Abstract
Subcutaneous daratumumab (DARZALEX®) co-formulated with recombinant human hyaluronidase (DARZALEX FASPRO®) is approved in several countries, including the USA and those of the EU, for use in combination with bortezomib, cyclophosphamide and dexamethasone for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis. Daratumumab is a CD38-targeting, human IgG1κ monoclonal antibody. In the pivotal phase III ANDROMEDA trial in adults with newly diagnosed systemic AL amyloidosis, the addition of daratumumab to bortezomib, cyclophosphamide and dexamethasone significantly increased the proportion of patients achieving a haematological complete response relative to bortezomib, cyclophosphamide and dexamethasone alone (primary endpoint). Daratumumab combination therapy produced rapid and deep haematological responses which were associated with improved major organ deterioration progression-free survival (PFS). The addition of daratumumab also led to higher cardiac and renal response rates at 6 and 12 months. Daratumumab had an acceptable tolerability profile when used as combination therapy. Therefore, daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone represents an important emerging first-line treatment option for patients with systemic AL amyloidosis.
Plain Language Summary
Systemic AL amyloidosis is a rare protein misfolding disease that causes serious damage to different organs, especially the heart and kidneys. Daratumumab (DARZALEX®) is a human monoclonal antibody that targets CD38, a protein expressed on clonal plasma cells. A subcutaneous formulation of daratumumab, co-formulated with recombinant human hyaluronidase (DARZALEX FASPRO®), is approved for use in adult patients with newly diagnosed AL amyloidosis. When used in combination with bortezomib, cyclophosphamide and dexamethasone, daratumumab was associated with higher rates of haematological complete response and prolongation of major organ deterioration PFS compared with bortezomib, cyclophosphamide and dexamethasone alone. The addition of daratumumab was also associated with near doubling of cardiac and renal response rates at 6 and 12 months. Subcutaneous daratumumab had an acceptable tolerability profile when used as combination therapy, with no new safety concerns. The combination of daratumumab with bortezomib, cyclophosphamide and dexamethasone is an important emerging treatment option for patients with newly diagnosed systemic AL amyloidosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.19365089. |
First-in-class CD38-targeting monoclonal antibody |
Associated with higher rates of haematological complete response and major organ deterioration PFS when combined with bortezomib, cyclophosphamide and dexamethasone |
Acceptable tolerability profile |
1 Introduction
Systemic light chain (AL) amyloidosis is a rare systemic disease characterized by the abnormal production of monoclonal immunoglobulin light chains [1,2,3,4], proteins made by clonal plasma cells [4]. The light chains misfold and aggregate to form insoluble amyloid fibrils that are deposited in tissues, causing progressive and largely irreversible organ damage [1,2,3]. The clinical features of AL amyloidosis are dependent on the organs involved [3, 4], with the heart and kidneys being the most commonly affected [1, 2]. Treatment of AL amyloidosis is generally based on a risk-adapted approach, taking into account clone characteristics, comorbidities and the severity of organ involvement [4].
Evidence suggests that CD138/38 plasma cells are involved in the production of monoclonal light chains [2]. CD38 is a transmembrane glycoprotein that is highly expressed on the surface of various haematopoietic cells, including clonal plasma cells in AL amyloidosis [5, 6]. It is involved in receptor-mediated adhesion, cell signaling and modulation of cyclase and hydrolase activity [5, 6]. Therefore, CD38 represents a promising target for the treatment of AL amyloidosis and other plasma cell disorders [2].
Daratumumab (DARZALEX®) is a first-in-class human IgG1κ monoclonal antibody against CD38. A subcutaneous formulation of daratumumab co-formulated with recombinant human hyaluronidase PH20 (rHuPH20; DARZALEX FASPRO®) has been approved in the USA [5] and the EU [6] for use in combination with bortezomib, cyclophosphamide and dexamethasone in adult patients with newly diagnosed AL amyloidosis. This article reviews the clinical efficacy and tolerability of daratumumab in this setting, with a brief overview of its pharmacological properties. Discussion of the use of daratumumab in other approved indications [i.e. relapsed and/or refractory multiple myeloma (MM) [7, 8], transplant-ineligible [9] and -eligible [10] newly diagnosed MM] is outside the scope of this article.
2 Pharmacological Properties of Daratumumab
The majority of data on the effects of targeting CD38 have been derived from in vivo and in vitro studies on MM [2]. AL amyloidosis and MM are both plasma cell disorders and therefore have certain similarities [2]. Daratumumab binds to the CD38 protein expressed on clonal plasma cells in MM and AL amyloidosis, thereby inhibiting the growth of CD38-expressing tumour cells [5, 6]. Daratumumab induces apoptosis directly via Fc-mediated cross-linking, as well as by immune-mediated tumour cell lysis through complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis [5, 6]. Daratumumab also modulates CD38 enzymatic activity by inhibiting cyclase activity and stimulating hydrolase activity [6]. Natural killer (NK) cells are known to express CD38, and daratumumab was associated with a decrease in total and activated NK cells in peripheral whole blood and bone marrow [5, 6].
The binding of daratumumab to CD38 on red blood cells (RBCs) may interfere with blood compatibility testing, resulting in a positive indirect antiglobulin test (indirect Coombs test) for up to 6 months after the last dose of daratumumab [5, 6]. As such, patients should be typed and screened before starting daratumumab therapy [6]. Strategies to mitigate daratumumab interference include treating reagent RBCs with dithiothreitol [5, 6]. ABO and Rh typing are not affected by daratumumab [6]. If an emergency blood transfusion is needed, non-cross-matched ABO/RhD-compatible RBCs can be given according to local blood bank practices [5, 6].
The pharmacokinetics of subcutaneous daratumumab are best described by a one-compartment model, with first-order absorption and parallel linear and non-linear elimination, according to a population pharmacokinetic analysis [11]. Following subcutaneous administration of daratumumab 1800 mg in patients with AL amyloidosis, the absolute bioavailability of daratumumab was not estimated, the absorption rate constant was 0.77/day and peak serum concentrations were reached in ≈ 3 days [5, 6]. The estimated apparent volume of distribution was 10.8 L [5, 6], indicating that daratumumab is primarily distributed in the vascular system; extravascular tissue distribution is limited [6]. The estimated apparent clearance was 210 mL/day and the estimated mean elimination half-life was 28 days [5, 6].
Age (33–92 years), sex, renal impairment [creatinine clearance (CLCR) 15–89 mL/min] and mild hepatic impairment [total bilirubin 1–1.50 × upper limit of normal (ULN) and AST > ULN] did not have clinically meaningful effects on daratumumab pharmacokinetics; therefore, no dosage adjustments are required [5, 6]. The effect of moderate and severe hepatic impairment on the pharmacokinetics of daratumumab is not known [5, 6]. In patients with AL amyloidosis, daratumumab exposure was higher in African-Americans and Asians than in whites [5]. Relative to patients weighing 51–85 kg, daratumumab plasma concentrations increased in patients with low bodyweight (≤ 50 kg) and decreased in those with high bodyweight (> 85 kg) [5].
3 Therapeutic Efficacy of Daratumumab
The efficacy of subcutaneous daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone in patients with newly diagnosed systemic AL amyloidosis was demonstrated in the randomized, open-label, active-controlled, multicentre, phase III ANDROMEDA trial [12]. Combination bortezomib, cyclophosphamide and dexamethasone was used as the active comparator [12].
ANDROMEDA enrolled patients aged ≥ 18 years with a histopathological diagnosis of systemic AL amyloidosis affecting one or more organs, measurable haematological disease and an ECOG performance status of 0–2 [12]. Patients were also required to have an absolute neutrophil count of ≥ 1.0 × 109/L, a haemoglobin level of ≥ 8.0 g/dL, a platelet count of > 50 × 109/L, ALT and AST levels of ≤ 2.5 × ULN, a total bilirubin level of ≤ 1.5 × ULN (or ≤ 2 × ULN in patients with Gilbert syndrome) and an estimated glomerular filtration rate of ≥ 20 mL/min/1.73 m2. Key exclusion criteria included symptomatic MM, previous therapy for AL amyloidosis, and evidence of a severe cardiovascular condition including an N-terminal pro-B-type natriuretic peptide (NT-proBNP) level of > 8500 ng/L, a systolic BP of < 90 mmHg, or a New York Heart Association (NYHA) classification of IIIB or IV [12].
After being stratified according to cardiac stage (I, II or IIIA on the basis of the European modification of the Mayo Clinic Cardiac Staging System), availability of transplantation in the local country (yes or no) and renal function (CLCR ≥ 60 or < 60 mL/min), 388 patients were randomized to receive daratumumab combination therapy or the active comparator (Fig. 1) [12]. After six cycles, patients in the daratumumab group continued to receive daratumumab monotherapy every 4 weeks for up to 24 cycles (see Table 1 for dosage regimens) and those in the active comparator group completed their treatment. The median duration of treatment was 9.6 and 5.3 months in the daratumumab and active comparator groups. Pre- and post-medications were given with daratumumab to prevent administration-related reactions. The primary endpoint was overall haematological complete response at the time of clinical cutoff in the intention-to-treat population. A haematological complete response was defined as negative serum and urine immunofixation and normalization of free light chain (FLC) levels and FLC ratios. However, if involved FLC (iFLC) was lower than the upper limit of normal, normalization of uninvolved FLC level and FLC ratio was not required to determine a complete haematological response. If the primary endpoint was significant, the major secondary endpoints [i.e. major organ deterioration progression-free survival (PFS) and overall survival (OS)] were tested hierarchically [12].
Trial design of the randomized, open-label, multinational phase III ANDROMEDA trial in adults with newly diagnosed systemic light chain amyloidosis [12]. Refer to Sect. 3 and Table 1 for details regarding treatment regimens. Efficacy results are reported in the animated figure (available online). VCd bortezomib + cyclophosphamide + dexamethasone
Supplementary file1 (MP4 6099 KB)
Baseline demographic and clinical characteristics were generally well balanced between treatment groups [12]. The median time since diagnosis was 43 days and the median patient age was 64 years. Most patients had an ECOG performance status of 0 or 1 (91%) and a cardiac stage of II or higher (77%). Overall, 66% of patients had involvement of two or more organs; 71% of patients had heart involvement and 59% had kidney involvement [12].
At the time of the primary analysis (median follow-up 11.4 months; data cutoff date 14 February 2020), the proportion of patients achieving a haematological complete response was significantly greater with daratumumab combination therapy than with the active comparator (primary endpoint; Table 1) [12]. Daratumumab combination therapy was also superior to the active comparator when haematological complete response was defined according to International Society of Amyloidosis (ISA) criteria (i.e. negative immunofixation and FLC ratio normalization or abnormal FLC ratio, if uninvolved FLC is higher than iFLC; Table 1) [12]. Prespecified subgroup analyses for the primary endpoint favoured daratumumab combination therapy over the active comparator in most subgroups, including sex, age, bodyweight, race, cardiac stage [13], availability of transplantation, CLCR, cardiac involvement, renal stage, alkaline phosphatase level, ECOG performance status, cytogenetic profile [12, 14] and presence of t(11;14) mutations [12]. The benefit of daratumumab combination therapy in the subgroup of Asian patients (Chinese, Japanese or Korean; n = 60) was consistent with that seen in the overall study population [15].
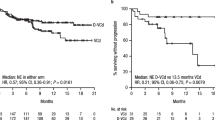
For secondary endpoints, major organ deterioration PFS (Table 1) favoured daratumumab combination therapy over the active comparator [12]. Similar results were seen in supportive analyses, including without censoring for subsequent treatment (HR 0.57; 95% CI 0.37–0.87). At the time of the primary analysis, OS did not differ significantly between the two treatment groups (HR 0.90; 95% CI 0.53–1.53) [12].
The median time to haematological complete response was 60 days with daratumumab combination therapy and 85 days with the active comparator [12]. The haematological complete response rate at 6 months was 50% with daratumumab combination therapy versus 14% with the active comparator (relative risk ratio 3.5, 95% CI 2.4–5.2; odds ratio 6.1, 95% CI 3.7–10.0). Deep haematological responses, including a very good partial response (VGPR) or better, an iFLC level of ≤ 20 mg/L, and a difference between involved and uninvolved FLC (dFLC) level of < 10 mg/L, were numerically higher in daratumumab combination therapy recipients than in active comparator recipients (Table 1) [12]. Further analyses demonstrated that rapid haematological responses (complete response and VGPR at 1 and 3 months) [16] and deep haematological responses (iFLC ≤ 20 mg/L and dFLC < 10 mg/L, regardless of FLC ratio) [17] were associated with improved major organ deterioration PFS.
Daratumumab combination therapy recipients were more likely than those in the active comparator group to have a cardiac or renal response at 6 and 12 months [12]. Cardiac response was defined as NT-proBNP response (> 30% and > 300 ng/L decrease in patients with baseline NT-proBNP ≥ 650 ng/L) or NYHA class response (≥ 2-class decrease in patients with baseline NYHA class III or IV), and renal response was defined as ≥ 30% decrease in proteinuria or drop in proteinuria below 0.5 g/24 h in the absence of renal progression. Among patients who were evaluated for cardiac response (n = 235), the cardiac response rate at 6 months was 42% with daratumumab combination therapy and 22% with the active comparator. Cardiac progression (i.e. NT-proBNP, cardiac troponin or ejection fraction progression) occurred in 3 and 8% of patients, respectively. Among patients who were evaluated for renal response (n = 230), the renal response rate at 6 months was 53% with daratumumab combination therapy and 24% with the active comparator. Renal progression (i.e. ≥ 25% decrease in eGFR) was seen in 4 and 12% of patients, respectively [12].
Daratumumab combination therapy was associated with some benefits over the active comparator in terms of health-related quality of life (HR-QOL), as assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30), the EuroQol 5-dimensions 5-level (EQ-5D-5L) visual analogue scale (VAS) and the 36-Item Short-Form Health Survey (SF-36) mental component summary (MCS) [18]. For EORTC QLQ-C30 global health status (GHS) and fatigue scales and EQ-5D-5L VAS, the median time to improvement was shorter and the median time to worsening was longer with daratumumab combination therapy versus the active comparator. Least squares mean scores for EORTC QLQ-C30 GHS and fatigue, EQ-5D-5L VAS and SF-36 MCS remained stable with daratumumab combination therapy and worsened with the active comparator, with the greatest between-group differences seen at week 16 (cycle 4). Improvements in GHS and fatigue were reported after cycle 6 in the daratumumab combination therapy group [18]. Similar improvements in fatigue-related symptoms (e.g. shortness of breath, feeling weak and tired) were observed with daratumumab combination therapy in the subgroups of patients with cardiac (n = 277) [19] or renal (n = 229) [20] involvement.
3.1 Updated Analysis
Improvements in clinical outcomes with daratumumab combination therapy relative to the active comparator were maintained over the longer term [21]. At the time of the updated analysis (median follow-up of 25.8 months; data cutoff date May 2021), when 11% of patients in the daratumumab group were still receiving treatment, the rates of haematological complete response and VGPR or better were higher in the daratumumab combination therapy group than in the active comparator group (Table 1). Cardiac and renal response rates at 18 months were numerically higher with daratumumab combination therapy than with the active comparator (53 vs 24% and 58 vs 26%) [21].
4 Tolerability and Safety of Daratumumab
Subcutaneous daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone had an acceptable tolerability profile in patients with newly diagnosed systemic AL amyloidosis participating in the ANDROMEDA trial discussed in Sect. 3 [12]. Subcutaneous daratumumab was well tolerated when used as combination therapy during the safety run-in phase of ANDROMEDA (n = 28), and no new safety concerns were identified compared with intravenous or subcutaneous daratumumab monotherapy or the active comparator [22].
In ANDROMEDA, the safety profiles of daratumumab, bortezomib, cyclophosphamide and dexamethasone were consistent with their known profiles and with the underlying disease [12]. In the safety population (n = 381), the exposure-adjusted incidence rates of overall adverse events (AEs) and grade 3 or 4 AEs were lower with daratumumab combination therapy than with the active comparator (154.23 vs 217.92 and 10.55 vs 18.96 per 100 patient-months at risk, respectively). The most common (≥ 20% incidence) AEs of any grade were peripheral oedema, diarrhoea, constipation, peripheral sensory neuropathy, fatigue, nausea and upper respiratory tract infection (Fig. 2). The most common (≥ 10% incidence) grade 3 or 4 AEs were infections (17% with daratumumab combination therapy vs 10% with the active comparator) and lymphopenia (13 vs 10%). Serious AEs occurred in 43% of daratumumab combination therapy recipients and 36% of active comparator recipients, with the most common of these being pneumonia (7 vs 5%). AEs led to treatment discontinuation in 4% of patients in each treatment group. Fatal AEs (in the absence of disease progression) occurred in 12% of patients receiving daratumumab combination therapy and 7% of those receiving the active comparator [12].
The most common adverse events of any grade (≥ 20% incidence in either treatment group) in ANDROMEDA [12]. DARA daratumumab, PSN peripheral sensory neuropathy, URTI upper respiratory tract infection, VCd bortezomib + cyclophosphamide + dexamethasone
4.1 Adverse Events of Special Interest
Serious or fatal cardiac AEs have been reported in patients with newly diagnosed AL amyloidosis receiving daratumumab combination therapy [5, 6]. In ANDROMEDA, serious cardiac disorders (including cardiac failure, cardiac arrest and atrial fibrillation [6]) occurred in 16% of patients receiving daratumumab combination therapy and 13% of those receiving the active comparator; corresponding rates of grade 3 or 4 cardiac disorders were 11 and 10%, respectively [5, 6]. Patients with NYHA class IIIA or Mayo stage IIIA disease may be at greater risk for cardiac toxicity [5]. In the USA, daratumumab is not indicated and is not recommended for the treatment of patients with AL amyloidosis who have NYHA class IIIB or IV cardiac disease or Mayo stage IIIB outside of controlled clinical trials. Patients with cardiac involvement of AL amyloidosis should be frequently monitored for cardiac toxicity, and appropriate supportive care should be administered [5].
Daratumumab may increase neutropenia and thrombocytopenia induced by background therapy [5, 6]. In ANDROMEDA, daratumumab combination therapy was associated with numerically higher rates of neutropenia (11 vs 6%) and thrombocytopenia (17 vs 12%) than the active comparator [12]. Complete blood counts should be monitored throughout the treatment period, and patients should be observed for signs of infection [5, 6]. Daratumumab dose delays may be required to allow recovery of neutrophils and platelets [5, 6].
Severe and/or serious infusion-related reactions (IRRs) and local injection-site reactions (ISRs) can occur with daratumumab [5, 6]. In ANDROMEDA, 7% of patients had systemic administration-related reactions to daratumumab [12]. Most (86%) of these reactions occurred during the first administration, and the median time to onset was 1.3 h (range 0.2–7.3 h). Local ISRs to daratumumab occurred in 11% of patients. All systemic and local reactions were grade 1 or 2 in severity [12]. To reduce the risk of reactions, patients should receive pre-medication (e.g. antihistamines, antipyretics and corticosteroids) and post-medication (e.g. corticosteroids) [5, 6].
Like all therapeutic proteins, daratumumab has the potential for immunogenicity [5]. However, < 1% of patients receiving daratumumab in clinical trials developed treatment-emergent anti-daratumumab antibodies [5, 6]. Although 7% of patients developed treatment-emergent anti-rHuPH20 antibodies, these did not appear to affect daratumumab exposure [5, 6].
5 Dosage and Administration of Daratumumab
In the USA [5] and the EU [6], daratumumab (in combination with bortezomib, cyclophosphamide and dexamethasone; 4-week cycle regimens) is indicated for the treatment of adult patients with newly diagnosed AL amyloidosis. The US approval of daratumumab in this indication was accelerated based on response rates, and continued approval may be contingent upon verification and description of clinical benefit in further confirmatory trials [5]. The recommended dosage is 1800 mg daratumumab and 30,000 units hyaluronidase (HuPH20) administered as a subcutaneous injection into the abdomen (over ≈ 3–5 min) weekly from weeks 1 to 8 (total of 8 doses), every 2 weeks from weeks 9 to 24 (total of 8 doses) and every 4 weeks from week 25 onwards until disease progression (or for a maximum of 2 years [5]) [5, 6]. Local prescribing information should be consulted for detailed information regarding preparation, storage and administration procedures, pre- and post-medication recommendations, warnings and precautions, drug interactions, and use in specific populations.
6 Place of Daratumumab in the Management of Newly Diagnosed Systemic Light Chain Amyloidosis
The goals of treatment in systemic AL amyloidosis are to eliminate the misfolded immunoglobulin light chains, to support the function of damaged organs, and to minimize toxicity [23]. Conventional systemic treatment options have included alkylator-based chemotherapy, including the combination of cyclophosphamide, bortezomib and dexamethasone [3]. In the most recent NCCN [23] and mSMART [24] guidelines, daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone is recommended as the preferred (category 1 [23]) regimen for the treatment of newly diagnosed AL amyloidosis.
Approval of daratumumab combination therapy in patients with newly diagnosed AL amyloidosis was based on data from the pivotal phase III ANDROMEDA trial, in which the addition of daratumumab to bortezomib, cyclophosphamide and dexamethasone significantly improved the proportion of patients achieving a haematological complete response (Sect. 3). Results of an updated analysis were generally consistent with that of the primary analysis (Sect. 3.1). It should be noted that the primary endpoint of haematological complete response used in ANDROMEDA was a study-specific endpoint. However, the superiority of daratumumab combination therapy was also evident for haematological complete response defined according to ISA criteria (Sect. 3). This is important, given that the ISA response criteria are validated and are widely used as surrogate endpoints in other studies of AL amyloidosis [25].
Organ dysfunction and damage are serious complications of systemic AL amyloidosis [4]. Daratumumab combination therapy produced rapid and deep haematological responses which were associated with improved major organ deterioration PFS (Sect. 3). When this endpoint was analysed without censoring for subsequent maintenance therapy, daratumumab combination therapy was still associated with prolonged major organ deterioration PFS (Sect. 3). The addition of daratumumab was associated with near doubling of 6-month cardiac and renal response rates (Sect. 3), a crucial finding considering that organ response rates are an important predictor of improved survival [12].
Achievement of both haematological and organ responses is expected to translate into improved OS in patients with systemic AL amyloidosis [3]. In ANDROMEDA, there were no differences in OS between daratumumab combination therapy and the active comparator after a median of 11.4 months (Sect. 3). Longer follow-up is needed to determine the effect of long-term daratumumab therapy on OS [12] and its potential role in patients with more severe cardiac or renal impairment. When comparing long-term results, it is important to consider that in ANDROMEDA, daratumumab could be continued for up to 2 years in the combination therapy group. Additional data are awaited with interest and will be important in further elucidating the place of daratumumab in patients with systemic AL amyloidosis.
Daratumumab had an acceptable tolerability profile when used in combination with bortezomib, cyclophosphamide and dexamethasone (Sect. 4). The overall safety profile of daratumumab combination therapy was consistent with the known safety profiles of the individual agents and with the underlying disease. Daratumumab combination therapy was well tolerated during the safety run-in phase of ANDROMEDA (Sect. 4), providing support for its use in the subsequent randomized portion of the trial [22]. When adjusted for treatment exposure, daratumumab combination therapy was associated with a lower incidence of AEs than with the active comparator (Sect. 4). Although IRRs and ISRs to daratumumab were observed, these were of mild or moderate severity, typically occurred during the first infusion, and may be mitigated with the use of pre- and post-medication (Sect. 4.1).
In conclusion, daratumumab is an effective addition to bortezomib, cyclophosphamide and dexamethasone in patients with newly diagnosed systemic AL amyloidosis, with an acceptable tolerability profile. Therefore, daratumumab combination therapy represents an important emerging first-line treatment option in this patient population.
Data Selection Daratumumab: 156 records identified
Duplicates removed | 43 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 55 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 33 |
Cited efficacy/tolerability articles | 13 |
Cited articles not efficacy/tolerability | 12 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Daratumumab, Darzalex, light-chain amyloidosis, AL amyloidosis. Records were limited to those in English language. Searches last updated 14 March 2022 | |
References
Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38.
Roccatello D, Fenoglio R, Sciascia S, et al. CD38 and anti-CD38 monoclonal antibodies in AL amyloidosis: targeting plasma cells and beyond. Int J Mol Sci. 2020;21(11):4129.
Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95(7):848–60.
Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136(23):2620–7.
Janssen Biotech. DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) injection, for subcutaneous use: US prescribing information. 2021. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX+Faspro-pi.pdf. Accessed 15 Mar 2022
European Medicines Agency. DARZALEX 800mg solution for injection: EU summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/darzalex-epar-product-information_en.pdf. Accessed 15 Mar 2022
Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013–24.
McKeage K. Daratumumab: first global approval. Drugs. 2016;76(2):275–81.
Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–54.
Lamb YN. Daratumumab: a review in combination therapy for transplant-eligible newly diagnosed multiple myeloma. Drugs. 2020;80(14):1455–64.
Luo MM, Zhu PP, Nnane I, et al. Population pharmacokinetics and exposure-response modeling of daratumumab subcutaneous administration in patients with light-chain amyloidosis. J Clin Pharmacol. 2021. https://doi.org/10.1002/jcph.1994.
Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58.
Minnema MC, Dispenzieri A, Merlini G, et al. Outcomes by cardiac stage in newly diagnosed AL amyloidosis: results from Andromeda [abstract]. Blood. 2020;136(Suppl. 1):44–5.
Kumar S, Dispenzieri A, Bhutani D, et al. Evaluating the impact of cytogenetic abnormalities on treatment outcomes in patients with AL amyloidosis: subanalyses from the ANDROMEDA study [abstract no. OAB-034]. In: 18th International Myeloma Workshop. 2021
Suzuki K, Wechalekar AD, Kim K, et al. Subcutaneous daratumumab (DARA SC) + bortezomib, cyclophosphamide, and dexamethasone (VCd) in Asian patients with newly diagnosed light chain (AL) amyloidosis: subgroup analysis from the phase 3 Andromeda study [abstract]. Blood. 2020;136(Suppl. 1):11.
Wechalekar AD, Palladini G, Merlini G, et al. Rapid and deep hematologic responses are associated with improved major organ deterioration progression-free survival in newly diagnosed AL amyloidosis: results from Andromeda [abstract]. Blood. 2020;136(Suppl. 1):6–7.
Comenzo RL, Kastritis E, Palladini G, et al. Reduction in absolute involved free light chain and difference between involved and uninvolved free light chain is associated with prolonged major organ deterioration progression-free survival in patients with newly diagnosed AL amyloidosis receiving bortezomib, cyclophosphamide, and dexamethasone with or without daratumumab: results from ANDROMEDA [abstract]. Blood. 2020;136(Suppl. 1):48–50.
Sanchorawala V, Palladini G, Minnema MC, et al. Health-related quality of life in patients with AL amyloidosis treated with daratumumab, bortezomib, cyclophosphamide, and dexamethasone: results from the phase 3 Andromeda study [abstract]. Blood. 2020;136(Suppl. 1):37–40.
Grogan M, Maurer MS, Witteles R, et al. Effect of daratumumab, bortezomib, cyclophosphamide, and dexamethasone on cardiac function and health-related quality of life in patients with newly-diagnosed AL amyloidosis with cardiac involvement: results from the phase 3 ANDROMEDA study [abstract]. J Am Coll Cardiol. 2021;77(18 Suppl. 1):3304.
Havasi A, Lachmann HJ, Leung N, et al. Effect of daratumumab/bortezomib/cyclophosphamide/dexamethasone on renal function and hrqol in patients with newly-diagnosed AL amyloidosis with renal involvement: results from the phase 3 ANDROMEDA study [abstract no. POS-799]. Kidney Int Rep. 2021;6(4 Suppl.):S347.
Comenzo R, Palladini G, Kastritis E, et al. Subcutaneous daratumumab with bortezomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed light chain (AL) amyloidosis: 18-month analysis of the phase 3 ANDROMEDA study [abstract no. 653]. Blood. 2021;138(Suppl. 1):159.
Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1):71–80.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NNCN Guidelines®): systemic light chain amyloidosis (version 1.2022). 2022. http://www.nccn.org. Accessed 15 Mar 2022
Mayo Clinic. mSMART Mayo Consensus on AL amyloidosis: diagnosis, treatment and prognosis. 2020. https://www.msmart.org. Accessed 15 Mar 2022
Palladini G, Schonland SO, Sanchorawala V, et al. Clarification on the definition of complete haematologic response in light-chain (AL) amyloidosis. Amyloid. 2021;28(1):1–2.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: M. Beksac, Department of Hematology, Ankara University, Ankara, Turkey; M. A. Dimopoulos, Department of Clinical Therapeutics, National & Kapodistrian University of Athens, Athens, Greece; G. Palladini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo and Department of Molecular Medicine, University of Pavia, Pavia, Italy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blair, H.A. Daratumumab: A Review in Newly Diagnosed Systemic Light Chain Amyloidosis. Drugs 82, 683–690 (2022). https://doi.org/10.1007/s40265-022-01705-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01705-3