Abstract
Background
Adherence to preventive medication is often poor, and current interventions have had limited success.
Purpose
This study was conducted to pilot a randomised controlled trial aimed at increasing adherence to preventive medication in stroke survivors using a brief, personalised intervention.
Methods
Sixty-two stroke survivors were randomly allocated to either a two-session intervention aimed at increasing adherence via (a) introducing a plan linked to environmental cues (implementation intentions) to help establish a better medication-taking routine (habit) and (b) eliciting and modifying any mistaken patient beliefs regarding medication/stroke or a control group. Primary outcome was adherence to antihypertensive medication measured objectively over 3 months using an electronic pill bottle.
Results
Fifty-eight people used the pill bottle and were analysed as allocated; 54 completed treatment. The intervention resulted in 10 % more doses taken on schedule (intervention, 97 %; control, 87 %; 95 % CI for difference (0.2, 16.2); p = 0.048).
Conclusions
A simple, brief intervention increased medication adherence in stroke survivors, over and above any effect of increased patient contact or mere measurement. (http://controlled-trials.com, number ISRCTN38274953.)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is estimated that, in developed countries, only 50 % of patients who suffer from chronic diseases adheres to treatment recommendations [1]. Adherence is lower in chronic conditions than in acute conditions and drops off dramatically after the first 6 months of treatment [2]. A recent large-scale survey of over 31,000 patients with hypertension in Italy found that only 41 % had >80 % adherence (measured by dispensed medications) 2–3 years after they were first prescribed, and poor adherence was associated with higher incidence of myocardial infarction, stroke and all-cause death [3].
The current Cochrane review of interventions to improve medication adherence concluded that ‘Current methods of improving adherence for chronic health problems are mostly complex and not very effective, so that the full benefits of treatment cannot be realized. High priority should be given to fundamental and applied research concerning innovations to assist patients to follow medication prescriptions for long-term medical disorders’ [4, p. 2].
This pilot study aimed to evaluate a brief intervention to increase medication adherence in the secondary prevention of stroke. Stroke is one of the most common causes of death in the USA and UK and is the most common cause of severe physical disability amongst adults. The risk of a recurrent stroke is 30–43 % within 5 years. Guidelines for secondary prevention after ischaemic stroke now recommend antiplatelet therapy and reduction of both blood pressure (BP) and cholesterol level as key components in reducing the risk of future vascular events [5]. Despite this, adherence to prescribed medication in stroke patients is often suboptimal. For instance, a recent US study of 2,888 stroke patients found that 25 % had discontinued one or more of their medicines at just 3 months post-discharge [6].
Poor adherence may be both non-intentional and intentional. Non-intentional non-adherence (e.g. forgetting) is often a consequence of cognitive impairment [7]. After a stroke, the impact of cerebrovascular disease on cognitive function, particularly memory, may mitigate against adherence, particularly if the patient is elderly and the drug regime is complicated [8].
Brief and easy-to-complete implementation intentions interventions have been shown to be effective at reducing forgetting and improving medication adherence [9]. These involve patients writing down exactly when and where they will take their medication, using the format of an if–then plan (‘If it is time X in place Y and I am doing Z, then I will take my pill dose’, e.g. first cup of tea at breakfast in the kitchen cues taking morning medication). If–then planning makes people highly sensitive to written environmental cues, establishes a habit and removes the burden of having to think about and remember when to act by reducing the load on prospective memory as habitual responses become established. Brown and colleagues showed that such an approach was successful in improving adherence in a randomised controlled trial (RCT) in patients with epilepsy (e.g. doses taken on schedule, 78.8 % in the intervention group versus 55.3 % in the control group, p < 0.01) [10].
Intentional non-adherence occurs when a patient deliberately chooses not to take their medication, against medical advice. This often depends on the patient’s beliefs concerning their condition and/or their medication. Leventhal’s self-regulation theory posits that patients have a common-sense model of their illness in terms of beliefs regarding how long it will last, whether it is acute or chronic, and so forth [11]. Patients also have beliefs about treatment, particularly the perceived necessity of medication versus concerns about any possible harmful effects [12]. A recent study in stroke patients found that patients’ concerns about their medication (e.g. dependence, toxicity, too many tablets) were key determinants of poor adherence, supporting the self-regulation theory [13].
We report the results of a pilot randomised trial of a brief intervention based on this theoretical framework. Our aim was to increase medication adherence via (a) developing an implementation intentions plan to reduce non-intentional non-adherence and (b) eliciting and modifying erroneous beliefs about medication and stroke to reduce intentional non-adherence. Although we were interested in patients’ experiences regarding all of their stroke medication, antihypertensive medication was targeted for measurement, as poor adherence to antihypertensives has been associated with significantly increased risk of stroke and death [14], and only 30–50 % of patients regularly take their antihypertensive drugs as prescribed [15].
Methods
Participants
In accordance with our published protocol [16], participants were recruited from consecutive discharges from the stroke clinic and stroke unit at the Western General Hospital in Edinburgh between January 2010 and October 2011. Inclusion criteria were first stroke or transient ischaemic attack (TIA), discharged to home and on any preventive stroke medication. All participants gave informed consent for the study which was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 (revised 2000) and had ethical approval from the South East Scotland Research Ethics Committee (REC ref. no. 09/S1102/36). The study is registered with Current Controlled Trials, http://controlled-trials.com, with the unique identifier number ISRCTN38274953.
Measures
Medication Events Monitoring System (MEMS® Aardex Ltd., Switzerland)
Our primary outcome measure was electronically recorded openings using MEMS pill bottles for 3 months. MEMS pill bottles were used in both treatment arms as MEMS measurement is not immune from the Hawthorne (or mere measurement) effect [17]. Following Brown and colleagues [10], we calculated the percentage of (a) prescribed doses taken, (b) days on which the correct dose was taken and (c) doses taken on schedule, i.e. within a 3-h window of the median time taken. We considered the latter as particularly important, as the implementation intentions intervention aims to establish regular behaviour patterns tied in to a specific time. Median rather than mean time was used to reduce the effect of any outliers.
The Medication Adherence Report Scale (MARS) [18]
The MARS consists of five items relating to taking medication, each scored from 1 to 5 and totalled to give an overall MARS score. The MARS is worded in order to make missing medication seem a normal behaviour, with the aim of reducing social desirability and eliciting honest responses. As the MARS was initially used to detect less than maximum adherence on patients’ antihypertensive medicine, patients were asked to answer each question for how they took their ‘blood pressure medicines’. The MARS has been used extensively to measure adherence in patients with chronic diseases and has shown good reliability (internal and test–retest) and validity (convergent and criterion).
Patient Beliefs About Medication and Illness
The Beliefs about Medication Questionnaire (BMQ) [19] was used to assess cognitive representations of medication. We only used the specific subscales (BMQ-specific) which relate to a patient’s prescribed medication, as the BMQ-general scales (medication in general) were not associated with adherence in our previous study [13]. The BMQ-specific has two five-item subscales representing (a) beliefs about the necessity of medication (necessity) and (b) concerns/beliefs about the risks and/or negative effects of taking medication (concerns). Patients were asked to give their personal views on the ‘medicines prescribed for your stroke or TIA (mini-stroke)’. The BMQ-specific subscales have shown good reliability and validity amongst patients with varied illnesses including heart conditions, asthma and diabetes [19].
Participants were also asked to indicate their perception of the benefits (0–100 %) provided by their stroke medication over the next 5 years following [20] and our previous study [13]. Finally, the Brief Illness Perception Questionnaire (BIPQ) [21] was used to assess patients’ views of their illness (i.e. stroke/TIA). The nine-item BIPQ provides an easy-to-complete, psychometrically robust measure of the major components of illness perceptions.
Frenchay Aphasia Screening Test [22]
This screening test, which is frequently used with stroke patients, was developed as a quick and simple method to identify the presence of language disturbance. It has shown good test–retest and inter-rater reliability as well as good construct and criterion validity [22]. In order to ensure that patients would be able to complete all self-report measures, those scoring <13 were excluded from the intervention.
Mini-Mental State Examination (MMSE) [23]
The MMSE is a brief, valid and reliable assessment of various components of cognitive function, which has been widely used in stroke research. We excluded patients scoring <23 from the intervention, as this could indicate cognitive difficulties which could affect study participation.
Procedures
The study was conducted in two stages: In part 1, participants completed a questionnaire, which was used to screen for eligibility for part 2, the pilot randomised trial. All participants gave separate written informed consent for parts 1 and 2. Full details of the procedures can be obtained from the study protocol [16].
Part 1: Screening for Intervention
Patients consenting to part 1 were sent the MARS, BMQ, BIPQ and perception of benefits questionnaires around 3 months after discharge. They were also asked to list all current medications taken and provide information on any help they currently received in remembering to take their pills.
All patients who reported less than maximum adherence (i.e. MARS scores <25) were considered for the brief intervention (part 2). Those indicating they were not responsible for their own medication, not on any antihypertensive medication or already using pharmacy-supplied Dosette boxes were excluded. Patients eligible for part 2 were sent an invitation letter plus information sheet, consent form and stamped addressed envelope. A power calculation indicated that a sample size of n = 30 in each treatment arm would detect a medium effect of the intervention on MEMS adherence [16].
Part 2: Intervention Versus Control
Two brief sessions, 2 weeks apart, were conducted by a trained research fellow for both the intervention and control groups, either in the patient’s home or at the hospital-based Wellcome Trust Clinical Research Facility. All interviews were timed, digitally audio-recorded and transcribed for a check on treatment fidelity.
All patients were screened (at session 1) using the MMSE and the Frenchay Aphasia Screening Test; there were no exclusions on either test. Patients in both treatment arms were told that we were using the MEMS containers to collect information about how patients took their medication.
Intervention Condition
Session 1 focussed on helping each patient establish a better medication-taking routine using an implementation intentions approach to develop an individually tailored coping plan [24]. The plan was introduced at session 1 so that patients could try it out over the 2-week period prior to review in session 2.
Following Brown et al. [10], patients were advised that it was a good idea to make taking their medicines part of a routine and were asked to make a plan to take their tablet(s) at the same time as something else that they did every day. They were then asked to write down the time, place and what they would be doing at the time they would take the first dose of their antihypertensive medicine (i.e. that which would be put in the MEMS pill bottle) on an individualised worksheet (see Fig. 1). This was repeated for all daily doses of this medication. Patients were then asked to repeat each plan up to three times, until they felt they were able to remember it without looking at what they had written down.
Examples of implementation intentions plans (following Brown et al. [10])
The research fellow also took baseline BP readings using an OMRON M10-IT BP monitor, according to a standard protocol. A single reading was taken from each arm; then, the arm with the highest systolic value was used to collect an average of three readings taken at 120-s intervals.
Session 2 first reviewed the effectiveness of the implementation intentions plan and any barriers/difficulties in following it, and any required changes were developed collaboratively, following the methods outlined by Sniehotta [24]. This approach helped ensure that the patient had developed a suitable implementation intentions plan before adherence measurement commenced.
The main focus of session 2 was to elicit and, if appropriate, challenge any mistaken beliefs about a patient’s illness (e.g. causes/effects of their stroke) and/or medication (e.g. beliefs regarding toxicity, dependence, etc.), using responses on the BMQ and BIPQ as a basis. This approach was based on the model of Petrie et al. [25] who elicited and modified patients’ dysfunctional beliefs regarding their recent myocardial infarction, resulting in faster return to work and lower angina symptoms at 3 months. Our aim was to correct any misperceptions and provide evidence so that participants’ medication necessity beliefs regarding their stroke medication came to outweigh their medication concerns beliefs. As an example, many patients did not understand why, as their cholesterol level was in the ‘normal’ range (i.e. 5.0 or less), they needed to take statins, which they believed were very likely to result in adverse side effects (resulting from negative reports regarding statins in the UK media). In these instances, the research fellow aimed to increase the patients’ belief in the necessity of their medication by informing them of the current recommendations for patients who have had a TIA or stroke (e.g. ‘A statin should be prescribed to patients who have had an ischaemic stroke, irrespective of cholesterol level’ [26]) and explaining that having a cholesterol level of 4.0 or lower was likely to further reduce their risk of having another TIA or stroke. The research fellow also provided information on the likelihood of experiencing any side effects mentioned (e.g. less than one in ten people report experiencing this side effect).
Control Condition
The use of an objective outcome measure of adherence in both groups, to control for any measurement effect of using the MEMS pill bottle, meant we were not able to have a ‘usual care’ group. Hence, control group participants received the same number of visits by the research fellow, and all measures including BP readings were collected at the same time points as the intervention group. During the first two sessions, the research fellow engaged the patient in non-medication-related conversation (e.g. what had happened when they had their stroke) to control for non-specific effects of attention/social contact.
Both Groups
At the end of session 2, the research fellow filled each participant’s MEMS pill bottle with 1 month’s supply of a single antihypertensive medication. For patients on more than one antihypertensive, the medication chosen was that taken most frequently (i.e. twice rather than once a day) or, if no difference, that which would most conveniently fit into the MEMS pill bottle. Patients were instructed to only take out one dose of their medication whenever they opened the pill bottle.
For each of the next 2 months, the research fellow made another brief visit to refill the MEMS pill bottle and also take an electronic reading from the MEMS cap, downloading the data onto a laptop PC for later analysis. The participant also completed the BIPQ, BMQ and perceptions of medication benefits measures at the first of these visits. At 3 months, the research fellow made a final visit to take a last MEMS cap reading, collect the MEMS pill bottle and final outcome measures and take the patient’s BP.
Randomisation
Patients were randomised by the web-based Edinburgh Clinical Trials Unit software to either the intervention or control arm using a minimisation algorithm, together with a random element giving a one in ten chance of allocation to the opposite treatment from that determined by the algorithm. Based on our previous study, the minimisation variables (chosen to ensure that the treatment arms did not differ on factors that might affect adherence) were age, MARS scores and complexity of the medication regime [13].
As this was a pilot study, the recruitment, intervention, data collection and analysis were all carried out by the same research fellow who was not blind to the treatment allocation. Patient contact time was controlled between treatment arms, and patients themselves were not informed which arm they were in.
Training of the Research Fellow
The research fellow was trained in the intervention by the principal investigator, who had been previously trained in the intervention used by Petrie et al. [25] in eliciting and addressing mistaken beliefs. Both the intervention and control group sessions (1 and 2) were piloted via role-play with the principle investigator acting as a pseudo-patient. These sessions were video-recorded, and feedback was given to the research fellow, after which minor modifications were made to the procedure.
Fidelity of Intervention Check
The principle investigator checked the transcriptions of sessions 1 and 2 for the first 11 participants in the intervention group (i.e. 38 % of all interviews) for adherence to the study protocol on an ongoing basis. Minor suggestions and modifications were made to subsequent sessions, but there were no violation issues. The fidelity check was terminated after the 11th interview, as no issues arose from the last four interviews checked (i.e. 8–11).
Statistical Analysis
Four people randomised to treatment did not use the MEMS pill bottle (three became ineligible between randomisation and the intervention and one declined to use the MEMS pill bottle at session 2) (Fig. 2). An a priori decision had been made to exclude all participants with no data on the primary outcome measure (MEMS) from the analysis, in accordance with our published protocol [16]. The remaining participants were analysed as allocated to the treatment arm (n = 29 in each group).
Missing Data
Four (6.9 %) of the 58 people included in the analysis discontinued participation in the study, all for reasons unrelated to the study aim, meaning that the data can be considered ‘missing at random’ [27] (i.e. 3 hospitalised for non-stroke reasons and 1 relocated). A further person chose to terminate a month early (for travel/time reasons) but completed all outcome measures at session 4 (defined completer).
There were no differences with regard to patterns of missing data and treatment group (χ 2(2) = 0.5, p = 0.766) nor with regard to any of the pretreatment outcome measures, gender, Scottish Index of Multiple Deprivation (SIMD) scores, MMSE scores or overall MEMS scores (data available from the authors). Dropouts had lower pretreatment MARS scores, but there were no differences by treatment group.
Analysis
Missing data were addressed by multiple imputation, which is currently the preferred method of imputing missing data. Multiple imputation has been shown to perform well with both longitudinal data and small samples [28]. We used Imputation of Chained Equations in the STATA software package to impute five datasets which were then analysed using SPSS version 19. T tests and χ 2 were used to test basic differences between treatment groups, and repeated-measures analysis of variance was used to compare changes in outcome measures over time. Where required, test statistics were pooled using Rubin’s rules [29].
Results
Part 1 (Screening for Intervention)
Overall, 494 people completed consent forms for part 1, 35 of these opted not to take part and 52 proved ineligible (50 previous stroke/TIA and 2 no stroke diagnosis). Questionnaires were, therefore, sent to 407 patients at a mean of 132 days (SD, 76.5) after their stroke/TIA; 355 questionnaires (87.2 %) were returned.
Two hundred seventy people were excluded from part 2 for reasons including maximum MARS adherence (53.5 %), not taking antihypertensives (20.6 %), receiving help taking their medication (11.0 %) and using a pharmacy-supplied Dosette box (23.7 %) (Fig. 2).
Part 2 (Intervention Versus Control)
Part 2: Participation
Eighty-five people were invited to take part in the intervention; 62 consented and were randomised to either the intervention or the control group (Fig. 2). Three people were excluded after randomisation and before starting the intervention: one (intervention) had started using a Dosette box before the research fellow’s first visit and two (both control) were no longer taking antihypertensive medication; therefore, 59 people started the intervention.
There were no major differences with respect to age, gender or any of the pretreatment beliefs measures between participants (n = 59) and non-participants (n = 296) (data available from the authors). However, part 2 participants (mean SIMD score = 10.5 (SD, 9.6)) were from areas of lower deprivation than non-participants (mean = 14.3 (SD, 13.3), 95 % CI for difference (0.9, 6.7); p = 0.011).
Part 2 participants reported taking a mean of 5.5 (SD, 2.3; range, 2–15) different regular oral medications, representing a mean of 6.8 (SD, 4.1; range, 2–24) tablets each day, and a mean of 1.7 (SD, 1.0; range, 1–4) antihypertensive medications per day.
Baseline characteristics were similar in the intervention and control groups, suggesting that the randomisation procedure was effective (Table 1). Total contact time was not significantly different between the intervention (mean, 224 min (SD, 50)) and control (mean, 197 min (SD, 45)) groups (95 % CI for difference (−1, 55); p = 0.056)). The slightly longer contact time in the intervention group largely occurred across sessions 1 and 2, when the intervention was delivered; we, therefore, estimate that the active part of the brief intervention lasted, on average, <30 min.
Part 2: Primary Outcome Measure (MEMS)
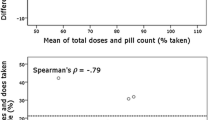
MEMS data were recorded for a period of 3 months (mean number of days = 82.2 (SD, 17.4); range, 16–90). The intervention group had higher adherence on all three MEMS outcome measures than the control group (Table 2), although this was only significant for doses taken on schedule (Fig. 3) (i.e. percentage of doses taken on schedule: mean difference, 9.8 %; 95 % CI (0.2, 16.2); p = 0.048; percentage of total doses taken: mean difference, 5.1 %; 95 % CI (−1.6, 9.0); percentage of days correct dose taken: mean difference, 5.4 %; 95 % CI (−1.8, 9.4)). There were no effects of time or the group × time interaction in any of the MEMS analyses.
Part 2: Secondary Outcome Measures
MARS Scores
Table 3 shows the changes in scores in self-reported adherence (MARS) from pre-intervention to follow-up. There were significant time and interaction effects of total MARS scores, with both groups reporting higher adherence at follow-up, but a significantly greater improvement in the intervention group (mean difference, 0.61; 95 % CI (0.1, 1.2); p = 0.027).
Self-reported adherence (i.e. MARS total scores) at follow-up was highly correlated with all objective MEMS measures (all p < 0.001), i.e. percentage of doses taken (r = 0.71), percentage of days correct dose taken (r = 0.70) and percentage of doses taken on schedule (r = 0.66).
Blood Pressure
Both groups showed a reduction in BP readings, with a significant effect of time on both systolic and diastolic measures (Table 3), but there were no differences between groups.
Beliefs About Illness and Medication
Table 4 shows the pre, post and follow-up scores on the BMQ-specific subscales. There was a significant effect of time with BMQ-necessity minus concerns increasing and BMQ-concerns decreasing from pretreatment to follow-up in both groups. BMQ-concerns also showed a significantly greater decrease by follow-up in the intervention versus the control group (mean difference, 1.3; 95 % CI (0.1, 2.5); p = 0.047). There were no significant effects of changes in beliefs about illness (BIPQ) or perceived benefit of medication as a result of the intervention (Table 4).
Discussion
This simple, brief, two-session intervention resulted in a 10 % increase in doses of antihypertensive medication taken on schedule. This level of increase is likely to be clinically important, as a large (n = 47,479) retrospective cohort study, using medical and pharmacy records to assess health outcomes and adherence over 1–5 years, concluded that ‘increasing adherence by one (anti-hypertensive) pill per week for a once-a-day regimen reduces the hazard of stroke by 8–9 % and death by 7 %’ [14]. Furthermore, the main effect was observed on doses taken on schedule, precisely what the implementation intentions intervention aimed to change. We also achieved our aim of a greater reduction in concerns about medication in the intervention versus the control group.
Previous interventions to improve adherence have had mixed results [4, 30], although adherence in clinical trials is often higher than expected [31]. Implementation intentions interventions in other health conditions have been successful in improving adherence to medication in some (e.g. antiepileptic drugs [10]), but not all (e.g. antibiotics [31]) studies. A recent RCT aimed at reducing concerns about medication in 136 non-compliant hypertensive patients in Jordan was strongly associated with higher adherence and reductions in BP [32]; however, this study did not control for patient contact time. In contrast, a controlled UK-based nurse-led support intervention which encouraged hypertensive patients to discuss their medication concerns showed no effects on either adherence or BP [33].
The current study, using electronic pill monitoring, found high levels of adherence overall, in contrast to some other studies of adherence in stroke patients [6]. Patients’ adherence did not appear to become worse over the 3 months of MEMS pill bottle usage nor were there any differences in adherence over time between groups, contrary to our expectation of a Hawthorne (or mere measurement) effect [17]. It is important to emphasise that we did not compare our brief intervention with treatment as usual; rather, our control condition involved significant additional contact and electronic recording of pill-taking for 3 months. It is highly likely that this resulted in improved adherence in the control group, e.g. when asked to rate the MEMS pill bottle during the follow-up interview, 46 % of control participants reported that they found it helpful regarding adherence. This may also account for the fact that, although the intervention group were significantly more regular in their pill-taking than the control group, the differences in pills taken (i.e. 5 % more in the intervention group) was non-significant. Although, due to cost, we were only able to use the MEMS pill bottle for one medication (a single antihypertensive), all patients took a number of medications (range, 2–15), often at the same time. From the detailed conversations the research fellow had with patients throughout the study, we have no reason to believe that using the MEMS pill bottle for only one antihypertensive negatively impacted on other medication-taking nor that it resulted in patients being any more or less adherent to their remaining medicines.
The intervention did not significantly increase patients’ beliefs in the necessity of their medication; rather, the effect was a reduction in concerns. However, as many of the patients had been on multiple preventive medications such as antihypertensives and statins for some years, many already viewed their treatment as ‘necessary’.
Baseline data were not recorded for the MEMS and so the apparent differences between groups from month 1 may well reflect an immediate effect of the intervention on adherence. The significantly greater improvement in self-report adherence (MARS) in the intervention group from pretreatment to follow-up supports this observation.
Although the greater increase in MARS total scores from pretreatment to follow-up in the intervention group did not translate into greater reductions in BP, both groups did show a significant reduction over time, which may be due to the relatively high adherence observed in both groups.
Limitations
A limitation of the current study was that the same research fellow delivered the intervention and conducted the analysis and so was not blind to the treatment arm of the patients. However, the main outcome measure was electronically recorded MEMS readings, and the remaining (self-report) outcome measures were posted out to and completed by patients in advance of the meetings with the research fellow, so we do not believe that this would have greatly affected the results. As we did not collect baseline MEMS readings, it is possible that the differences found may have been observed at pretreatment and not as a result of the intervention. However, there were no differences between groups in baseline self-reported adherence (MARS) and we did observe significantly higher increases in MARS scores in the intervention group, suggesting that this was not the case.
We planned to recruit patients 3 months after their stroke, to allow time for the establishment of medication routines; however, many patients were prescribed antihypertensives before their stroke and so the intervention may be more effective if delivered earlier. All participants were White British, had had an ischaemic stroke and tended towards higher socio-economic status; thus, the intervention warrants evaluation with more diverse populations. However, although those from more deprived areas reported lower adherence overall, socio-demographic status was not related to increases in self-reported adherence from pretreatment to follow-up, suggesting that our intervention may be effective across social domains. We also acknowledge that other factors that were not measured in the current study, such as depression and social support, may also contribute to non-adherence of medication.
Conclusions
The current study has shown that a combined brief intervention which addresses patients’ erroneous beliefs about medication and stroke to reduce intentional non-adherence, in conjunction with introducing an implementation intentions plan to reduce forgetting, can improve adherence to preventive medication by 10 % in an older population of stroke patients, over and above any effects of measurement or high therapeutic contact. The effect found was equivalent to taking one additional dose in ten within ±3 h of a regular time.
Using plans to establish routines may help older adults adhere to time-regulated tasks by making them automatic habits, whilst addressing patients’ concerns may result in less reluctance to take medication. It has also been suggested that negative views about treatment may underlie unintentional, as well as intentional, reasons for not taking medication [34]; thus, it is plausible that the observed reduction in medication concerns led to decreases in both forgetting and choosing not to take medication in the current study.
This pilot was conducted in stroke survivors; however, it may well be generalizable to other patients on multiple medications. We estimate that the active part of the intervention took no more than 30 min, spread over sessions 1 and 2. Although our approach may be more resource-intensive than simpler interventions, such as telephone reminders or mobile phone alarms, our findings suggest that it is important to address not just non-intentional adherence but also intentional adherence by eliciting and addressing patients’ underlying beliefs which are likely to affect their medication-taking. Hence, there remains a need for personalised interventions to increase adherence. We believe that this simple, relatively brief intervention could address this need, particularly if delivered in a healthcare setting at the time when medication for a chronic condition was first prescribed.
We observed small to medium (0.17 to 0.59) effects on all the main outcome measures. Using G*Power [35] to estimate the sample sizes required to detect these effects in a larger-scale study, sample sizes varied from n = 102 to n = 240. Allowing for 20 % attrition, this would mean a total sample size of 288 patients would be large enough to detect the effects found in the current study. The next step, therefore, is to confirm this effect in an adequately powered RCT to demonstrate that the intervention can be successfully delivered by trained health professionals (e.g. nurses) in different healthcare settings.
References
World Health Organisation. Adherence to long-term therapies: Evidence for action. Geneva: WHO; 2003.
Osterberg L, Blaschke T. Adherence to medication. New Eng J Med. 2005;353:487-497.
Esposti LD, Saragoni S, Benemei S, et al. Adherence to antihypertensive medications and health outcomes among newly-treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47-54.
Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011.
Scottish Intercollegiate Guidelines Network. Management of patients with stroke or TIA: Assessment, investigation, immediate management and secondary prevention—A national guideline. Guideline 108. Edinburgh: SIGN; 2008.
Bushnell CD, Zimmer LO, Pan W, et al. Persistence with stroke prevention medications 3 months after hospitalization. Arch Neurol. 2010;67:1456-1463.
Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: Small cognitive changes make a big difference. J Aging Health. 2009;21:567-580.
Fischer B, Lehrl S, Weber E, Gundert-Remy U, Fischer U. Cerebrovascular insufficiency and compliance with drug therapy. Z Gerontol. 1981;14:145-152.
Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull. 2006;132:249-268.
Brown I, Sheeran P, Reuber M. Randomized controlled trial of an implementation intention intervention to enhance adherence with antiepileptic drug treatment. Epilepsy Behav. 2009;16:634-639.
Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cognitive Ther Res. 1992;16:143-163.
Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: Application of the Necessity-Concerns Framework. J Psychosom Res. 2008;64:41-46.
O’Carroll RE, Whittaker J, Hamilton B, Johnston M, Sudlow C, Dennis M. Predictors of adherence to secondary prevention medication in stroke patients. Ann Behav Med. 2011;41:383-390.
Bailey JE, Wan JY, Tang J, Ghani MA, Cushman WC. J Gen Intern Med. 2010;25:495-503.
Stephenson J. Noncompliance may cause half of antihypertensive drug “failures”. JAMA. 1999;282:313-314.
O’Carroll RE, Dennis M, Johnston M, Sudlow C. Improving Adherence to Medication in Stroke Survivors (IAMSS): A randomised controlled trial: Study protocol. BMC Neurol. 2010;10:15.
Wetzels GE, Nelemans PJ, Schouten JS, van Wijk BL, Prins MH. All that glisters is not gold: A comparison of electronic monitoring versus filled prescriptions-an observational study. BMC Health Serv Res. 2006;6:8.
Horne R. Measuring adherence: The case for self-report. Int J Behav Med. 2004;11(suppl):75.
Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1-24.
Trewby PN, Reddy AV, Trewby CS, Ashton VJ, Brennan G, Inglis J. Are preventive drugs preventive enough? A study of patients’ expectation of benefit from preventive drugs. Clin Med. 2002;2:527-533.
Broadbent E, Petrie KJ, Weinman J. The Brief Illness Perception Questionnaire (BIPQ): Validity and reliability. Int J Behav Med. 2004;11:278.
Enderby PM, Wood VA, Wade DT, Hewer RL. The Frenchay Aphasia Screening Test: A short, simple test for aphasia appropriate for non-specialists. Int Rehabil Med. 1987;8:166-170.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
Sniehotta FF, Scholz U, Schwarzer R. Action plans and coping plans for physical exercise: A longitudinal intervention study in cardiac rehabilitation. Brit J Health Psychol. 2006;11:23-37.
Petrie KJ, Cameron LD, Ellis CJ, Buick D, Weinman J. Changing illness perceptions after myocardial infarction: An early intervention randomized controlled trial. Psychosom Med. 2002;64:580-586.
SIGN: Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention. A national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2008.
Lane P. Handling drop-out in longitudinal clinical trials: A comparison of LOCF and MMRM approaches. Pharm Stat. 2008;7:93-106.
Graham J. Missing data analysis: Making it work in the real world. Ann Rev Psychol. 2009;60:549-576.
Rubin DB. Multiple imputation for non-response in surveys. New York: Wiley; 1987.
Hughes CM. Medication non-adherence in the elderly. How big is the problem? Drugs Aging. 2004;21:793-811.
Jackson C, Lawton RJ, Raynor DK, et al. Promoting adherence to antibiotics: A test of implementation intentions. Patient Educ Couns. 2006;61:212-218.
Alhalaqia F, Nawafleh AH, Clark A, et al. Adherence therapy for medication non-compliant patients with hypertension: A randomised controlled trial. J Hum Hypertens. 2012;26:117-126.
Ellis G, Rodger J, McAlpine C, Langhorne P. The risk of stroke nurse specialist input on risk factor modification: A randomised controlled trial. Age Aging. 2005;34:389-392.
Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: How unintentional is it really? BMC Health Serv Res. 2012;12:98.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behav Res Methods. 2007;39:175-191.
Acknowledgments
We would like to thank the doctors and nurses at the Western General Hospital stroke clinic and ward for their help in patient recruitment and the patients for giving us their time to take part.
Conflict of Interest
The authors have no conflict of interest to disclose.
Sources of Funding
This project was funded by a grant from the Scottish Government, Department of Health, Chief Scientist Office; reference number CZH/4/569.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
O’Carroll, R.E., Chambers, J.A., Dennis, M. et al. Improving Adherence to Medication in Stroke Survivors: A Pilot Randomised Controlled Trial. ann. behav. med. 46, 358–368 (2013). https://doi.org/10.1007/s12160-013-9515-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-013-9515-5