Abstract
Purpose
To explore patients’ willingness to have medication adherence measured using different methods and evaluate the feasibility and validity of their combination (i.e., pill counts, a medication diary and a questionnaire assessing adherence two months post-discharge).
Methods
(1) A cross-sectional evaluation of the willingness of patients with polypharmacy to have their medication adherence measured post-discharge. (2) Medication adherence was monitored during two months using pill counts based on preserved medication packages and a diary in which patients registered their adherence-related problems. During a home visit, the Probabilistic Medication Adherence Scale (ProMAS) and a questionnaire on feasibility were administered.
Results
A total of 144 participants completed the questionnaire at discharge. The majority was willing to communicate truthfully about their adherence (97%) and to share adherence-related information with healthcare providers (99%). More participants were willing to preserve medication packages (76%) than to complete a medication diary (67%) during two months. Most participants reported that preserving medication packages (91%), completing the diary (99%) and the ProMAS (99%) were no effort to them. According to the majority of participants (60%), pill counts most accurately reflected medication adherence, followed by the diary (39%) and ProMAS (1%). Medication adherence measured by pill counts correlated significantly with ProMAS scores, but not with the number of diary-reported problems. However, adherence measured by the medication diary and ProMAS correlated significantly.
Conclusion
Combining tools for measuring adherence seems feasible and can provide insight into the accordance of patients’ actual medication use with their prescribed regimen, but also into problems contributing to non-adherence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medication adherence defined as “the process by which patients take their medications as prescribed” [1], is indispensable to maximise therapeutic effectiveness and disease management. Optimization of adherence is needed, however, as approximately 50% of patients with chronic conditions do not adhere to prescribed medication regimens [2]. Moreover, polypharmacy, usually defined as the concomitant intake of at least five medicines [3], is found to be associated with poorer medication adherence because of the larger number of medicines and the complexity of the medication regimen [4,5,6]. Non-adherence rates between 6% and 55% were reported in older patients with polypharmacy [7], contributing to poorer health outcomes, increased healthcare service utilization and higher healthcare expenditures [8,9,10,11,12].
To improve medication adherence, a multitude of interventions have been developed in recent years [13,14,15,16]. An accurate measurement of medication adherence is crucial to correctly assess the effectiveness of such interventions, but remains a major challenge in research and clinical practice, despite the different methods being available. To date, there is no ‘gold standard’ for the evaluation of adherence in patients with polypharmacy. The best strategy seems to be a combined use of at least two methods [2, 17,18,19].
A systematic review concluded that a simultaneous use of multiple instruments validating adherence to polypharmacy from various perspectives would be beneficial for future researchers [20]. However, the patient’s perspective is hardly taken into account when measuring adherence to medication. Currently, there is a lack of information on the willingness of patients with polypharmacy to have their medication adherence measured by healthcare providers. Moreover, it is unclear whether a combination of subjective and objective methods measuring adherence is considered feasible by patients with polypharmacy in practice.
The combination of subjective (e.g., self-report scales) and objective measures (e.g., measurements of clinical outcomes, pill counts, pharmacy records, electronic monitoring of medication administration) is considered as the current state of the art [17, 18]. Previous research compared medication adherence measured by pill count with adherence measured by several self-report scales [21,22,23], pharmacy data [21] and electronic monitoring [24, 25]. Furthermore, the Medication Adherence Report Scale [26] and Electronic Monitoring data [27] were used to validate the Probabilistic Medication Adherence Scale (ProMAS). To our knowledge, the ProMAS has so far not been validated using pill counts. A medication diary, used to report problems related to medication adherence, has been developed in previous research, but was not validated yet [28]. Therefore, in this study, the combination of pill counts, a patient diary and a self-report questionnaire for assessment of medication adherence has been evaluated during two months post-discharge.
The aims of the study were to explore the willingness of patients with polypharmacy to have medication adherence measured using different methods after hospital discharge; to evaluate the feasibility and validity of a combination of pill count, a medication diary and a questionnaire for assessment of medication adherence in patients with polypharmacy two months post-discharge.
Methods
Design
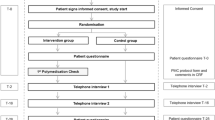
A quantitative prospective descriptive study was conducted. The first part of the study consisted of a survey assessing the willingness of patients with polypharmacy to have their medication adherence measured after hospital discharge. For this purpose, patients completed a questionnaire at the day of hospital discharge. At the end of this survey, patients could indicate whether they were willing to participate in the second part of the study. In part two, medication adherence was evaluated based on empty medication packages kept by patients for two months and a diary in which patients reported their adherence-related problems. During a home visit two months post-discharge, the Probabilistic Medication Adherence Scale (ProMAS) and a survey on the feasibility of the different methods were administered.
Setting and participants
Participants were recruited from internal medicine (n = 4), surgical (n = 5) and geriatric wards (n = 2) of two hospitals in Belgium. Hospitalised patients were eligible for inclusion if they were at least 18 years old, took at least five chronic medicines daily (i.e., polypharmacy), returned home after discharge and could understand and speak Dutch. Patients not responsible for their own medication administration at home, those incapable of providing consent to participate in the study or having a diagnosis of dementia were excluded. Between January and March 2022, all patients who met the inclusion criteria and were willing to participate were included (i.e., consecutive sampling).
Data collection
In this study, data were collected at the day of hospital discharge (i.e., moment of inclusion) and during two months post-discharge.
Study part 1: at hospital discharge
Patients who consented to participate in the study were asked to complete a self-developed questionnaire at the day of hospital discharge. Socio-demographic characteristics (age, sex, education level, living situation, type and reason for hospitalisation) and data on medication management at home (number of medicines, preparation and administration of medication, medication aids) were collected. Additionally, statements regarding truthfulness of communicating adherence-related information to healthcare providers, the willingness of patients to have their adherence measured by various methods after discharge, and the expected feasibility of these methods were surveyed using 4-point Likert scales. Finally, factors that may influence truthful communication about medication adherence, taking prescribed medication and the feasibility of the methods according to the patient were questioned. At the end of this questionnaire, patients could indicate whether they were willing to participate in the second part of the study, in which medication adherence was measured using different methods over a course of two months post-discharge. If patients refused to participate in the second part, the reasons for non-participation were surveyed.
At discharge, the medication schedule of patients, who consented to participate in part two, was copied to collect data about medication use. At this time, patients received instructions on the use of the medication diary and the preservation of their medication packages for a period of two months (± 60 days).
Study part 2: two months post-discharge
Within the two-month period, participants had to fill out a self-developed medication diary 28[]. Participants were asked to record (changes in) their medication schedule and to indicate on a daily basis whether they experienced any problems related to their medication use. In case of problems, participants had to fill out a structured problem sheet indicating the problems they were experiencing (e.g., no medication left in stock, forgot to take, took an incorrect dose, …), which medication the problems relate to (all or specific ones) and whether they took any action to address the problem. Participants were not supposed to record every medication intake. Participants were contacted three times (i.e., 1 day, 3 weeks and 5 weeks post-discharge) to find out if completing the diary and preserving medication packages went well and to collect information on drop-outs.
Approximately 60 days after hospital discharge, a home visit was conducted by one of the researchers (LM, MDG, JVD). At this moment, the Probabilistic Medication Adherence Scale (ProMAS) was administered [26]. The instrument was developed in Dutch and consisted of 18 questions assessing a wide range of medication adherence behaviours (i.e., taking less or more medicines than prescribed, forgetting to take medicines, adapting the medication regimen, fulfilling the prescription too late and stopping or not starting prescribed medication). All questions could be answered with yes or no. Based on the sum score of the ProMAS, a distinction between four categories of medication adherence can be made: low (0–4), medium-low (5–9), medium-high (10–14) and high (15–18) [26].
Furthermore, a self-developed questionnaire with statements regarding the feasibility of preserving medication packages, filling out the medication diary and the ProMAS was administered. Statements could be answered using 4-point Likert scales (1 = strongly disagree to 4 = strongly agree). Patients were asked to rank the different methods (i.e., pill count, diary and ProMAS) from the one that most accurately reflected their medication adherence over a course of two months to the one that least accurately reflected this. Finally, the preserved medication packages were collected to perform a pill count and the medication diary was collected for analysis. During the home visit, the diary was already reviewed by the researcher. Additional questions could be posed, if clarification was needed.
Study outcomes
The main outcomes of this study were: willingness to have medication adherence monitored by healthcare providers including willingness to communicate about medication adherence, feasibility and validity of methods measuring medication adherence.
Sample size calculation
No power analysis was conducted for the outcomes ‘willingness’ and ‘feasibility’ given their descriptive nature. However, an a priori power analysis was performed using G-power version 3.1 to explore the correlation between the degree of medication adherence measured by pill counts, the number of problems reported in the medication diary and the ProMAS score. A sample size of 84 participants would be required considering a power of 80%, an alpha of 5% and an expected correlation of 0.3 [29].
Data analysis
Data were analysed using IBM SPSS version 28. A significance level of 0.05 was used. Discontinuous data were described using absolute numbers and percentages, continuous data were described using mean and standard deviations or median and range. Normality of the distribution was tested using Z-scores. Differences in patient characteristics and willingness between the group of patients who participated and the group who did not participate in study part 2 were tested using Chi-square test (categorical variables) and independent-T test (continuous variables).
Medication adherence measured by pill counts was calculated using the following formula: \(\frac{the\,number\,of\,pills\,taken\,based\,on\,the\,preserved\,medication\,packages}{the\,number\,of\,pills\,to\,be\,taken }\,X\,100\). Both the average overall adherence rate and the adherence rate per medicine were calculated. Patients were considered adherent to their medication if their medication adherence percentage was between 90% and 110%. To obtain an adherence score of the ProMAS, items marked with (R) in the original questionnaire were first reverse coded. Afterwards, the items were summed whereby higher scores represent a higher degree of medication adherence. In terms of adherence measured by the diary, the number of problems reported in the diary as well as the percentage of days with problems leading to non-adherence were considered.
Correlation between adherence rates measured by pill counts, the medication diary and the ProMAS was assessed using Pearson R in case of normal distribution of the data or using Spearman’s Rho if non-normally distributed. Cases with missing data (e.g., drop-outs) were excluded for analysis.
Ethics
The Ethics Committee of the Antwerp University Hospital (Belgian registration number B3002021000224) provided ethical approval. This study was conducted in accordance with the Declaration of Helsinki. All participants received written information on the purpose, design and execution of the study. All respondents gave written informed consent before participating in the study. Data were registered and analysed after pseudonymisation to ensure privacy.
Results
The research population
In the first part of the study, 144 patients consented to participate and completed the survey at discharge. The characteristics of the research population are shown in Table 1. The mean age of the participants was 70 years [SD 9.96] and 58% were men. The majority of participants (63%) had at least a higher secondary education degree. Almost one-third (27%) lived alone and 44% needed help to reside in their own home. Most hospital admissions were planned (56%). Surgery was the main reason for hospitalisation (46%), followed by treatment (22%) and examination (19%). Participants took on average seven chronic medicines at discharge [SD 2.32, range 5–15] and 67% used medication aids.
Of the 144 participants, 85 consented to participate in the second part of the study (59%). Participants were significantly more often male (68% vs. 42%, p = 0.002), needed significantly less help to reside at home (37% vs. 54%, p = 0.035), were less often unexpectedly hospitalised (35% vs. 58%, p = 0.008) and used fewer medication aids (61% vs. 77%, p = 0.041) compared to those who did not participate in part 2. Reasons for non-participation are shown in Supplementary Table 1.
Of the 85 participants enrolled in the second part of the study, 16 dropped out (18.8%) leaving a population for analysis of 69 participants. Reasons for drop-out were: (deteriorating) health status (n = 7), personal reasons (n = 4), diary or medication packages not kept consistently (n = 3), getting nervous of participating in the study (n = 2), not wanting a home visit once returned to home (n = 1), participating is too much of an effort (n = 1), not returned home (n = 1), not wanting to participate once returned home (n = 1), no medication self-management anymore applicable (n = 1). No differences in population characteristics were found between the group of dropouts and non-dropouts (See Supplementary Table 2).
Willingness to communicate about medication adherence
Most participants with polypharmacy were willing to communicate truthfully about their medication adherence (97%), to share this kind of information with healthcare providers (99%) and to ask healthcare providers for help if problems with medication intake arise (94%). Participants who were willing to have their medication adherence monitored post-discharge were more likely to indicate that they actually communicate honestly about medication use (92% vs. 75%, p = 0.005), considered it more important for healthcare providers to be aware of their medication adherence (99% vs. 86%, p = 0.003), were more likely to report problems concerning medication intake (95% vs. 85%, p = 0.030), to tell healthcare providers if medicines are not being taken as prescribed (95% vs. 83%, p = 0.015) and found it easier to admit incorrect medication intake (95% vs. 83%, p = 0.015) compared to those who were unwilling to have their medication adherence monitored (Table 2). According to participants, some factors may influence truthful communication: fear of reaction from healthcare providers (57%), not wanting to disappoint healthcare providers (43%) and not having a good relationship with healthcare providers (14%).
Willingness to have medication adherence measured after discharge and the expected feasibility of different measurement methods
At the time of hospital discharge, more participants with polypharmacy indicated they were (rather) willing to preserve medication packages (76%) than to complete a medication diary (67%) over a course of two months. The majority of participants (65%) considered the combination of both measurement methods feasible in practice (Fig. 1). However, participants indicated several factors that may influence the feasibility of keeping a medication diary: duration (44%) and frequency of diary completion (45%), the busyness of daily life (39%), forgetfulness (34%) and deteriorating health status (32%). The same factors were mentioned considering the feasibility of preserving medication packages: duration (47%), the busyness of everyday life (35%), forgetfulness (35%) and deteriorating health status (31%).
Medication adherence measured using different methods during two months post-discharge
Pill count
The mean medication adherence rate, measured by pill counts based on empty medication packages, was 96% [minimum 69%, maximum 124%]. Applying a cut-off of 90–110% of medicines taken, 78% of the participants was found to be adherent according to the average overall adherence rate, while only 32% of the participants appear to be adherent taking into account the adherence rate per medicine.
Medication diary
About 60% of the participants reported no problems with medication intake over a two-month period. Of those who experienced problems, forgetting to take medicines was most commonly reported in the medication diary (Table 3).
Probabilistic medication adherence scale
Participants had a mean score of 13 out of 18 on ProMAS ([SD 2.7], minimum 6/18, maximum 17/18). Based on the ProMAS scores, 10% of the participants were categorized as being medium-low adherent, 49% were medium-high adherent and 41% were high adherent to their medication. Analysis of the individual questions included in the ProMAS can be found in (See Supplementary Table 3).
Correspondence between measurement results and actual medication adherence according to participants
Respectively 90%, 91% and 97% of the participants indicated that the medication diary, the pill count based on empty medication packs and the self-report questionnaire (ProMAS) correctly reflected how well the participant had taken his/her medication during a two-month period. According to the majority of participants (60%), pill counts most accurately reflected medication adherence, followed by the medication diary (39%) and the ProMAS (1%).
Feasibility of methods measuring medication adherence in practice
Most participants reported that preserving medication packages (91%), completing the medication diary (99%) and the ProMAS (99%) were no effort to them. Furthermore, filling out the medication diary and the ProMAS was found to be easy for most participants, respectively 88% and 99%. All participants reported that they completed the PROMAS and the diary truthfully. However, one participant indicated he did not preserve all medication packages honestly. Approximately 87% of participants indicated that they would be willing to preserve medication packages once again. Only 19% would like to continue using the medication diary in the future.
Validity of methods measuring medication adherence
The higher the medication adherence according to ProMAS, the higher the mean percentage of medication adherence according to pill counts (r = 0.333, p = 0.005) and the higher the percentage of medicines with which participants are adherent to (threshold of ≥ 90% - ≤ 110%) (r = 0.251, p = 0.037).
Furthermore, medication adherence measured by the ProMAS correlated negatively with the total number of problems participants reported in the medication diary (r= -0.492, p < 0.001) and with the percentage of days participants experienced problems (r= -0.516, p < 0.001). Thus, the more problems participants experienced according to the diary, the less adherent they were according to the self-report questionnaire.
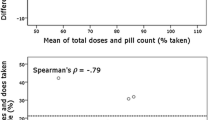
A weak negative correlation was found between mean percentage of adherence based on pill count and total number of problems reported in the diary (r= -0.168). The correlation was not significant (p = 0.181). The same applies to the correlation between the average adherence rate and the percentage of days with problems experienced (r= -0.182, p = 0.147). Additionally, no correlation was found between the percentage of medicines to which participants were adherent and problems reported in the diary.
Discussion
Identifying and monitoring medication adherence in patients with polypharmacy remains a challenge despite several measurement methods being currently available [17, 18, 20]. This study evaluated a combination of pill counts and self-reported measures assessing medication adherence (i.e., medication diary and ProMAS). Using self-reports, truthful communication about medication adherence by patients is indispensable requiring a certain level of willingness and cooperation from the patient. To the best of our knowledge, this is the first study exploring patients’ willingness to communicate about medication intake and willingness to have medication adherence monitored.
The results of this study showed an overall high willingness of participants with polypharmacy to communicate truthfully about medication use and to share medication adherence-related information with healthcare providers. However, despite this high willingness, only 59% of patients participated in the second part of the study. There seems to be a discrepancy between this willingness and the actual act of having medication adherence monitored. It is noteworthy that patients who were not willing to communicate problems concerning medication intake, including not taking medication correctly, were also not willing to have their adherence monitored after discharge and did not participate in part two of the study. These patients are likely to be less adherent. Furthermore, some subgroups were less willing to participate in the second part of the study and, as a result, were less well presented. These subgroups included women, patients using medication aids and those needing help to reside at home. Research showed that women are less likely to be adherent to medications as compared to men [30,31,32,33,34]. Patients using medication aids or requiring assistance from others may already face challenges with medication intake, affecting medication adherence as well. The fact that these subgroups are not duly represented and that only ‘willing’ or ‘dedicated’ patients participated in this study may lead to a potential misinterpretation of the results, could limit generalisability and should, therefore, be considered as a limitation. Overestimation of medication adherence should be considered, given that the second part of the study consisted of a self-selecting sample of dedicated patients.
This may also explain the overall high adherence rates in the study sample. Based on pill counts, patients had taken 96% of their medicines on average. We would have used the conventional threshold of ≥ 80% to differentiate between adherent and non-adherent patients [35,36,37,38]. However, analysis showed only four patients in our sample would be classified as non-adherent. Given this result and given the historical cut-off of 80% can be questioned as ‘standard’ [38], it was chosen to use a stricter threshold of ≥ 90% in this sample.
Two methods were applied to differentiate between adherent and non-adherent patients, based on pill counts. The first method consisted of calculating the average overall adherence rate and resulted in 78% adherent patients. However, this method potentially overestimates multiple medication adherence when a patient is more adherent to one treatment over another and hence averaging introduces an estimation bias where the observed value appears higher than the true value [39]. Therefore, the adherence rate for each medicine was calculated as well, resulting in only 32% adherent patients. This proves that even different approaches to one measurement method produce different results. The high variation in adherence rates between medicines taken by a patient underscores the importance of measuring adherence for individual medications.
Researchers and healthcare providers often have to balance reliability and practicability when choosing appropriate measurement tools [18]. The pill count was chosen since it is a low cost method, which can be used for different types of formulations (e.g., tablets, capsules and inhalers). However, it offers no insight into behaviours predicting non-adherence and problems causing non-adherence. Patient self-reports can be complementary in this regard: the medication diary can provide on a daily basis information on problems leading to not taking medicines as prescribed while a self-report questionnaire can provide additional information on adherence-related behaviours and attitudes [17, 18]. Furthermore, patient self-reports are inexpensive, well-validated and straightforward and are, therefore, often used in research and clinical practice [17, 18].
Practicability of the combination of these three methods was demonstrated as the combination was feasible and easy to use in practice according to patients. Nevertheless, our results imply that preserving medication packages for two months was highly acceptable to patients, more so than completing a medication diary. This is evident, as the majority of patients expressed a willingness to preserve medication packages once again, while less than a fifth would like to continue using the diary. When interpreting the feasibility of the methods, it is important to consider that among those who declined adherence monitoring, almost half did so due to the need of completing a diary, and a third refused because of the need to preserve medication packages. This suggests that these methods were perceived as too demanding for them.
Combining the different methods in practice also revealed some difficulties. First, a few patients already had a filled pill box at home at time of hospital discharge. The packages of these medicines were no longer available for pill count. Although this unavailability was recorded and taken into account in the actual pill count two months post-discharge, this may lead to a less accurate measurement of adherence. Second, some patients indicated that they did not experience any problems with their medication and therefore did not report any problems in the diary while the ProMAS questionnaire showed that they, for example, sometimes forgot to take a medicine. In the medication diary, problems with medication were possibly underreported because there may be a difference in what the patient himself perceives as a problem and the actual presence of a problem. Last, self-reported adherence may be subject to recall bias and social desirability bias [18, 19, 40, 41]. Efforts were made to minimise the risk of recall bias by asking patients to report problems in the medication diary on a daily basis. However, some patients indicated that they did not complete the medication diary daily, but weekly or monthly. Daily completion of the diary is probably too burdensome for these patients. Not only the feasibility of a combination of pill counts, the medication diary and the ProMAS but also their respective validity was tested. Regrettably, a perfect reference standard for the validation of adherence measures does not exist at this moment [42, 43]. Previous studies have already contrasted different methods of measuring adherence [21,22,23,24,25,26,27]. To the best of our knowledge, this was the first study validating the ProMAS using pill counts. Our study showed a significant positive correlation between adherence measured by both methods: the less medication adherence according to the pill count, the less medication adherence according to the ProMAS. The ProMAS can provide insight into the behaviour underlying the failure to take medicines as prescribed. Considering the patients’ perspective on validity, only 1% of the participants deemed ProMAS as the most accurate reflection of their medication adherence. ProMAS provides a snapshot of medication adherence and is more susceptible to recall bias, while the diary and pill count enable the daily and longitudinal measurement of medication adherence. The latter potentially instils greater confidence in patients regarding the accuracy of the measured adherence.
The medication diary was recently developed and, therefore, not previously validated. The self-reported adherence measurement tools, i.e., the medication diary and the ProMAS, correlated significantly. This result indicate that the medication diary can be used as a complementary measurement in order to gain insight into problems leading to non-adherence behaviours. In contrast, no significant relationship could be demonstrated between pill count and problems (leading to non-adherence) reported in the medication diary. The a priori calculated sample size of 84 participants was not achieved, due to dropouts which possibly explains the non-significant result. Our study showed a dropout rate of 19% over two months. Researchers should take attrition into account when calculating the sample size needed, considering that the longer the follow-up period, the higher the dropout rate will be.
Conclusion
Despite the high willingness to communicate truthfully about medication adherence and to share adherence-related information with healthcare providers, only 59% of patients had their adherence effectively monitored during two months. The combination of pill counts, a medication diary and the ProMAS assessing medication adherence two months post-discharge seems feasible in practice. The different methods are complementary to each other. While a pill count can assess the extent to which a patient’s actual medication use corresponds to the prescribed medication regimen, self-reports can give additional information about adherence-related behaviours. Moreover, the medication diary has the potential to provide insight into daily problems and patterns contributing to non-adherence.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Vrijens B et al (2012) A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73(5):691–705. https://doi.org/10.1111/j.1365-2125.2012.04167.x
Sabate E (2003) Adherence to long-term therapies: evidence for action. World Health Organization, Geneva, Switzerland
Masnoon N et al (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17(1):230. https://doi.org/10.1186/s12877-017-0621-2
Kardas P, Lewek P, Matyjaszczyk M (2013) Determinants of patient adherence: a review of systematic reviews. Front Pharmacol 4:91. https://doi.org/10.3389/fphar.2013.00091
Marcum ZA, Gellad WF (2012) Medication adherence to multidrug regimens. Clin Geriatr Med 28(2):287–300. https://doi.org/10.1016/j.cger.2012.01.008
Hovstadius B, Petersson G (2012) Factors leading to excessive polypharmacy. Clin Geriatr Med 28(2):159–172. https://doi.org/10.1016/j.cger.2012.01.001
Zelko E, Klemenc-Ketis Z, Tusek-Bunc K (2016) Medication adherence in elderly with polypharmacy living at home: a systematic review of existing studies. Materia socio-medica 28(2):129–132. https://doi.org/10.5455/msm.2016.28.129-132
Cutler RL et al (2018) Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 8(1):e016982. https://doi.org/10.1136/bmjopen-2017-016982
De Vera MA et al (2014) Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol 78(4):684–698. https://doi.org/10.1111/bcp.12339
Iuga AO, McGuire MJ (2014) Adherence and health care costs. Risk Manage Healthc Policy 7:35–44. https://doi.org/10.2147/RMHP.S19801
Simpson SH et al (2006) A meta-analysis of the association between adherence to drug therapy and mortality BMJ (Clinical research ed.), 333(7557): p. 15–15 https://doi.org/10.1136/bmj.38875.675486.55
van Boven JF et al (2014) Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med 108(1):103–113. https://doi.org/10.1016/j.rmed.2013.08.044
Anderson LJ et al (2020) A systematic overview of systematic reviews evaluating medication adherence interventions. Am J Health-System Pharm 77(2):138–147. https://doi.org/10.1093/ajhp/zxz284
Cross AJ et al (2020) Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications Cochrane Database Syst Rev, 5(5): p. Cd012419 https://doi.org/10.1002/14651858.CD012419.pub2
Verloo H et al (2017) Nurse interventions to improve medication adherence among discharged older adults: a systematic review. Age Ageing 46(5):747–754. https://doi.org/10.1093/ageing/afx076
Presley B, Groot W, Pavlova M (2019) Pharmacy-led interventions to improve medication adherence among adults with diabetes: a systematic review and meta-analysis. Res Social Adm Pharm 15(9):1057–1067. https://doi.org/10.1016/j.sapharm.2018.09.021
Anghel LA, Farcas AM, Oprean RN (2019) An overview of the common methods used to measure treatment adherence. Med Pharm Rep 92(2):117–122. https://doi.org/10.15386/mpr-1201
Lam WY, Fresco P (2015) Medication Adherence Measures: An Overview BioMed research international, 2015. : p. 217047–217047 https://doi.org/10.1155/2015/217047
Basu S et al (2019) Improving the assessment of medication adherence: challenges and considerations with a focus on low-resource settings. Ci Ji Yi Xue Za Zhi 31(2):73–80. https://doi.org/10.4103/tcmj.tcmj_177_18
Pednekar PP et al (2019) Methods for measuring multiple medication adherence: a systematic review-report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health 22(2):139–156. https://doi.org/10.1016/j.jval.2018.08.006
Grymonpre RE et al (1998) Pill Count, Self-Report, and Pharmacy Claims Data to measure Medication Adherence in the Elderly. Ann Pharmacother 32(7–8):749–754. https://doi.org/10.1345/aph.17423
Elm JJ et al (2007) Self-reported adherence versus pill count in Parkinson’s disease: the NET-PD experience. Mov Disord 22(6):822–827. https://doi.org/10.1002/mds.21409
Wu P et al (2014) The combination of pill count and self-reported adherence is a strong predictor of first-line ART failure for adults in South Africa. Curr HIV Res 12(5):366–375. https://doi.org/10.2174/1570162x1205141121102501
van Onzenoort HAW et al (2010) Assessing Medication Adherence simultaneously by electronic monitoring and pill count in patients with mild-to-moderate hypertension. Am J Hypertens 23(2):149–154. https://doi.org/10.1038/ajh.2009.207
El Alili M et al (2016) A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 82(1):268–279. https://doi.org/10.1111/bcp.12942
Kleppe M et al (2015) The development of the ProMAS: a probabilistic medication adherence scale. Patient Prefer Adherence 9:355–367. https://doi.org/10.2147/PPA.S76749
Kleppe M (2016) Understanding medication adherence: a self-report measure and a dual-process framework
Mortelmans L, Dilles T (2024) The development and evaluation of a medication diary to report problems with medication use. Heliyon e26127. https://doi.org/10.1016/j.heliyon.2024.e26127
Liu XS, Carlson R, Kelley K (2019) Common language effect size for correlations. J Gen Psychol 146(3):325–338. https://doi.org/10.1080/00221309.2019.1585321
Rea F et al (2020) Women discontinue antihypertensive drug therapy more than men. Evidence from an Italian population-based study. J Hypertens 38(1):142–149. https://doi.org/10.1097/hjh.0000000000002222
Chen SL et al (2014) Factors associated with gender differences in medication adherence: a longitudinal study. J Adv Nurs 70(9):2031–2040. https://doi.org/10.1111/jan.12361
Rebić N et al (2023) What’s sex and gender got to Do with it? A scoping review of sex- and gender-based analysis in Pharmacoepidemiologic studies of Medication Adherence. Value Health 26(9):1413–1424. https://doi.org/10.1016/j.jval.2023.04.002
Radic J et al (2023) Medication adherence and aender difference in hypertensive patients. J Hypertens, 41(Suppl 3).
Franchi C et al (2022) Multiple medication adherence and related outcomes in Community-Dwelling Older people on chronic polypharmacy: a retrospective cohort study on administrative Claims Data. Int J Environ Res Public Health 19(9):5692
Haynes RB et al (1980) Can simple clinical measurements detect patient noncompliance? Hypertension 2(6):757–764. https://doi.org/10.1161/01.hyp.2.6.757
Kleinsinger F (2018) The Unmet Challenge of Medication Nonadherence. Perm J 22:p18–033. https://doi.org/10.7812/tpp/18-033
Brown MT, Bussell JK (2011) Medication adherence: WHO cares? Mayo Clin Proc 86(4):304–314. https://doi.org/10.4065/mcp.2010.0575
Baumgartner PC et al (2018) A systematic review of Medication Adherence Thresholds Dependent of Clinical outcomes. Front Pharmacol 9. https://doi.org/10.3389/fphar.2018.01290
Pednekar P et al (2017) Measuring multiple medication adherence–which measure when? Value & Outcomes Spotlight, pp 17–20
Stirratt MJ et al (2015) Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 5(4):470–482. https://doi.org/10.1007/s13142-015-0315-2
Wagner G, Miller LG (2004) Is the influence of social desirability on patients’ self-reported adherence overrated? J Acquir Immune Defic Syndr 35(2):203–204. https://doi.org/10.1097/00126334-200402010-00016
Culig J, Leppée M (2014) From Morisky to Hill-bone; self-reports scales for measuring adherence to medication. Coll Antropol 38(1):55–62
Lehmann A et al (2014) Assessing medication adherence: options to consider. Int J Clin Pharm 36(1):55–69. https://doi.org/10.1007/s11096-013-9865-x
Funding
L.M. was supported by the Research Foundation Flanders through grant 11L0522N. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.M., E.G., M.D.G., J.V.D., AM.D.C. , M.P., P.v.d.B and T.D.; Methodology, L.M., E.G., M.D.G., J.V.D., AM.D.C. , M.P., P.v.d.B and T.D.; Funding acquisition: L.M.; Formal analysis; L.M., Writing—Original Draft, L.M., Writing—Review and Editing, L.M., E.G., M.D.G., J.V.D., AM.D.C. , M.P., P.v.d.B and T.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mortelmans, L., Goossens, E., De Graef, M. et al. Evaluation of methods measuring medication adherence in patients with polypharmacy: a longitudinal and patient perspective. Eur J Clin Pharmacol 80, 891–900 (2024). https://doi.org/10.1007/s00228-024-03661-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03661-1