Abstract
Objective
The aim of this study was to examine the practicality and accuracy of using an electronic monitoring device as a means of measuring medication adherence in elderly stroke survivors, with emphasis on patients’ experiences.
Methods
The Medication Event Monitoring System (MEMS), which records date and time of pill-bottle openings, was used to measure adherence to antihypertensive medication in a randomized controlled trial (RCT) of a brief psychological intervention with 58 stroke survivors. Patients were asked to describe and rate their experiences of using the MEMS pill bottle.
Results
MEMS adherence was related to both pill count and self-reported adherence (Medication Adherence Report Scale). Most patients found the MEMS acceptable and easy to use, although some found it cumbersome and/or experienced difficulties with the cap. Nearly half (48 %) reported at least one instance where MEMS data did not reflect their pill-taking behavior (e.g. taking a tablet out the day before to take on a flight); 55 % of patients indicated that the MEMS helped them remember their medication, suggesting a mere measurement effect.
Conclusion
Electronic pill monitoring has many flaws, including practical difficulties and data inaccuracies. There was evidence of a measurement effect, indicating that MEMS should be used in both intervention and control arms when used to measure adherence within RCTs. We also observed that the MEMS pill bottle is not suitable for measuring adherence in patients who use their own ‘days of the week’ box for sorting medication, as we found poorer adherence at follow-up in this group. Despite these limitations, we conclude that electronic monitoring presents the best method currently available for objective measurement of adherence, especially where detailed timing information is required. Accuracy may be improved by the concurrent use of other measures (e.g. pill count, self-report).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Failure to take medicine as prescribed can reduce the effectiveness of treatment regimens, yet adherence to medication is often sub-optimal; thus, interventions which improve medication adherence are required [1]. However, accurately measuring medication adherence remains a problem for both researchers and health professionals. Adherence is often measured by patient self-report, tallying pill counts with prescribed doses or using medications dispensed as a proxy for medications taken; all of these have scope for inaccuracies, including memory failure (self-report) and not recording the time or day on which the tablets were taken (pill tally and medications dispensed). Currently, the closest method to a ‘gold standard’ is the use of electronic pill-bottle caps, which record the time and date of cap openings. It is presumed that, if patients are given correct instructions (e.g. “Only open the bottle when you are due to take your tablet[s], and only take out one dose at a time”), these will provide an objective measure of when and how patients take their tablets. However, there is no guarantee that patients actually swallow the tablet(s) at the time of opening, if at all.
Electronic devices have been shown to significantly correlate with other methods of measuring adherence (including self-report and pill counts) and generally show lower levels of adherence than self-report, which may be expected as patients may favorably self-report their pill-taking [2]. This suggests that electronic methods may provide an accurate record of actual adherence. Nonetheless, a number of problems with the use of electronic pill monitoring have been highlighted [3]. These include patients not using the pill bottle as instructed (e.g. taking out a week’s tablets at a time), interference with existing routines, and malfunctioning of the electronic device itself. Further, it is difficult to estimate to what degree the use of such devices increases adherence in patients simply because they know their medication taking is being monitored (i.e. mere measurement effect) [4]. In some instances, data recorded by electronic devices may be a poor indication of medication-taking behavior; for example, in HIV patients living in difficult conditions [5]. A review of electronic devices in elderly patients supported their validity as a measure of medication adherence, but this review did not evaluate their use from the patients’ perspective [6].
In this paper, we report patients’ experiences of the use of an electronic pill bottle, the MEMS (Medication Event Monitoring System, MEMS®, Aardex Ltd, Switzerland), in a randomized controlled trial (RCT) of a brief, psychological intervention aimed at increasing adherence in stroke survivors. The MEMS cap contains a computer chip which records the date and time of each opening, which is then downloaded to a personal computer for subsequent analysis. Antihypertensive medication was targeted for MEMS measurement, as poorly treated blood pressure significantly increases the risk of future vascular events [7], and only 30–50 % of patients regularly take their antihypertensive drugs as prescribed [8]. In order to ensure that any increases in adherence were due to the brief psychological intervention and not the use of the MEMS pill bottle, we used the MEMS in both control and intervention treatment arms, as adherence has been shown to increase simply as a consequence of patients knowing it is being measured [4]. The proportion of tablets taken on schedule was higher in the intervention group than in the control group (97 vs. 87 %; between-group difference 10 % [95 % CI 0.2–16.2]) [9].
We had high levels of contact with each patient during the study, hence we were not only able to monitor their use of the MEMS containers, but also to appraise the accuracy of the MEMS recordings in relation to the time patients actually swallowed their pills. These reported experiences did not appear to differ by treatment arm. We also compared MEMS adherence to self-report and pill count measures, and report the use of the MEMS pill bottle and its effect on medication-taking behavior from the patients’ personal viewpoints.
Methods
Participants
Participants were recruited from consecutive discharges from the stroke unit at the Western General Hospital in Edinburgh. Inclusion criteria for the intervention were first stroke or transient ischemic attack; discharged to home; on any antihypertensive medication; and had self-reported sub-maximal adherence (using the Medication Adherence Report Scale [MARS]). Exclusion criteria were using a pharmacy-supplied dosette box or not responsible for their own medication (e.g. given to them by a carer). Ethical approval was given by the South East Scotland Research Ethics Committee (REC ref. no. 09/S1102/36).
Fifty-eight people used the MEMS pill bottle (n = 29 in each treatment arm), and were analyzed as allocated [9]. All were white British, 64 % were male, and the mean age was 69.2 ± 10.7 years (range 51–85). Patients were of higher socioeconomic status than the average for the region (i.e. 51.7 % were from the least deprived quintile of Scottish Index of Multiple Deprivation vs. 44.1 % for Edinburgh).
Measures
Our primary outcome measure was electronically recorded openings using MEMS pill bottles. Following Brown et al. [10], we used MEMS readings to calculate percentage of doses taken, days on which the correct dose was taken, and doses taken on schedule (i.e. within a 3-h window of the median time taken). Although the MEMS itself did not form part of the intervention, we used the MEMS pill bottle in both treatment arms as it has been shown to have a possible measurement effect on adherence [4].
The MARS [11] consists of five items (each scored on a scale of 1−5) relating to taking medication. The wording of the MARS reflects missing medication as a normal behavior, with the aim of eliciting honest responses. We calculated three scores: total adherence (i.e. sum of all items); non-intentional non-adherence (i.e. forgetting; item 1 score); and intentional non-adherence (i.e. choosing not to take medicine as prescribed; sum of items 2−5). Sub-maximal adherence was defined as a MARS total score <25.
The total number of tablets put into the MEMS pill bottle by the research fellow at visits 2, 3, and 4 minus any remaining tablets at visit 5 was used to calculate pill count (tablets dispensed), which was then expressed as a percentage of total days of pill recording.
Procedure
Patients were screened at first interview using the Mini-Mental State Examination (MMSE) [11] to ensure that they did not have cognitive difficulties (i.e. scores <23) which could affect study participation. There were no exclusions.
The brief psychological intervention was conducted during two home visits, 2 weeks apart. For the intervention group, the aim of the first session (visit 1) was to develop an implementation intentions plan (i.e. if/then) to aid the patient in remembering to take their medication (e.g. “if it is 8 am and I am in the kitchen and I am having breakfast, then I will take my first tablets of the day”). The second session (visit 2) aimed to elicit and where appropriate address any mistaken beliefs that the patient may have had about their medication (e.g. “My blood pressure is now within the normal range, so I can stop taking my pills”). The control group received an equivalent amount of contact from the same researcher who conducted the intervention, in order to control for therapeutic contact. In the two control group sessions, the discussion was steered to general topics regarding the patient’s stroke (e.g. how they were coping, their experience in hospital). Further details of the intervention are reported in the paper discussing the primary results of the study [9].
The MEMS pill bottle was used for 3 months (mean 82.2 ± 17.4 days; range 16–90) in both treatment arms, and was re-filled with one of the patient’s antihypertensive medications at ≈1-month intervals (at visits 2−4) by the research fellow, who also conducted the intervention. The cost of the MEMS cap (≈£65/$US105 at the time of our research) prohibited its use with more than one medication per patient. The medication chosen was either that most frequently taken (e.g. twice rather than once daily) or, if no difference, that which was most convenient to the patient. Patients were instructed that when they were ready to take their medication, they should open the bottle, take out a single dose, and reclose the bottle. They were given the opportunity to practice on a spare bottle before being given their own MEMS pill bottle filled with their antihypertensive, at the end of visit 2. Patients in both treatment arms were told that we were using the MEMS pill bottle to gather information about how people took their medication for the purposes of research, and their data would not be relayed to anyone else, including the doctors and nurses responsible for their medical care. The monitoring period commenced on the day following visit 2, therefore openings carried out at visit 2 were not included in the analysis. Openings relating to the refilling of the bottle by the research fellow at visits 3 and 4 were also excluded from any analysis.
At the final interview (visit 5), patients were asked to rate (using a 7-point Likert scale that ranged from ‘strongly agree’ to ‘strongly disagree’) the ease of use, acceptability and helpfulness of using the MEMS pill bottle. They were also invited to recount their experiences of using the MEMS pill bottle. All interviews were recorded and fully transcribed.
Analysis
Analysis of the main intervention effects was conducted on an ‘analyzed as allocated’ basis [9]. Missing data was handled by multiple imputation (five datasets), and results were pooled according to Rubin’s rules. Analysis of variance was used to test differences between groups. Spearman correlations were used to assess associations between measures of adherence. Limits of agreement (LOA) were calculated for differences between measures of pills taken (i.e. MEMS and pill count). LOA provide an assessment of the variation in between-measures differences for individual patients, with 95 % of differences expected to fall within the defined limits [13]. Agreement is considered good if the mean difference is close to zero and the LOA are relatively narrow. If there is good agreement, it may be concluded that the two measures could be used interchangeably.
Results
There were significant correlations between pill count and MEMS adherence (0.56 < ρ < 0.73) [Table 1]. The mean differences between pill count, MEMS total doses taken, and days correct dose taken were small and the LOA between these three measures were narrow (Table 1), indicating good levels of agreement. The plot of differences between total doses and pill count against the mean of these measures supports high agreement (Fig. 1); further, the association of the difference and mean is very small (Spearman’s ρ = −0.07), indicating that the agreement holds at all levels of adherence [13]. In contrast, both correlations and LOA indicate lower agreement between percentage of doses taken on schedule with total doses, days correct doses taken, and/or pill count (Table 1). The plot of differences between total doses and doses taken on schedule against the mean of these two measures (Fig. 1) also suggests that agreement is relatively poor, particularly for low levels of adherence (as indicated by a significant association of difference and mean [i.e. Spearman’s ρ = −0.79; p < 0.001]). These findings indicate that total doses taken, days correct dose taken, and pill count appeared interchangeable, but that none of these measures were a good estimate of regularity of pill taking, particularly for low adherers.
Pill count and MEMS adherence were moderately correlated with both non-intentional non-adherence (forgetting) and total MARS scores (0.32 < ρ < 0.46), but the associations with intentional non-adherence were not significant, nor were intentional and non-intentional MARS non-adherence significantly related. Non-adherence in the current RCT appeared to be more due to patients forgetting to take their medication, rather than choosing not to take it.
Ease and acceptability of using the MEMS pill bottle
Fifty-six patients (28 in each treatment arm) completed the Likert scales regarding using the MEMS pill bottle and commented on its use. The pill bottle presented a number of challenges for both participants and researchers. Patients reported finding it large, cumbersome, top heavy and easy to knock over (this did not result in any electronic failures) (e.g. “I would hate to have one for every tablet I’m taking. I mean the bulk of the thing!” [male aged 81 years, intervention group]), although its size could also act as a useful visual reminder (“Well, [it was helpful due to] the fact it was large and you could see it all the time.” [male aged 85 years, control group]).
Some patients had difficulties opening or closing the bottle, including the spring-loaded cap flying out of their hands (e.g. “Well… to start off with, unscrewing it, I was a bit wobbly with that.” [male aged 64 years, intervention group]). Pharmacy advice made it a requirement to leave pills in blister strips, as removing some medication could result in deterioration as a result of air contact.Footnote 1 However, the blister strips were frequently too large for the pill bottle (despite using the largest available bottle), meaning they had to be cut into smaller strips. As a result, patients sometimes had difficulty removing the strips (“It was difficult to dig these out; they tend to get stuck under the lip.” [male aged 56 years, control group]); and a few reported having difficulty pressing their pills out of the (smaller than usual) strip (“There’s nothing much to push through.” [female aged 65 years, intervention group]).
Most patients were on multiple medications and this also posed problems for some, as it meant one of their medications was being treated differently from the others (“Yes, it gave me another bottle to open and another thing to think about. Yes, it was harder for me.” [female aged 82 years, control group]; “[It’s not easy to use] because there was only one tablet in it. Perhaps if I had all my tablets in it.” [female aged 76 years, intervention group]). Patients who looked after young grandchildren were concerned that the MEMS cap had no child-safety mechanism.
Patients frequently expressed interest in the workings of the MEMS cap (e.g. “So the wee spring, when you take the lid off, the spring expands or something” [male aged 79 years, control group]), and quite a few commented on the potential flaws in the system as an accurate record of pill-taking (“It doesn’t even tell you how many tablets you are taking at a time. It’s restrictive.” [male aged 74 years, control group]; “You might get a craftier person opening the lid and not taking the tablet.” [male aged 79 years, control group]).
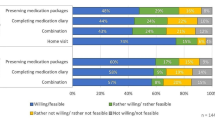
However, despite the concerns and difficulties mentioned above, only 4 % of patients did not agree that the MEMS pill bottle was easy or acceptable to use (Fig. 2), and the vast majority agreed or strongly agreed with both statements (e.g. “I found the pill bottle easy to use. Yes, perfectly acceptable.” [male aged 55 years, intervention group]).
Helpfulness of using the MEMS pill bottle
Although it was not intended that the MEMS pill bottle would increase adherence, 31 patients (55 %) agreed that they had found the MEMS pill bottle helpful in remembering to take their tablets, whilst only 16 (29 %) disagreed that it was helpful (Fig. 2). Reasons given for helpfulness included that it was something different or more noticeable (e.g. “it was helpful because… it’s attracting you, it is sitting there and you say, ‘My tablet!’, ken.” [male aged 77 years, intervention group] and “I found it a good thing, yes… because the bottle is there and… I think you’re more inclined to say, ‘Yes’, if I see it, I take it.” [female aged 74 years, control group]). Some patients from both groups remarked that knowing it was being measured made them more conscientious in taking their medication (e.g. “but certainly… it reminded me I had to undo the bottle. Otherwise, I could hear you saying, ‘My God, he’s still in his bed at 11 o’clock in the morning!’” [male aged 85 years, intervention group] and “Even though I was pretty bad at taking them …I did know that it was being recorded sort o’ thing …… so I did try harder.” [female aged 56 years, control group]). Some patients reporting feeling guilty if they had missed a tablet, as the research fellow would know this from the MEMS recordings (e.g. “I just felt that I was being watched all the time. It’s like big brother!” [male aged 79 years, control group]). However, others expressly said that knowing it was being recorded had not made any difference to them (e.g. “No, no, no I never worried about that at all.” [female aged 74 years, control group]).
Mere measurement effect
We did not collect MEMS readings pre-treatment and there were no changes over time in MEMS adherence across the 3 months of data collection, contrary to our expectation that adherence would tail off in the control group due to an initial measurement effect from patients knowing their pill-taking was being monitored [9]. Self-reported MARS adherence significantly increased from pre-treatment to follow-up across both treatment arms (mean increase 0.62, 95 % CI 0.3−0.9, p < 0.001) and patients in both groups said the MEMS was helpful at remembering their tablets, suggesting a degree of measurement effect in both treatment arms, which was still evident at 3 months. Importantly, there was no association between treatment group and how helpful patients viewed the MEMS pill bottle to be in remembering their tablets (χ 2(6) = 4.4; p = 0.620) and patients views of the helpfulness of MEMS were not directly related to any adherence scores (MEMS, MARS or pill count) (−0.09 < ρ < 0.09; 0.491 < p < 0.976).
Patients who used their own ‘days-of-the-week’ box
Patients consenting to the intervention who reported using their own ‘days-of-the-week’ box (which they filled with all of their tablets on a weekly, fortnightly or monthly basis) were asked if they wanted to continue with the study as this would mean one of their antihypertensives being moved from their own box to the MEMS pill bottle. Of these patients, 16 (seven in the intervention group and nine in the control group) agreed to continue, whilst only four declined. Participants who used a ‘days-of-the-week’ box (n = 16) did not differ from those not using a box (n = 42) with regard to pre-treatment MARS adherence (mean 23.0 ± 1.3 vs. 23.3 ± 1.4; 95 % CI for difference −0.5 to 1.1; p = 0.488). Further, there was no evidence that using a ‘days-of-the-week box’ relative to not using a box was related to greater cognitive impairment (mean MMSE 28.3 ± 1.5 vs. 28.5 ± 1.3; 95 % CI for difference −0.6 to 1.0; p = 0.631) or age (69.8 ± 12.3 vs. 69.0 ± 10.2 years; 95 % CI for difference −7.1 to 5.6; p = 0.808).
However, some of those using their own ‘days-of-the-week’ box commented that using the MEMS pill bottle had made it harder for them (e.g. “Well, all my other tablets were taken apart from that one day, so what I’ve done is took my normal tablets and I forgot tae open the bottle…” [female aged 55 years, control group, talking about a missed MEMS tablet]). In addition, those who had previously used a ‘days-of-the-week’ box had lower scores on all adherence measures at 3-month follow-up, and significantly lower adherence on percentage of doses taken on schedule than those who had not (83.9 ± 17.4 vs. 94.1 ± 13.9 %; 95 % CI for difference 1.2–19.1; p = 0.026).
MEMS recording: data issues
As well as practical difficulties highlighted above (i.e. getting the medication into the pill bottle), there were a number of recording discrepancies that presented challenges in the data set. First, almost half (n = 27) of the patients travelled away from home during the recording period; and 12 % (n = 7) travelled abroad to different time zones (including a round-the-world-trip). Our intervention was aimed at developing medication-taking habits, linked to patients associating their medication with a specific time of day and regular activity (e.g. 8 am, breakfast); therefore, we were most interested in regularity of pill taking, which was clearly affected by time zone changes resulting from foreign travel. Because of its size, patients also sometimes chose not to take their MEMS pill bottle with them when away from home either on holiday or for overnight stays with friends, resulting in ‘missed’ days.
A number of patients did not use the pill bottle as instructed. For example, one patient who used their own ‘days-of-the-week’ box took out a whole week’s worth of tablets to fill their own box; another consistently failed to fully screw the cap back onto the pill bottle, meaning it did not record openings over an extended period; and a few patients opted to use the pill bottle as a convenient pill container when travelling away (“When I went away to Spain, I just put all the tablets in that.” [male aged 50 years, control group]), resulting in extra openings to add in (or take out) other medications to the one being recorded. In some instances, patients attributed extra openings to inquisitive family members, who they believed had been interested in its workings or contents. Patients often reported taking out pills to swallow at a later time, including the next day (research fellow: “So you’d already got your tablets out [of the containers] and then you went downstairs and then you came back and took them?”; patient: “Oh, they were… all out waiting for me coming back to have my breakfast. But I think on one occasion, you know, it was about 11 o’clock before I got my breakfast.” [male aged 84 years, intervention group]; “The early shift… I took it out, put the three tablets in there [small pill box] and just took them to work and had them about… half-past nine.” [male aged 59 years, control group]).
Overall, only 30 patients (52 %) reported no incidents which may have affected the accuracy of the MEMS recordings as a reflection of actual pill-taking behavior. Therefore, 28 patients (48 %) had at least 1 day during the 3-month period when they reported taking their tablets as prescribed, but for which there was no MEMS recording (five patients ran out of tablets in the pill bottle before the re-fill date; three failed to use the MEMS pill bottle as instructed; two removed tablets the day before to take the next day [e.g. when travelling by plane]; and seven chose not to use the MEMS pill bottle at particular times [e.g. overnight stays]) and/or at least 1 day with an extra MEMS recording (eight patients took tablets out in advance of swallowing them; three reported openings made by other people in their household; one opened it to check they had taken an earlier tablet; two added other tablets for a trip away; and one opened it because they had forgotten to put the blister pack back in). As the research fellow had extensive contact with patients during the course of the study, we were certain that in all of these instances, the resulting recorded electronic data was not an accurate representation of the patient’s actual medication-taking behavior. Fortunately, these incidents did not differ by treatment group and so have not affected our overall findings.
Discussion
Although the vast majority of patients in our RCT reported that using the MEMS pill bottle was easy and acceptable, a number of physical and practical problems emerged. Patients found the bottle large and cumbersome, and some reported that it was an extra thing to remember, mirroring the experiences of older renal patients [14]. There was no direct relationship between measured adherence and how helpful patients viewed the MEMS pill bottle in remembering their medication, supporting earlier findings that patients’ positive views of the MEMS did not result in biased measurement [14]. In contrast, however, patients’ comments in the current study regarding the effect of knowing their adherence was being measured plus a self-reported increase in adherence in both treatment arms suggests there was a degree of measurement effect (i.e. adherence may have increased as a direct result of it being measured by the MEMS cap).This contradicts the results of a recent study in 226 adults taking diabetic medication, which concluded there was a non-significant increase in MARS adherence when using a MEMS container, and hence there was no measurement effect [15]. However, 39 % of patients reported maximum adherence at baseline; thus, there was no scope for increase in a sizeable proportion of the sample. Importantly, for the current RCT, there was no relationship between patients’ views of helpfulness of the MEMS and treatment group, thus, by using the MEMS pill bottle in both treatment arms, we have successfully controlled for any measurement effect in this pilot study.
It has been recommended that using a range of different measures within studies will produce the best chance of generating an accurate picture of actual adherence [16], and the current RCT was able to compare the MEMS pill bottle with other measures of adherence. Known violations of MEMS pill bottle usage mean we are certain that the data were not always an accurate reflection of actual pill taking in almost half of our patients. Nonetheless, the high association of MEMS with both self-reported adherence and pill count suggests that, when patients are given clear instructions as to its use, the MEMS cap provides a good method of adherence measurement. The very high agreement between pill count and MEMS doses in this RCT may be due to the fact that pill count was controlled by the research fellow across the five study visits; levels of agreement are likely to be lower for pharmacy pill counts, which rely on patients remembering to bring their pills to appointments. The detailed information provided by the MEMS recordings means we were able to examine the regularity of pill taking within a ±3-h window, which was important to our intervention, and may be critical for other stroke medications, such as warfarin. In addition, the relatively poor levels of agreement between doses taken on schedule with the measures of pills taken suggest that, where regularity of pill taking is important, overall measures such as pill count are unlikely to provide an accurate picture of adherence behavior, which may lead to biased conclusions.
A limitation of this research is it was conducted in a small sample of higher socioeconomic, elderly stoke patients, meaning the findings might not generalize to other populations. Although older patients may have poorer adherence and greater difficulty in using the MEMS bottle, potentially due to physical or cognitive impairment, socioeconomic status has not been shown to be consistently related to medication taking [1]. A further limitation is that, due to the high cost of the MEMS pill bottle, we only measured adherence for one medication, and elderly patients are likely to be on multiple medications for chronic illnesses. Nonetheless, we observed that where possible, most patients tended to take their medications at the same time, particularly their morning pills; thus, if they remembered to take one medication, they were likely to remember them all.
Conclusion
The current paper assessed the practicalities and accuracy of using an electronic measuring device as an objective measure of adherence to antihypertensive medication in an RCT. Such an assessment was possible because of the extensive contact with patients in our RCT, including the opportunity for participants to discuss their personal experiences of using the MEMS pill bottle. These results form part of a pilot RCT that aimed to increase adherence via a psychological intervention [9]. We found that there were a number of occasions where the MEMS reading would not reflect actual pill taking for around half of the patients in our sample. There was also evidence of a measurement effect, with an increase in self-reported adherence across both the intervention and control groups. We deduce that, in RCTs, electronic measurement should be used in all treatment arms, so that any observed increases in adherence can be attributed to the intervention and not the use of the MEMS. In addition, patients using their own ‘days-of-the-week’ box had poorer MEMS adherence at follow-up and so changing the routines of these patients for the purposes of recording medication usage was not helpful. Thus, we would advise that people who already use their own ‘days-of-the-week’ box are excluded from research using the MEMS pill bottle to measure adherence, as their medication routines may be adversely affected. Despite these issues, we conclude that electronic pill monitoring is still likely to present the best method of objectively recording adherence currently available, particularly where detailed information regarding timing is important. Accuracy of measurement may be improved by combining electronic measurement with a range of different measures (e.g. pill count, self-report).
Notes
We appreciate that, in normal circumstances, some patients may remove their own medication from blister packaging (e.g. to put in their own ‘days of the week’ boxes); however, we were constrained to keep tablets in blister packs within the current research in accordance with pharmacy advice.
References
Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011.
Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs. 2003;2(3):219–28.
Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? J Clin Psychol Med Settings. 2002;9:25–34.
Wetzels GE, Nelemans PJ, Schouten JS, et al. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions-an observational study. BMC Health Serv Res. 2006;6:8.
Samet JH, Sullivan LM, Traphagen ET, et al. Measuring adherence among HIV-infected persons: is MEMS consummate technology? AIDS Behav. 2001;5:21–30.
MacLaughlin EJ, Raehl CL, Treadway AK, et al. Assessing medical adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005;22(3):231–55.
Bailey JE, Wan JY, Tang J, et al. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010;25(6):495–503.
Stephenson J. Noncompliance may cause half of antihypertensive drug “failures”. JAMA. 1999;28(4):313–4.
O’Carroll RE, Chambers JA, Dennis M, et al. Improving adherence to medication in stroke survivors: a pilot randomized controlled trial. Ann Behav Med. 2013;46(3):358–68.
Brown I, Sheeran P, Reuber M. Randomized controlled trial of an implementation intention intervention to enhance adherence with antiepileptic drug treatment. Epilepsy Behav. 2009;16(4):634–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Horne R. Measuring adherence: the case for self-report. Int J Behav Med. 2004;11(Suppl):75.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60.
Russell CL, Owens S, Hamburger KQ, et al. Medication adherence and older renal transplant patients’ perceptions of electronic medication monitoring. J Gerontol Nurs. 2009;35(10):17–21.
Sutton S, Kinmonth A-L, Hardeman W, et al. Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med. 2014;48(3):293–9.
Chambers JA, O’Carroll RE. Adherence to medical advice. In: Benyamini Y, Johnston M, Karademas EC, editors. Assessment in health psychology. Göttingen/Boston: Hogrefe; 2015 (in press).
Acknowledgments
This project was funded by a grant from the Scottish Government, Department of Health, Chief Scientist Office, reference number CZH/4/569. We would like to thank the doctors and nurses at the Western General Hospital stroke clinic and ward for their help in recruitment, and the participants for giving up their time to take part.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standards
All participants gave informed consent for the study which was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 (revised 2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chambers, J.A., O’Carroll, R.E., Dennis, M. et al. Personal experiences of electronic measurement of medication adherence in elderly stroke survivors. Drugs Ther Perspect 31, 167–174 (2015). https://doi.org/10.1007/s40267-015-0200-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-015-0200-6