of Chl a, ranging from 0.5 to 9.5 μg l−1 (averaged values from 0 to 60 m water depth; Fig. 2c). Strong deep tidally induced mixing in the outer sill region dispersed the bloom throughout the water column. Increased freshwater run-off from GrIS in the start of July strengthened water column stratification and produced a pycnocline within the upper 60 m, which could withstand strong tidal mixing in the outer sill region (Fig. 2). This stratification was maintained until September, when maximum surface water temperatures and minimum salinities were recorded above the pycnocline (Fig. 2a, b). From July to October, a second bloom was observed in the upper water column, showing a rise in concentrations of Chl a, ranging from 0.5 to 2.5 μg l−1 (averaged values from 0 to 60 m water depth; Fig. 2c). Towards winter, the freshwater run-off decreased, resulting in increased surface salinities and weakening stratification.
Microplankton
Eleven taxonomic groups were identified (phylum to class, after Boxshall et al. 2013), i.e. Bacillariophyceae (diatoms), Haptophyta (haptophytes), Dictyochophyceae (silicoflagellates), Dinoflagellata (dinoflagellates), Ciliophora (ciliates), Chrysophyceae (chrysophytes), Ebridiaceae (ebridians), Chlorophyta, Heliozoa, and Radiolaria. Among these, 170 species were identified, mostly diatoms and 58 undetermined taxa (determined only to a generic level). In particular, haptophytes and many dinoflagellates were very difficult to identify in Lugol’s fixed samples and were thus identified mainly to a genus level. A detailed list of all identified groups and species with affiliations is presented in Table 1. The microplankton assemblage from net hauls was generally dominated by diatoms, which occurred every month throughout the study period (annual average of 65.6–86.6 % in all years).
The nMDS plot for relative species abundances of microplankton shows a clustering of samples into three groups: winter (October–March), spring (April–May), and summer/autumn seasons (June–October) (Fig. 3). The following paragraphs describe the annual succession of major microplankton groups (i.e. diatoms, haptophytes, silicoflagellates, dinoflagellates, ciliates, and chrysophytes) for the three identified seasonal assemblage groups, including some deviations from a general recurring pattern. Seasonal microplankton assemblage composition (%) patterns are shown in Fig. 4 with a general scheme shown in Fig. 5.
Non-metric multidimensional scaling (nMDS) ordination for square root-transformed relative microplankton species abundance data for each sampling date, based on Bray–Curtis similarities. The distances between samples in the plot reflect their similarity in species composition. Samples are named according to month and year. Different symbols are used to represent samples belonging to three marked seasons: triangle—winter (October–March); circle—spring (April–May); and square—summer/autumn (June–October). The figure gives similarity levels (%) between samples according to each season
Seasonal patterns of the microplankton assemblage composition (%) at GF3 in a 2006, b 2007, c 2008. The group ‘other’ includes ebridians, Chlorophyta, cyanobacteria, Heliozoa, and Radiolaria. All species used in this figure are listed in Table 1. Seasonal patterns of the microplankton assemblage composition (%) at GF3 in d 2009, e 2010. The group ‘other’ includes ebridians, Chlorophyta, cyanobacteria, Heliozoa, and Radiolaria. All species used in this figure are listed in Table 1
Simplified graphical presentation of seasonal patterns of dominant microplankton groups (above line) and dominant diatom genera and species (below line) (>0.2 %—see legend in Fig. 6 for particular species). These seasonal patterns are based on clustering from Bray–Curtis similarities (averaged values from 2006 to 2010): thick solid lines—>20 %, solid lines—20–5 %, and dashed lines—<5 %. Average daylight hours in Godthåb are plotted on top of the figure
Winter
Winter season in Godthåbsfjord was represented by four microplankton groups: diatoms, silicoflagellates, dinoflagellates, and ciliates. Diatoms were dominant in all years (average of 83.3 % in 2006–2010; Figs. 4, 5), represented by genus Chaetoceros (mainly Chaetoceros decipiens and Chaetoceros convolutus) and genus Thalassiosira (mainly Thalassiosira antarctica var. borealis and Thalassiosira rotula). In addition, Thalassionema nitzschioides showed a dominance pattern in early winter (i.e. October–December), while Nitzschia frigida occurred in late winter, prior to the spring blooms (Fig. 5). Silicoflagellates, mainly Dictyocha speculum, contributed significantly from January to March, with maxima in February 2006 (36.2 %; Fig. 4a) and February 2009 (9.8 %; Fig. 4d). The dinoflagellates were often present in winter months but generally in low relative abundances, except for the winters of 2009 (average of 7.6 %; Fig. 4d) and 2010 (average of 13.2 %; Fig. 4e). This group was mostly represented in winter by genus Protoperidinium. Ciliates, represented mainly by the tintinnids, i.e. Favella serrata and Acanthostomella norvegica, contributed significantly in winters 2009 and 2010 (average of 5.8 and 8.6 %, respectively; Fig. 4d, e).
Spring
Diatoms dominated the microplankton assemblage in spring and were complemented by haptophytes (Fig. 5). Haptophytes, represented by Phaeocystis sp., were mainly observed as solitary, non-motile cells and not as colonies, which might be an effect of fixation as fixatives are known to dissociate colonial cells from the colony matrix (A. Davidson, personal communication). The percentage of identified haptophytes may be biased as these cells are smaller than 20 μm, whereas the sampling method only accurately collects cells larger than 20 μm. Nevertheless, Phaeocystis sp. was observed during spring seasons with a maximum contribution recorded in April–May 2010 (average of 71.5 %; Fig. 4e). The year 2009 is an exception, with no haptophyte cells being observed in any samples (Fig. 4d). Diatoms showed highest relative abundances in April 2008 (98.6 %; Fig. 4c) and in April–May 2009 (average of 99.6 %; Fig. 4d). Spring 2008 was mainly represented by genus Fragilariopsis, i.e. Fragilariopsis cylindrus and Fragilariopsis oceanica, while spring 2009 was mainly represented by genus Thalassiosira, i.e. Thalassiosira antarctica var. borealis, Thalassiosira nordenskioeldii, Thalassiosira rotula, and Thalassiosira anguste-lineata. In May 2009, all of the above-listed Thalassiosira spp. appeared almost solely as long, massive chains together with a high relative abundance of resting spores of T. antarctica var. borealis. In contrast, spring 2010 revealed a low relative abundance of diatoms compared to previous years (i.e. average of 26.5 %; Fig. 4e).
Summer/autumn
Summer/autumn season was represented mainly by diatoms and occurrence of chrysophytes. Diatoms contributed most from July to October, reaching averages of over 85 % for each year studied (2006–2010; Figs. 4, 5). Highest relative abundances of this group were recorded in summer/autumn 2006 (average of 92.5 %; Fig. 4a) and summer/autumn 2010 (average of 91.4 %, excluding June—missing data; Fig. 4e). Diatoms were mainly represented by Chaetoceros spp. (Fig. 5), i.e. Chaetoceros decipiens, Chaetoceros curvisetus, and Chaetoceros wighamii. Thalassionema nitzschioides also showed importance during this season in all years (Fig. 5). Additionally, Fragilariopsis cylindrus and Nitzschia frigida were recorded in late summer/autumn 2010, unlike in the previous years studied. Another important algal species, Dinobryon balticum (chrysophytes), showed high relative abundances in June 2006 and June–July 2009 (averages of 16.2 and 22.8 % in Fig. 4a, d, respectively). D. balticum was primarily identified in colonies (i.e. >20 μm). Dinoflagellates also contributed significantly to the microplankton assemblage in summer 2008 (average of 16.3 %; Fig. 4c), represented by Protoperidinium brevipes and Peridiniella catenata. The highest variety of species belonging to the dinoflagellate genera Protoperidinium was observed in September 2010, mainly consisting of Protoperidinium cf. pellucidum, Protoperidinium brevipes, and Protoperidinium ovatum.
Species–environment relationships
The environmental, species (>0.2 %), and sample values generated by CCA for axes 1 and 2 are plotted in Fig. 6. The measured variables used in this analysis are temperature, salinity, PAR, Chl a, and nutrients. The measured variables explain 48 % of the variance in species data with P value <0.0001. In contrast, nutrients show no statistical significance in this study, with P value >0.06. The combination of CCA axes 1 and 2 explains 51.7 % of the variance in microplankton data.
Ordination diagram of the dominant microplankton species (>0.2 %) and variables, i.e. salinity, temperature, irradiance, and Chlorophyll a along the two CCA axes. Three groups of species can be identified: one influenced by higher water temperature (cluster to the left), one influenced by higher salinity (upper right cluster), and one influenced by increased irradiance (lower right cluster). The legend gives average relative abundance of species calculated from all samples in brackets
On the biplot, the direction of the variables’ lines represents their approximate correlation to the ordination axis, other variables, and species. The correlation of variables with axes 1 and 2 indicates that PAR is positively correlated with axis 1, temperature is negatively correlated with axis 1, and salinity and Chl a are positively correlated with axis 2 (Fig. 6). A species’ location along variables’ lines presents its approximate weighted optima along each variable. Generally, silicoflagellates, dinoflagellates, and ciliates show preferences for more saline waters. Haptophytes and diatoms, such as Thalassiosira spp. and Fragilariopsis spp., are correlated with higher light intensities. Thalassiosira spp. and Fragilariopsis spp. are also correlated with cooler and fresher waters. Chrysophytes and diatom species Nitzschia frigida are associated with fresher waters. Diatoms, such as Chaetoceros spp. and Thalassionema nitzschioides, are correlated with warmer waters, with the latter species also related to more saline waters (Fig. 6).
Discussion
The microplankton assemblage from net hauls reveals three seasonal groupings throughout the year, i.e. winter (October–March), spring (April–May), and summer/autumn (June–October) (Fig. 3). Spring and summer/autumn seasons depict separate blooms (i.e. peaks in phytoplankton biomasses; Fig. 2c), which are mainly produced by diatoms and the haptophyte Phaeocystis sp. The winter season, in contrast, shows the lowest phytoplankton biomasses, yet the highest microplankton diversity, represented by diatoms, silicoflagellates, dinoflagellates, and ciliates.
Annual patterns
The established monthly sampling depicts two annual bloom patterns, but this may not give a full picture of the beginning and/or end of blooms as bloom phases may vary in duration, usually lasting only a matter of weeks (e.g. Rat’kova and Wassmann 2002; Hill and Cota 2005). The two identified blooms were also observed and described in previous planktonic studies from this sub-Arctic area (Smidt 1979; Arendt et al. 2013; Juul-Pedersen et al. 2015). Generally, all studied seasons and years are characterised by a dominance of diatoms, particularly the diatom genera Chaetoceros and Thalassiosira (Fig. 5). It should be noted that only diatoms were studied in greater detail using LM and SEM (mainly identified to a species level), while other potentially important microplankton groups, such as haptophytes and dinoflagellates (mainly identified to a genus level), should be treated with caution (for a complete list of species and groups, see Table 1).
Seasonal succession
During winter, weak light conditions prevail in Godthåbsfjord, with a minimum day length of 4 h in December (Fig. 5). Here, the diverse microplankton assemblage is represented by diatom species and genera showing variable environmental preferences: from fresher waters (Nitzschia frigida) to more saline waters (Thalassionema nitzschioides) and from cooler waters (Thalassiosira spp.) to warmer waters (Chaetoceros spp.). These affinities to variable temperatures and salinities indicated by dominant diatoms are linked to weaker water column stratification, typically observed in Godthåbsfjord during the winter season (Mortensen et al. 2011, 2013). This weak stratification, driven by low freshwater run-off and deep tidal mixing, is reinforced by dense coastal inflows pushing upper fjord waters outwards (Mortensen et al. 2011). These dense winter inflows are known to affect the basin waters in the inner fjord system as well (Smidt 1979). T. nitzschioides is reported in association with temperate waters of Atlantic origin, i.e. carried by the Irminger Current (Jiang et al. 2001) and the Atlantic–Norwegian Current (Koç Karpuz and Schrader 1990). Water at the sampling station (GF3) is influenced by waters of Atlantic origin found offshore in the WGC (see also Arendt et al. 2010; Juul-Pedersen et al. 2015) through intermediate and dense coastal inflows. In contrast, N. frigida is considered a cryophilic species common in sea ice edge blooms in the Arctic (Hasle and Heimdal 1998; Wiktor and Wojciechowska 2005; Degerlund and Eilertsen 2010; Poulin et al. 2011) and has been observed at the lower base of sea ice off the West Greenland coast (Jensen 2003). N. frigida has also been reported from Arctic sea ice during dark winter period (Niemi et al. 2011) as this species is known to be highly adapted to a wide range of light regimes (Poulin et al. 2011). Sea ice and glacial ice are present in the inner parts of Godthåbsfjord during winter, and both ice types pass through the sampling area in periodic bursts, affecting the sampled surface waters. Other microplankton groups associated with more saline waters are also significant during winter, i.e. silicoflagellates as well as dinoflagellates and ciliates during the last 2 years studied (2009–2010). In Arctic waters of the Canadian Beaufort Sea, diatoms (mostly N. frigida), dinoflagellates and ciliates, characterised both surface waters and bottom sea ice during dark winter period, showing a diverse winter community (Niemi et al. 2011), similar to that in the present study. In contrast, in the Atlantic-influenced coastal waters of Northern Europe, silicoflagellates are mainly found during late winter and spring, whereas dinoflagellates and ciliates are common during the summer season (Kraberg et al. 2010).
Spring bloom is the most pronounced annual bloom event in Godthåbsfjord with the exception of 2010, as described below. Highest phytoplankton biomasses are recorded during spring, favoured by increasing light intensities and incipient stratification inside the fjord due to an onset of freshwater input from snowmelt and melting of sea ice. The spring microplankton assemblage is represented by species associated with higher light intensities, i.e. Phaeocystis sp. (2006–2008, 2010) and diatom genera such as Thalassiosira and Fragilariopsis (2006–2009) (Fig. 5). Species belonging to these diatom genera are also associated with cooler and fresher waters. Fragilariopsis spp. are typically considered species associated with sea ice and sea ice melt (Hasle and Syvertsen 1996; Quillfeldt 1996, 2001; Lovejoy et al. 2002). The outer parts of the Godthåbsfjord system remain largely free of sea ice, but during shorter periods (i.e. days to weeks), drifting and melting sea ice originating from the inner parts of the fjord system pass through the study area. This diatom genus thus seems to be better characterised as ‘directly and/or indirectly’ associated with sea ice, e.g. meltwater originating from sea ice. Fragilariopsis spp. recorded in Godthåbsfjord during spring seasons are therefore related to cold and freshwater in the surface layer (in common with Fragilariopsis cylindrus in Quillfeldt 2004), influenced by freshwater input from snowmelt as well as meltwater from sea ice. Generally, the haptophytes and diatoms dominant during spring blooms are also observed at most stations on the shelf outside the fjord (‘Fyllas Banke’) and in the central parts of Godthåbsfjord in May 2006 (Arendt et al. 2010). Furthermore, similar species compositions to the one observed in this study are typical of microplankton blooms reported farther north in Arctic regions (Quillfeldt 1996; Hasle and Heimdal 1998; Wiktor and Wojciechowska 2005; Degerlund and Eilertsen 2010).
During the initial part of the summer/autumn bloom (June–July; Fig. 5), colonies of chrysophyte Dinobryon balticum are found in association with fresher waters. D. balticum is regarded as preferring low-saline summer surface waters in Arctic regions (e.g. Hasle and Heimdal 1998; Keck et al. 1999). The summer months show a strong stratification of the water column in Godthåbsfjord driven by freshwater run-off from the GrIS. The summer/autumn bloom is mainly characterised by diatoms associated with warmer waters, i.e. Chaetoceros spp. and Thalassionema nitzschioides, corresponding with higher temperatures due to summer solar heating and air–sea heat exchange. Chaetoceros spp. dominate from July to October, and this diatom genus is the main contributor to the summer/autumn peak in biomass. This seasonal dominance of Chaetoceros spp. reflects the renewal of nutrients due to the onset of the major freshwater run-off from GrIS (see Mortensen et al. 2011). Generally, Chaetoceros spp. has been reported as the last successive stage (late summer) of the diatom growth season in West Greenland coastal waters (Grøntved and Seidenfaden 1938). Juul-Pedersen et al. (2015) show that the summer run-off is a key driver of the summer phytoplankton bloom in Godthåbsfjord, which, unlike in the classical Arctic pattern, compares to and in some years even exceeds the production in spring. Depending on various environmental factors (e.g. freshwater input), a summer/autumn bloom might be a more pronounced event in the microplankton succession than the spring bloom, as recorded in 2010 (see below).
Inter-annual variations
Inter-annual variations in the dominant groups of the microplankton assemblage and bloom dynamics related to environmental conditions were observed during the study in Godthåbsfjord. We must note, however, that the monthly sampling procedure could have missed short-lived blooms (e.g. 1–2 weeks), which should not be interpreted as inter-annual variability.
In 2009, a distinct change in microplankton species composition was observed, one that was recorded only once throughout the entire study period (2006–2010). A complete absence of the significant component of spring blooms, the haptophyte Phaeocystis sp. (Fig. 4d), was accompanied by a mass occurrence of unusually long and massive chains of the diatom genus Thalassiosira, with almost no single cells (e.g. Navicula cf. distans). This change towards mass diatom chain formation may be explained by physiological adaption to nutrient conditions. Only chains with specialised structures, such as spaces between cells (e.g. Thalassiosira spp.), can obtain a higher nutrient supply relative to solitary cells (Pahlow et al. 1997). Both high nutrient concentrations and high levels of turbulence are favourable for chain formation and survival (Pahlow et al. 1997). High nutrient levels were observed just prior to the spring bloom in 2009, with maximum nitrate plus nitrite (12.5 μM), phosphate (1.0 μM), and silicate (6.2 μM) concentrations (in Juul-Pedersen et al. 2010). Resting spores, particularly of the species Thalassiosira antarctica var. borealis, occurred simultaneously with the spring bloom (May 2009), which might be a response to rapid nutrient depletion towards the end of May (annual minima of 0.8, 0.1, and 0.8 μM for nitrate plus nitrite, phosphate, and silicate, respectively; Juul-Pedersen et al. 2010). The strong nutrient depletion during the 2009 spring bloom thus probably resulted from high nutrient uptake by chain-forming and spore-forming diatoms. Resting spores are often formed by certain species, e.g. Thalassiosira antarctica var. borealis (Quillfeldt 2001), when nutrients are nearing exhaustion. This mechanism ought to increase the species’ survival potential by creating hibernating spores that sink to the sediment following the bloom and remain there until re-suspension (Hasle and Syvertsen 1996). These unusual trends observed in the microplankton assemblage in spring 2009 are possibly linked to an unusually long period of dense coastal inflow, which began in February and continued for at least 3 months (Mortensen et al. 2011; Rysgaard et al. 2012). This extensive inflow of dense coastal water continuously lifted nutrient-rich water into the photic zone (above approximately 40 m), thereby influencing the zone in which the bloom occurs. We hypothesise that microplankton cells found in the nutrient-rich dense coastal waters were introduced to the surface layers prior to the spring bloom and may have produced the unusual species composition and succession observed in 2009. Another hypothesis is related to the single-cell stage of Phaeocystis sp., which might have been favoured by environmental conditions occurring prior to the 2009 spring bloom. Such a single-cell bloom could have been missed in the samples due to (1) cell size smaller than the mesh net size and/or (2) a possibly short-lived bloom occurring outside of the sampling scheme.
Nevertheless, Phaeocystis sp. reappeared in 2010, suggesting resilient microplankton species composition assemblages within these fjord systems. The absence of Phaeocystis sp. in spring 2009 and low relative abundance in spring 2006 coincide with the highest recorded phytoplankton biomass values (Fig. 2c and compare to Figs. 4, 5), probably linked to a higher contribution of diatoms during these two spring blooms compared with other years.
Phaeocystis spp. colonies and single cells have both been shown to be a food source for copepods (Nejstgaard et al. 2007), though they appear to represent a poor diet (Tang et al. 2011). In contrast, Phaeocystis spp. have been found to retain particular organic carbon within the pelagic to a greater extent relative to diatoms (Reigstad and Wassman 2007). The occurrence of Phaeocystis sp. in Godthåbsfjord is therefore important for the energy pathway through the food web, sustaining food for ciliates and likely the very abundant copepod within the fjord system, Microsetella norvegica (Arendt et al. 2013), which are thought to feed on particulate carbon aggregates (Koski et al. 2005, 2007).
In 2010, the most pronounced bloom event occurred in summer/autumn, as recorded by the late maximum peak in biomass. A similarly late annual peak in biomass (and primary production) has also been reported from previous annual surveys conducted during the relatively warm period of the 1950–1960s farther north, in Disko Bay, West Greenland (Andersen 1981). Summer/autumn 2010 in Godthåbsfjord (missing data for June; Fig. 4e) showed unusual occurrence of diatom species, typically inhabiting surface waters influenced by inter alia melting/drifting ice and/or snowmelt, i.e. Fragilariopsis cylindrus and Nitzschia frigida (Fig. 5). Exceptionally, high air temperatures in SW Greenland in 2010 contributed to markedly greater glacial ice melt (Mortensen et al. 2013). This resulted in a strong freshening of surface waters during summer, supported by the lowest surface salinity recorded throughout the study period (minimum of 18; Fig. 2a). The strong freshwater signal in summer 2010 was responsible for surface water properties suitable for the typically ice-associated species. Additionally, a high variety of dinoflagellate genus Protoperidinium was observed in summer/autumn 2010, coinciding with the unusually late diatom bloom, possibly serving as a food source for these dinoflagellates. Dinoflagellates, coexisting with diatoms throughout the study period, have been shown to be important grazers of diatoms, such as the dominant genus Protoperidinium as well as the genera Gymnodinium and Gyrodinium (Hansen 1991; Bralewska and Witek 1995). These dinoflagellates typically occur at the peak of the diatom bloom or immediately after the bloom (e.g. Smetacek 1981; Tiselius and Kuylenstierna 1996; Juul-Pedersen et al. 2012).
In 2009–2010, dinoflagellates as well as predatory ciliates contributed significantly to the microplankton assemblage (Fig. 4d, e), which might be linked to the distinct changes recorded in the diatom bloom dynamics, i.e. mass chain formation in 2009 and pronounced summer/autumn bloom in 2010. Sherr et al. (1986) suggest that ciliates and dinoflagellates act as trophic intermediaries between small prey and larger predators. It is also known that ciliates, especially the tintinnids dominant in this study, feed on diatoms as well as build their shells by agglutinating particles suspended in the water column, e.g. leftovers of grazed diatom frustules, to their organic membranes (Karleskint et al. 2010).
Conclusions
The present paper highlights the importance of studying species–environment relationships at a temporal scale of months and multiple years in order to determine and identify key drivers for microplankton succession in sub-Arctic waters. In Godthåbsfjord, the annual succession patterns alongside measured biomasses confirm the existence of two blooms observed in previous studies. The more detailed seasonal patterns in microplankton succession reveal shifts from a high variety of microplankton groups, including small predators, in winter to predominantly phytoplankton during spring and summer/autumn blooms. Our study shows that species composition of microplankton assemblage is mainly influenced by physical variables such as salinity, temperature, and light, while chemical variables (nutrients) seem to be less decisive. Such detailed planktonic studies are extremely important in complex sub-Arctic systems such as Godthåbsfjord, where local and regional forces affect fjord circulation, e.g. local subglacial freshwater discharge from GrIS and large-scale circulation systems affecting dense coastal inflows. The ocean forcing via the West Greenland Current (WGC) versus a local freshwater input from GrIS subject to tidal mixing creates a highly productive system that is sensitive to changing environmental conditions. An identified increased inflow of dense coastal water (2009) and increased freshwater input (2010) influenced physical and chemical water properties, which likely resulted in distinct changes in the microplankton assemblage structure during the observation period.
References
Andersen OGN (1981) The annual cycle of phytoplankton primary production and hydrography in the Disko Bugt area, West Greenland. Medd Grønland 6:1–68
Arendt KE, Nielsen TG, Rysgaard S, Tönnesson K (2010) Differences in plankton community structure along the Godthåbsfjord, from the Greenland Ice Sheet to offshore waters. Mar Ecol Prog Ser 401:49–62
Arendt KE, Juul-Pedersen T, Mortensen J, Rysgaard S (2013) A 5-year study of seasonal patterns in mesozooplankton community structures in a sub-Arctic fjord reveals dominance of Microsetella norvegica (Crustacea, Copepoda). J Plankton Res 35:105–120
Booth BC, Smith WO (1997) Autotrophic nanoflagellates and diatoms in the Northeast Water Polynya, Greenland: summer 1993. J Mar Syst 10:241–261
Boxshall GA, Mees J, Costello MJ, Hernandez F, Gofas S, Hoeksema BW et al (2013) World Register of Marine Species. http://www.marinespecies.org at VLIZ. Accessed 2013-11-05
Bralewska JM, Witek Z (1995) Heterotrophic dinoflagellates in the ecosystem of the Gulf of Gdansk. Mar Ecol Prog Ser 117:241–248
Braman RS, Hendrix SA (1989) Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescense detection. Anal Chem 61:2715–2718
Degerlund M, Eilertsen HC (2010) Main species characteristics of phytoplankton spring blooms in NE Atlantic and Arctic waters (68–80°N). Estuaries Coasts 33:242–269
Gradinger RR, Baumann MEM (1991) Distribution of phytoplankton communities in relation to the large-scale hydrographical regime in the Fram Strait. Mar Biol 111:311–321
Grasshoff K, Erhardt M, Kremling K (1983) Methods of seawater analysis. Verlag Chemie, Weinheim
Grøntved B, Seidenfaden G (1938) The phytoplankton of the waters west of Greenland. Medd Grønland 82:5–380
Hansen PJ (1991) Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagial food web. Mar Ecol Prog Ser 73:253–261
Hasle GR, Heimdal BR (1998) The net phytoplankton from Kongsfjorden, Svalbard, July 1988, with general remarks on species composition of arctic phytoplankton. Polar Res 17:31–52
Hasle GR, Syvertsen EE (1996) Marine diatoms. In: Tomas CR (ed) Identifying marine diatoms and dinoflagellates. Academic Press, San Diego
Hill V, Cota G (2005) Spatial patterns of primary production on the shelf, slope and basin of the Western Arctic in 2002. Deep-Sea Res Part II 52:3344–3354
Horner R (2002) A taxonomic guide to some common marine phytoplankton. Biopress Ltd., Bristol
Jensen KG (2003) Holocene hydrographic changes in Greenland coastal waters. Dissertation, Denmarks og Grønlands Geologiske Undersøgelse Rapport 2003/58, Denmark
Jiang H, Siedenkrantz MS, Knudsen KL, Eiríksson J (2001) Diatom surface sediment assemblages around Iceland and their relationship to oceanic environmental variables. Mar Micropaleontol 41:73–96
Juul-Pedersen T, Rysgaard S, Batty P, Mortensen J, Retzel A, Nygaard R, Burmeister A, Søgaard DH et al (2010) Nuuk Basic: The MarineBasis programme. In: Jensen LM, Rasch M (eds) Nuuk ecological research operations, 3rd annual report, 2009. National Environmental Research Institute, Aarhus University, Denmark, pp 45–66
Juul-Pedersen T, Arendt KE, Mortensen J, Retzel A, Nygaard R, Burmeister A, Sejr MK, Blicher ME et al (2012) Nuuk basic: the marinebasis programme. In: Jensen LM, Rasch M (eds) Nuuk ecological research operations, 5th annual report, 2011. National Environmental Research Institute, Aarhus University, Denmark, pp 47–67
Juul-Pedersen T, Arendt KE, Mortensen J, Blicher ME, Søgaard DH, Rysgaard S (2015) Seasonal and interannual phytoplankton production in a sub-arctic tidewater outlet glacier fjord, SW Greenland. Mar Ecol Prog Ser 524:27–38. doi:10.3354/meps11174
Karleskint G, Turner R, Small J (2010) Introduction to marine biology. Brooks/Cole, Cengage Learning, Canada
Katsuki K, Takahashi K, Onodera J, Jordan RW, Suto I (2009) Living diatoms in the vicinity of the North Pole, summer 2004. Micropaleontology 55:137–170
Keck A, Wiktor J, Hapter R, Nilsen R (1999) Phytoplankton assemblages related to physical gradients in an arctic, glacier-fed fjord in summer. ICES J Mar Sci 56:203–214
Koç Karpuz N, Schrader H (1990) Surface sediment diatom distribution and Holocene paleotemperature variations in the Greenland, Iceland and Norwegian Sea. Paleoceanography 5:557–580
Koski M, Kiørboe T, Takahashi K (2005) Benthic life in the pelagic: aggregate encounter and degeneration rates by pelagic harpactoid copepods. Limnol Oceanogr 50:1254–1263
Koski M, Møller EF, Maar M (2007) The fate of discarded appendicularian houses: degradation by the copepod, Microsetella norvegica, and other agents. J Plankton Res 29:641–654
Kraberg A, Baumann M, Dürselen C-D (2010) Coastal phytoplankton, photo guide for Northern European Seas. Verlag Dr. Friedrich Pfeil, München
Lovejoy C, Legendre L, Martineau MJ, Bâcle J, Quillfeldt CH (2002) Distribution of phytoplankton and other protists in the North Water. Deep Sea Res Part II Top Stud Oceanogr 49:5027–5047
Mortensen J, Lennert K, Bendtsen J, Rysgaard S (2011) Heat sources for glacial melt in a sub-Arctic fjord (Godthåbsfjord) in contact with the Greenland Ice Sheet. J Geophys Res 116. doi:10.1029/2010JC006528
Mortensen J, Bendtsen J, Motyka RJ, Lennert K, Truffer M, Fahnestock M, Rysgaard S (2013) On the seasonal freshwater stratification in the proximity of fast-flowing tidewater outlet glaciers in a sub-Arctic sill fjord. J Geophys Res 118:1382–1395. doi:10.1002/jgrc.20134
Nejstgaard JC, Tang KW, Steinke M, Dutz J, Koski M, Antajan E, Long J (2007) Zooplankton grazing on Phaeocystis: a quantitative review and future challenges. Biogeochemistry 83:147–172
Niemi A, Michel C, Hille K, Poulin M (2011) Protist assemblages in winter sea ice: setting the stage for the spring ice algal bloom. Polar Biol 34:1803–1817
Pahlow M, Riebesell U, Wolf-Gladrow DA (1997) Impact of cell shape and chain formation on nutrient acquisition by marine diatoms. Limnol Oceanogr 42:1660–1672
Poulin M, Daugbjerg N, Gradinger R (2011) The pan-Arctic biodiversity of marine pelagic and sea-ice unicellular eukaryotes: a first-attempt assessment. Mar Biodiv 41:13–28. doi:10.1007/s12526-010-0058-8
Poulsen LK, Reuss N (2002) The plankton community on Sukkertop and Fylla Banks off West Greenland during a spring bloom and post-bloom period: hydrography, phytoplankton and protozooplankton. Ophelia 56:69–85
Quillfeldt CH (1996) Ice algae and phytoplankton in north Norwegian and arctic waters: species composition, succession and distribution. Dissertation, University of Tromsø, Norges fiskerihøgskole, Norway
Quillfeldt CH (2001) Identification of some easily confused common diatoms species in Arctic spring blooms. Bot Mar 44:375–389
Quillfeldt CH (2004) The diatom Fragilariopsis cylindrus and its potential as an indicator species for cold water rather than for sea ice. Vie et Mil 54:137–143
Quillfeldt CH (2005) Common diatom species in Arctic spring blooms: their distribution and abundance. Bot Mar 43:499–519. doi:10.1515/BOT.2000.050
Rat’kova TN, Wassmann P (2002) Seasonal variation and spatial distribution of phyto- and protozooplankton in the central Barents Sea. J Mar Syst 38:47–75
Reigstad M, Wassman P (2007) Does Phaeocystis spp. contribute significantly to vertical export of organic carbon? Biogeochemistry 83:217–234
Richter A, Rysgaard S, Dietrich R, Mortensen J, Petersen D (2011) Coastal tides in West Greenland derived from tide gauge records. Ocean Dyn. doi:10.1007/s10236-010-0341-z
Rysgaard S, Mortensen J, Juul-Pedersen T, Sørensen LL, Lennert K, Søgaard DH, Arendt KE, Blicher ME, Sejr MK, Bendtsen J (2012) High air–sea CO2 uptake rates in nearshore and shelf areas of Southern Greenland: temporal and spatial variability. Mar Chem 128–129:26–33. doi:10.1016/j.marchem.2011.11.002
Sherr EB, Sherr BF, Paffenhöffer GA (1986) Phagotrophic protozoa as food for metazoans: a ‘missing’ trophic link in marine pelagic food webs. Mar Microb Food Webs 1:61–80
Smetacek V (1981) The annual cycle of protozooplankton in the Kiel Bight. Mar Biol 63:1–11
Smidt ELB (1979) Annual cycles of primary production and of zooplankton at Southwest Greenland. Medd Grønland Bioscience 1:1–53
Strickland JDH, Parsons TR (1972) A practical handbook of sea-water analysis. Bull Fish Res Board Can 167:1–310
Tang KW, Nielsen TG, Munk P, Mortensen J, Møller EF, Arendt KE, Tönnesson K, Juul-Pedersen L (2011) Metazooplankton community structure, feeding rate estimates, and hydrography in a meltwater-influenced Greenlandic fjord. Mar Ecol Prog Ser 434:77–90
Thomsen HA (1982) Planktonic choanoflagellates from Disko Bugt, West Greenland, with a survey of the marine nanoplankton of the area. Meddelelser om Grønland. Bioscience 8:1–35
Throndsen J, Hasle GR, Tangen K (2007) Phytoplankton of Norwegian coastal waters. Almater Forlag As, Oslo
Tiselius P, Kuylenstierna M (1996) Growth and decline of a diatom spring bloom: phytoplankton species composition, formation of marine snow and the role of heterotrophic dinoflagellates. J Plankton Res 18:133–155
Wassmann P, Ratkova T, Andreassen I, Vernet M, Pedersen G, Rey F (1999) Spring bloom development in the marginal ice zone and the central Barents sea. Mar Ecol 20:321–346
Wiktor J, Wojciechowska K (2005) Differences in taxonomic composition of summer phytoplankton in two fjords of West Spitsbergen, Svalbard. Pol Polar Res 26:259–268
Acknowledgements
The authors wish to express their gratitude to Dr. Józef Wiktor for helping identify and classify diatoms, dinoflagellates, and ciliates. We wish to thank Dr. Teresa Radziejewska and Dr. Andrew Davidson for constructive comments and consultations. We also acknowledge Genowefa Daniszewska-Kowalczyk for technical assistance as well as Thomas Krogh, Louise Mølgaard, Ditte Marie Mikkelsen, and Morten Frederiksen for participating in fieldwork. We thank Martin Blicher for assisting with data analysis and interpretation. We thank the Polish Narodowe Centrum Nauki (NCN) in Krakow for financial support for the Greenland studies (Grant No. 2011/03/N/ST10/05794). The MarineBasic-Nuuk programme, part of the Greenland Ecosystem Monitoring Programme, was funded by the Danish Energy Agency as part of its climate support programme for the Arctic. Financial support was also received from the Greenland Institute of Natural Resources, the Aage V. Jensen Charity Foundation, and the Canadian Excellence Research Chair (CERC) programme. This publication is a contribution to the MarineBasic-Nuuk programme, part of the Greenland Ecosystem Monitoring Programme, and to the Greenland Climate Research Centre. We wish to thank the Translation Centre at University of Copenhagen for language improvement. Finally, we appreciate the constructive critiques of the Editor and three anonymous reviewers, which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krawczyk, D.W., Witkowski, A., Juul-Pedersen, T. et al. Microplankton succession in a SW Greenland tidewater glacial fjord influenced by coastal inflows and run-off from the Greenland Ice Sheet. Polar Biol 38, 1515–1533 (2015). https://doi.org/10.1007/s00300-015-1715-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1715-y