Abstract
Intravenous daratumumab (DARZALEX®) is a first-in-class human IgG1κ monoclonal antibody against CD38 available for use in patients with relapsed and/or refractory multiple myeloma. In phase I/II and II trials and a pooled analysis of these studies, daratumumab monotherapy induced an overall response (partial response or better) in approximately one-third of patients; responses were rapid, deep and durable. An overall survival (OS) benefit was seen with daratumumab monotherapy, including in patients with a minimal response or stable disease. In phase III trials, daratumumab in combination with either bortezomib plus dexamethasone or lenalidomide plus dexamethasone significantly prolonged progression-free survival and induced deep and durable responses compared with bortezomib plus dexamethasone or lenalidomide plus dexamethasone. An OS benefit with daratumumab triple combination therapy is yet to be demonstrated (as the OS data were not mature at the time of the last analysis). Daratumumab was generally well tolerated when used as monotherapy and had a generally manageable tolerability profile when used in combination therapy. Infusion-related reactions (IRRs) were the most common adverse events; these were predominantly grade 1 or 2 and mostly occurred during the first infusion. The most common grade 3–4 adverse events associated with daratumumab triple combination therapy were thrombocytopenia, neutropenia and anaemia. Although final OS data are awaited, current evidence indicates that daratumumab is a valuable addition to the treatment options currently available for patients with relapsed or refractory multiple myeloma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First-in-class CD38 monoclonal antibody |
As monotherapy, induces an overall response (partial response or better) in approximately one-third of patients; responses are rapid, deep and durable |
In combination with bortezomib plus dexamethasone or lenalidomide plus dexamethasone, significantly prolongs progression-free survival compared with bortezomib plus dexamethasone or lenalidomide plus dexamethasone |
Manageable tolerability profile, with IRRs and haematological adverse events occurring most commonly |
1 Introduction
Multiple myeloma is a neoplastic malignancy characterized by the proliferation of plasma cells in the bone marrow [1]. Although the overall incidence of multiple myeloma has increased over the past decade, mortality rates associated with the disease have declined due to the availability of novel and more effective treatment options [1]. The introduction of proteasome inhibitors (PIs; e.g. bortezomib) and immunomodulatory drugs (IMiDs; e.g. lenalidomide) has contributed to extended survival for patients with multiple myeloma; however, the disease remains incurable [2]. Patients with disease refractory to both PIs and IMiDs have a particularly poor prognosis, with a median overall survival of ≈ 8–9 months [2, 3]. Given the success of targeted immunotherapy with monoclonal antibodies in other cancers, recent research has focused on the development of this class of drugs for multiple myeloma [4].
Daratumumab (DARZALEX®) is a first-in class human IgG1κ monoclonal antibody against the transmembrane glycoprotein CD38 that is approved for the treatment of multiple myeloma in several countries worldwide, including the USA [5], Japan [6] and those of the EU [7]. This article reviews pharmacological, clinical efficacy and tolerability data relevant to the use of intravenous daratumumab in this setting.
2 Pharmacodynamic Properties of Daratumumab
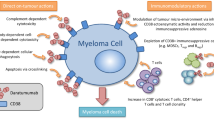
CD38 is highly expressed on the surface of multiple myeloma cells and is involved in receptor-mediated adhesion, cell signalling and enzymatic activity [8]. Daratumumab binds to CD38, thereby inhibiting the growth of CD38-expressing tumour cells [5, 7]. As well as directly inducing apoptosis via Fcγ receptor-mediated cross-linking, daratumumab also induces tumour cell lysis through complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-mediated cellular phagocytosis [8,9,10]. Daratumumab also works through immunomodulatory mechanisms, including modulation of the tumour microenvironment, depletion of immunosuppressive cells, enhancement of T-cell responses and increased T-cell clonality [11]. The direct on-tumour actions of daratumumab may contribute to the rapid responses observed in some patients following treatment, while the immunomodulatory actions may explain the durable or sustained responses seen with daratumumab [9, 11].
In preclinical studies, the cytotoxicity of daratumumab in CD38+ tumour cells was synergistically increased when used in combination with other multiple myeloma therapies, particularly lenalidomide [12, 13]. Daratumumab-dependent ADCC against multiple myeloma cells was significantly (p < 0.001) augmented by pretreating peripheral blood mononuclear cells (from healthy subjects) with lenalidomide [13]. Similarly, the combination of daratumumab and lenalidomide significantly increased multiple myeloma cell lysis compared with daratumumab (p < 0.05) or lenalidomide (p < 0.001) alone. A mixed model analysis demonstrated that the observed effect (% lysis) of daratumumab plus lenalidomide was significantly (p = 0.01) higher than the predicted additive effect [13]. Daratumumab also induced lysis in multiple myeloma cell lines resistant to lenalidomide and/or bortezomib; the combination of daratumumab plus lenalidomide (but not bortezomib) was synergistic, with a significant (p < 0.01) difference between observed and predicted results [12].
CD38 expression on multiple myeloma cells was associated with clinical response to daratumumab monotherapy in patients with multiple myeloma, while an increased expression of complement-inhibitory proteins accompanied resistance to daratumumab [14]. In a cohort of patients receiving daratumumab 16 mg/kg (n = 102), pretreatment levels of CD38 expression on multiple myeloma cells were significantly (p = 0.005) higher in patients with partial response or better than in patients with less than partial response. Conversely, expression levels of the complement-inhibitory proteins CD46, CD55 and CD59 were not associated with clinical response to daratumumab. CD38 expression was significantly (p < 0.001) reduced on both bone marrow-localized and circulating multiple myeloma cells following the first daratumumab infusion, and gradually increased ≈ 6 months after daratumumab treatment. CD55 and CD59 expression levels were significantly (p < 0.05) increased on bone marrow-localized and circulating multiple myeloma cells only at the time of progression [14].
The binding of daratumumab to CD38 on red blood cells (RBCs) interferes with blood compatibility testing, thereby complicating the safe provision of blood products to daratumumab-treated patients [15]. This may result in a positive indirect antiglobulin test (indirect Coombs test) for up to 6 months after the last daratumumab infusion [5, 7]. Patients may also present with a positive direct Coombs test [15]. Daratumumab can be detected on serum protein electrophoresis and immunofixation assays used for the clinical monitoring of endogenous myeloma protein [5, 7]. False positive results may affect the assessment of complete response and disease progression in some patients with IgGκ myeloma protein. To mitigate daratumumab interference, alternative methods for evaluating depth of response should be considered in patients with persistent very good partial response [5, 7].
3 Pharmacokinetic Properties of Daratumumab
Daratumumab demonstrated non-linear pharmacokinetics following intravenous administration in patients with relapsed or refractory multiple myeloma [16]. Administration of daratumumab 16 mg/kg weekly for 8 weeks then every 2 weeks for 16 weeks and every 4 weeks thereafter rapidly saturated target-mediated clearance during weekly administration, with saturation maintained during the every 2- and 4-week dosing periods [16].
Across a dose range of 1–24 mg/kg (monotherapy) or 1–16 mg/kg (combination therapy), increases in the area under the concentration-time curve of daratumumab are more than dose-proportional [5, 7]. Following weekly administration of daratumumab 16 mg/kg for 8 weeks as monotherapy or combination therapy, the mean serum maximal concentration (Cmax) is ≈ 2.7- to 3-fold higher than that seen after the first dose. Steady-state concentrations of daratumumab are attained after ≈ 5 months of 4-weekly dosing (by the 21st infusion) and the mean ratio of Cmax at steady state to Cmax after the first dose is 1.6 [5, 7].
The estimated mean central volume of distribution of daratumumab is 4.7 L when administered as monotherapy and 4.4 L when administered as combination therapy [5]. At a dose of 16 mg/kg, the estimated mean linear clearance of daratumumab as monotherapy is 171.4 mL/day [5]. The estimated mean terminal half-life based on linear clearance of daratumumab is ≈ 18 days when administered as monotherapy and ≈ 23 days when administered as combination therapy [5, 7]. Clearance decreases and the terminal half-life increases with increasing dose and with repeated dosing. Increasing bodyweight increases the central volume of distribution and clearance of daratumumab; thus, the drug is administered on a mg/kg basis (Sect. 6) [5, 7]. Hepatic enzyme-mediated metabolism and renal excretion are unlikely to be the primary elimination pathways for intact daratumumab [7].
The pharmacokinetics of daratumumab are not affected to a clinically relevant extent by age, gender, race, renal impairment [creatinine clearance (CLCR) 15–89 mL/min], mild hepatic impairment [total bilirubin 1–1.5 × upper limit of normal (ULN)] or moderate hepatic impairment (total bilirubin 1.5–3 × ULN) [5, 7]. No dosage adjustments are considered necessary in elderly patients or in patients with renal or hepatic impairment [7].
Daratumumab is not expected to have metabolic drug–drug interactions, given that it is unlikely to inhibit/induce enzymes involved in drug metabolism [7]. While formal drug interaction studies have not been conducted, no clinically relevant drug–drug interactions have been observed between daratumumab and pomalidomide or thalidomide [7]. The pharmacokinetics of daratumumab are not affected by the coadministration of lenalidomide or bortezomib [5]. Concomitant administration of daratumumab plus bortezomib does not alter the pharmacokinetics of bortezomib [5].
4 Therapeutic Efficacy of Daratumumab
4.1 Monotherapy
The efficacy of intravenous daratumumab 16 mg/kg monotherapy in adults with relapsed or refractory multiple myeloma was demonstrated in two open-label, multicentre, two-part trials (GEN501 [17] and SIRIUS [18]) and a pooled analysis of data from both trials [19]. Patients in the phase I/II GEN501 trial had relapsed after or were refractory to ≥ 2 prior lines of therapy, including PIs, IMiDs, chemotherapy and autologous stem cell transplantation (ASCT) [17]. Patients in the phase II SIRIUS trial had been previously treated with ≥ 3 lines of therapy, including a PI and an IMiD, or were double refractory to the most recently received PI and IMiD [18]. All patients were aged ≥ 18 years and had an Eastern Cooperative Oncology Group performance status of ≤ 2 [17, 18]. GEN501 was primarily designed to evaluate safety [17], while the primary endpoint in the SIRIUS trial was overall response rate (ORR) [18]. The data cut-off date was 9 January 2015 for GEN501 and SIRIUS and 31 December 2015 for the pooled analysis [19].
GEN501 was a two-part study consisting of a dose-escalation period (n = 32) and a dose-expansion period (n = 72) [17]. In part 1, patients in 10 cohorts received daratumumab at doses of 0.005 to 24 mg/kg. In part 2, patients received daratumumab 8 or 16 mg/kg for 8 weeks (with a 3-week washout period after the initial 16 mg/kg infusion), then every 2 weeks for 14 or 16 weeks and every 4 weeks for up to 24 months. Treatment continued until disease progression or unacceptable toxicity [17]. SIRIUS was a two-part study, with part 1 consisting of two stages [18]. In part 1 stage 1 of SIRIUS, 34 patients were randomized to receive daratumumab 8 mg/kg every 4 weeks, or 16 mg/kg weekly for 8 weeks, then every 2 weeks for 16 weeks and every 4 weeks thereafter. Treatment with daratumumab 8 mg/kg was discontinued after the first interim analysis. In part 1 stage 2 (n = 25) and part 2 (n = 65), all patients received daratumumab 16 mg/kg according to the schedule used in part 1 stage 1 [18]. Discussion in this section focuses on the approved daratumumab dosage of 16 mg/kg.
At baseline, patients enrolled in the daratumumab 16 mg/kg arms of GEN501 (n = 42) and SIRIUS (n = 106) had received a median of four [17] and five [18] lines of previous therapy. The proportion of patients who were refractory to both PIs and IMiDs was 64% in GEN501 and 95% in SIRIUS. Previous ASCT had been performed in 76 and 80% of patients. The median time since initial diagnosis was 5.7 years in GEN501 and 4.8 years in SIRIUS. The median age of patients was 64 years [17, 18].
Monotherapy with daratumumab 16 mg/kg was effective in patients with relapsed or refractory multiple myeloma, with approximately one-third of patients achieving an ORR (partial response or better) (Fig. 1) [17,18,19]. Most responses were partial responses or very good partial responses (Fig. 1). The proportion of patients achieving clinical benefit (defined as ORR plus minimal response) was 45% in GEN501, 34% in SIRIUS and 37% in the pooled analysis [17,18,19]. The median time to first response was 0.9 months in GEN501 [17] and 1.0 month in SIRIUS [18]. In the pooled analysis, the median duration of response was 7.6 months [19]. Of note, responses were seen in all prespecified patient subgroups, regardless of refractory status or the number of prior lines of therapy [17, 18]. In 14 patients, a deepening response was observed with continued daratumumab treatment [19]. For example, among 10 patients who achieved an initial partial response, seven went on to achieve a very good partial response, one achieved a complete response and two achieved a stringent complete response [19]. The proportion of patients achieving stable disease was 52% in GEN501, 43% in SIRIUS and 46% in the pooled analysis [17,18,19].
After a median follow-up of 20.7 months in the pooled analysis, daratumumab was associated with a median progression-free survival (PFS) of 4.0 months and a 12-month PFS rate of 22% [19]. According to a prespecified exploratory analysis, median PFS was 15.0 months in patients who achieved a partial response or better compared with 3.0 months in patients with minimal response or stable disease and 0.9 months in patients with progressive disease or who were not evaluable. Median overall survival (OS) was 20.1 months and the 24-month OS rate was 45%. Median OS was 18.5 months in patients who achieved a minimal response or stable disease compared with 3.7 months in patients with progressive disease or who were not evaluable [19].
Results from a small observational study in Poland support the efficacy of daratumumab monotherapy in a real-world setting (available as an abstract) [20]. Patients who had received ≥ 2 cycles of daratumumab were included in the preliminary efficacy analysis (n = 22 evaluable). At the time of this analysis (median follow-up 5.1 months), the ORR was 32%; median PFS and OS had not been reached [20].
4.2 Combination Therapy
Key data supporting the therapeutic efficacy of daratumumab combination therapy in patients with relapsed and/or refractory multiple myeloma are available from open-label, multicentre phase I/II [21] and III (CASTOR [22] and POLLUX [23]) trials. In the phase I/II trial, combination therapy with daratumumab, lenalidomide and dexamethasone demonstrated 18-month PFS and OS rates of 72 and 90%, with an ORR of 81% [21]. Because of the availability of data from the phase III trials, data from the phase I/II trial are not discussed further.
All patients in CASTOR and POLLUX were required to have measurable disease and documented disease progression according to International Myeloma Working Group (IMWG) criteria during or after completion of their last regimen [22, 23]. They had received ≥ 1 prior line of therapy and had achieved a response to ≥ 1 of their previous treatments. Patients with bortezomib-refractory disease were excluded from CASTOR, while POLLUX excluded patients with disease refractory to lenalidomide [22, 23].
The primary endpoint of both trials was PFS [22, 23]. If results for the primary endpoint were significant at the interim analysis, the major secondary endpoints were tested sequentially in the following order: time to disease progression, very good partial response rate, rate of results below the threshold for minimal residual disease (MRD; POLLUX only), ORR and OS. Response rates were assessed in patients with measurable disease at baseline or screening who received ≥ 1 dose of study medication and had ≥ 1 post-baseline disease assessment; all other efficacy analyses were performed in the intent-to-treat populations [22, 23].
4.2.1 With Bortezomib Plus Dexamethasone
In CASTOR, the assignment of patients to randomized treatment arms [daratumumab plus bortezomib and dexamethasone (n = 251) or bortezomib plus dexamethasone (n = 247)] was stratified by International Staging System (ISS) disease stage at screening (I, II or III), the number of lines of previous therapy (1, 2 or 3, or > 3) and previous treatment with bortezomib (no or yes) [22]. Intravenous daratumumab 16 mg/kg was administered (via infusion) weekly during cycles 1–3, then every 3 weeks during cycles 4–8 and every 4 weeks thereafter until disease progression, unacceptable toxicity or withdrawal of consent, with each cycle 21 days in length. During cycles 1–8, bortezomib 1.3 mg/m2 was administered subcutaneously on days 1, 4, 8 and 11, and dexamethasone 20 mg was administered orally or intravenously on days 1, 2, 4, 5, 8, 9, 11 and 12 (i.e. 160 mg per cycle); the dose of dexamethasone could be reduced to 20 mg once weekly in patients aged > 75 years, those with a BMI of < 18.5 kg/m2 or those with a history of unacceptable adverse events (AEs) to glucocorticoid therapy. At baseline, the proportion of patients with stage I, II or III disease was 39, 39 and 22%, respectively. The median age of patients was 64 years and the median time since initial diagnosis was 3.8 years. Patients had received a median of two previous lines of therapy, and 61% of patients had undergone ASCT [22].
At the time of the prespecified interim analysis (data cut-off date of 11 January 2016; median follow-up 7.4 months), the addition of daratumumab to bortezomib and dexamethasone significantly prolonged median PFS relative to bortezomib plus dexamethasone, reducing the risk of disease progression or death by 61% (Table 1) [22]. Because the prespecified statistical boundary for the primary endpoint was crossed, the trial was unblinded early and daratumumab monotherapy was offered to patients in the bortezomib plus dexamethasone group who had disease progression. In the time-to-event analysis of disease progression, 65% of patients in the daratumumab plus bortezomib and dexamethasone group were free from disease progression after 12 months compared with 29% of patients in the bortezomib plus dexamethasone group [hazard ratio (HR) 0.30; 95% CI 0.21–0.43; p < 0.001] [22]. In subgroup analyses, some of which are available as abstracts [24,25,26,27,28] plus a poster [27], daratumumab plus bortezomib and dexamethasone significantly prolonged PFS relative to bortezomib plus dexamethasone regardless of age, sex, ISS disease stage, previous ASCT, refractoriness to prior therapy with an IMiD or the last line of previous therapy, type of multiple myeloma, baseline creatinine clearance, time since last therapy, prior exposure to bortezomib, number of prior lines of therapy and cytogenetic risk status [22, 24,25,26,27,28]. Estimated 12-month PFS rates are reported in Table 1.
Median OS was not reached in either group over 7.4 months’ follow-up (Table 1) [22]. Daratumumab plus bortezomib and dexamethasone was significantly (p < 0.001) more effective than bortezomib plus dexamethasone with regard to rates of overall response (Table 1), complete response or better (19 vs. 9%) and very good partial response or better (59 vs. 29%). The median time to first response was 0.9 months in the daratumumab plus bortezomib and dexamethasone group and 1.6 months in the bortezomib plus dexamethasone group. The median duration of response was not reached in the daratumumab plus bortezomib and dexamethasone group, compared with 7.9 months in the bortezomib plus dexamethasone group. Rates of stable disease and progressive disease are presented in Table 1 [22].
At all evaluated sensitivity thresholds (1 tumour cell per 104, 105 or 106 white cells), rates of MRD negativity were significantly (p < 0.01) higher with daratumumab plus bortezomib and dexamethasone than with bortezomib plus dexamethasone (available as an abstract) [29]. For example, at a sensitivity threshold of 1/105 cells, 10% of patients in the daratumumab plus bortezomib and dexamethasone group were MRD-negative compared with 2% of patients in the bortezomib plus dexamethasone group. MRD negativity was reached earlier for patients receiving daratumumab plus bortezomib and dexamethasone versus bortezomib plus dexamethasone. Across all thresholds, MRD-negative patients demonstrated prolonged PFS compared with MRD-positive patients [29].
4.2.1.1 Updated Analysis
Longer term, daratumumab combination therapy continued to provide clinical benefit (available as an abstract plus poster) [30]. After a median follow-up of 19.4 months, median PFS was still significantly (p < 0.0001) prolonged with daratumumab plus bortezomib and dexamethasone compared with bortezomib plus dexamethasone (16.7 vs. 7.1 months; HR 0.31; 95% CI 0.24–0.39). Median OS was not reached in either treatment group; a prespecified interim analysis for OS is planned after 160 OS events. A significantly (p < 0.0001) higher ORR was observed with daratumumab plus bortezomib and dexamethasone versus bortezomib plus dexamethasone (84 vs. 63%); similar results (p < 0.0001) were seen with regard to very good partial response or better (62 vs. 29%) and complete response or better (29 vs. 10%) [30].
4.2.2 With Lenalidomide Plus Dexamethasone
Patients in POLLUX received daratumumab plus lenalidomide and dexamethasone (n = 286) or lenalidomide plus dexamethasone (n = 283); randomization was stratified by the number of lines of previous therapy (1, 2 or 3, or > 3), ISS disease stage (I, II or III) and previous treatment with lenalidomide (no or yes) [23]. Intravenous daratumumab 16 mg/kg was administered (via infusion) weekly for 8 weeks during cycles 1 and 2, then every 2 weeks for 16 weeks during cycles 3–6 and every 4 weeks thereafter, with each cycle 28 days in length. Lenalidomide 25 mg (CLCR > 60 mL/min) or 10 mg (CLCR 30–60 mL/min) was administered orally on days 1–21 of each cycle, with weekly dexamethasone 40 mg (20 mg in patients aged > 75 years or with a BMI of < 18.5 kg/m2). Treatment was continued until disease progression, unacceptable toxicity, withdrawal of consent or death. The median age at baseline was 65 years and the median time since initial diagnosis was 3.6 years. Patients had received a median of one line of prior therapy, and 63% of patients had undergone ASCT [23].
In the preplanned interim analysis of this study (data cut-off date of 7 March 2016; median follow-up 13.5 months), daratumumab plus lenalidomide and dexamethasone recipients had significantly longer median PFS than lenalidomide plus dexamethasone recipients, with a 63% lower risk of disease progression or death (Table 1) [23]. In the time-to-event analysis of disease progression, the proportion of patients who were free from disease progression after 12 months was 86% in the daratumumab plus lenalidomide and dexamethasone group versus 63% in the lenalidomide plus dexamethasone group (HR 0.34; 95% CI 0.23–0.48; p < 0.001) [23]. In subgroup analyses, some of which are available as abstracts [27, 28, 31,32,33] plus a poster [27], daratumumab plus lenalidomide and dexamethasone significantly prolonged median PFS relative to lenalidomide plus dexamethasone regardless of age, ISS disease stage, time since last therapy, the number of previous lines of therapy, prior exposure to lenalidomide or PI, refractoriness to PI or the last line of previous therapy, type of multiple myeloma and cytogenetic risk status [23, 27, 28, 31,32,33]. Estimated 12-month PFS rates are reported in Table 1.
Median OS was not reached in the daratumumab plus lenalidomide and dexamethasone group (Table 1) [23]. Estimated 12-month OS rates are reported in Table 1. The ORR was significantly higher with daratumumab plus lenalidomide and dexamethasone than with lenalidomide plus dexamethasone (Table 1). Similar results were seen with regard to rates of complete response or better (43 vs. 19%; p < 0.001) and very good partial response or better (76 vs. 42%; p < 0.001). The median duration of response was not reached in the daratumumab plus lenalidomide and dexamethasone group compared with 17.4 months in the lenalidomide plus dexamethasone group. Rates of stable disease and progressive disease are presented in Table 1 [23].
Rates of MRD negativity were significantly (p < 0.001) higher with daratumumab plus lenalidomide and dexamethasone than with lenalidomide plus dexamethasone at all thresholds [23]. For example, at the IMWG-recommended threshold of 1/105 cells, 22% of patients in the daratumumab plus lenalidomide and dexamethasone group had results below the threshold for MRD compared with 5% of patients in the lenalidomide plus dexamethasone group (odds ratio 5.99; 95% CI 3.21–11.15) [23]. Patients receiving daratumumab plus lenalidomide and dexamethasone reached MRD negativity earlier than those receiving lenalidomide plus dexamethasone (available as an abstract) [34]. MRD-negative patients had longer PFS than MRD-positive patients across all sensitivity thresholds [34].
4.2.2.1 Updated Analysis
Daratumumab combination therapy continued to demonstrate a clinical benefit over the longer term (available as an abstract plus poster) [35]. After a median follow-up of 24.5 months, daratumumab plus lenalidomide and dexamethasone was still associated with significantly (p < 0.0001) prolonged median PFS relative to lenalidomide plus dexamethasone (not reached vs. 17.5 months; HR 0.41; 95% CI 0.31–0.53). Median OS was not reached in either treatment group; a prespecified interim analysis for OS is planned after 165 OS events. Significantly (p < 0.0001) more daratumumab plus lenalidomide and dexamethasone recipients than lenalidomide plus dexamethasone recipients achieved an ORR (93 vs. 76%), with 79 and 48% having achieved a very good partial response or better, and 51 and 21% having achieved a complete response or better (p < 0.0001) [35].
5 Tolerability of Daratumumab
5.1 Monotherapy
Intravenous daratumumab was generally well tolerated when used as monotherapy in patients with relapsed or refractory multiple myeloma in the GEN501 [17] and SIRIUS [18] trials discussed in Sect. 4.1. In a pooled analysis of these studies (n = 148), the treatment-emergent adverse events of any grade that occurred most frequently (≥ 20% incidence) with daratumumab 16 mg/kg were fatigue (42%), nausea (30%), anaemia (28%), back pain (27%), cough (26%), thrombocytopenia (22%), upper respiratory tract infection (URTI; 22%) and neutropenia (21%) [19]. Across both trials, 4% of patients discontinued treatment because of AEs [19].
Infusion-related reactions (IRRs) have been reported with daratumumab [5, 7]. In the pooled analysis, IRRs occurred in 48% of patients, with 3% of patients experiencing grade ≥ 3 IRRs (bronchospasm, dyspnoea, hypoxia and hypertension) [19]. IRRs were predominantly grade 1 or 2 [17, 18], and most (96%) were observed with the first infusion [19]. To reduce the risk of IRRs, patients should be pre-medicated prior to treatment with daratumumab [5, 7]. Post-infusion medications are recommended to reduce the risk of delayed IRRs. Daratumumab therapy should be interrupted in patients who experience IRRs of any grade/severity and permanently discontinued in patients who experience life-threatening (grade 4) IRRs [5, 7]. Patients who experienced IRRs in GEN501 and SIRIUS were safely managed with pre- and post-infusion medications [19].
5.2 Combination Therapy
Daratumumab, when combined with bortezomib plus dexamethasone [22] or lenalidomide plus dexamethasone [23], had a generally manageable tolerability profile in patients with relapsed and/or refractory multiple myeloma in the phase III CASTOR and POLLUX trials discussed in Sect. 4.2.
In CASTOR, the most frequently reported AEs of any grade (occurring in ≥ 20% of patients and with a numerically higher incidence in the daratumumab plus bortezomib and dexamethasone group than the bortezomib plus dexamethasone group) were thrombocytopenia (59 vs. 44%), peripheral sensory neuropathy (47 vs. 38%), diarrhoea (32 vs. 22%), URTI (25 vs. 18%), cough (24 vs. 13%) and fatigue (21 vs. 25%) [22]. Overall, 7% of daratumumab plus bortezomib and dexamethasone recipients and 9% of bortezomib plus dexamethasone recipients discontinued treatment because of AEs [22]. In POLLUX, the most common AEs of any grade (occurring in ≥ 20% of patients and with a numerically higher incidence in the daratumumab plus lenalidomide and dexamethasone group than the lenalidomide plus dexamethasone group) were neutropenia (59 vs. 43%), diarrhoea (43 vs. 25%), fatigue (35 vs. 28%), URTI (32 vs. 21%), constipation (29 vs. 25%), cough (29 vs. 13%), muscle spasms (26 vs. 19%), nasopharyngitis (24 vs. 15%), nausea (24 vs. 14%) and pyrexia (20 vs. 11%) [23]. AEs resulted in discontinuation in 7% of patients in the daratumumab plus lenalidomide and dexamethasone group and 8% of patients in the lenalidomide plus dexamethasone group [23].
Daratumumab has been reported to increase neutropenia and thrombocytopenia induced by background therapy [5, 7]. In both CASTOR and POLLUX, the most frequent (≥ 12% incidence) grade 3 or 4 AEs reported with daratumumab-based therapy were thrombocytopenia, neutropenia and anaemia [22, 23]. In CASTOR, daratumumab plus bortezomib and dexamethasone was associated with numerically higher rates of grade 3 or 4 thrombocytopenia (45 vs. 33%) and neutropenia (13 vs. 4%) than bortezomib plus dexamethasone [22]. In POLLUX, daratumumab plus lenalidomide and dexamethasone was associated with a numerically higher rate of grade 3 or 4 neutropenia than lenalidomide plus dexamethasone (52 vs. 37%) [23]. Blood counts should be monitored regularly during treatment, and patients with neutropenia should be observed for signs of infection [5, 7]. Daratumumab dose delays may be required to allow recovery of neutrophils and platelets; no dose reductions are recommended [5, 7].
In CASTOR, grade 3 or 4 infections and infestations occurred in 21% of patients in the daratumumab plus bortezomib and dexamethasone group and 19% of those in the bortezomib plus dexamethasone group; the most common grade 3 or 4 infection was pneumonia [22]. In POLLUX, the incidence of grade 3 or 4 infection was 28% with daratumumab plus lenalidomide and dexamethasone and 23% with lenalidomide plus dexamethasone [23].
Daratumumab-associated IRRs of any grade (most commonly cough and dyspnoea) occurred in 45% of patients in CASTOR and 48% of patients in POLLUX [22, 23]. The majority of IRRs were grade 1 or 2 in severity and occurred during the first infusion. Corresponding rates of daratumumab-associated grade 3 IRRs were 9 and 5%, respectively. Two patients in CASTOR and one patient in POLLUX discontinued daratumumab-based therapy because of IRRs [22, 23].
Although rare, second primary cancers are an important clinical consideration in the treatment of multiple myeloma [22]. In CASTOR, the incidence of a second primary cancer was 2.5% in the daratumumab plus bortezomib and dexamethasone group and 0.4% in the bortezomib plus dexamethasone group. Most of these cancers developed ≤ 6 months after the initiation of study medication and occurred in patients with prior exposure to IMiDs and alkylating agents. No haematological secondary cancers were reported [22]. In POLLUX, 2.8% of patients in the daratumumab plus lenalidomide and dexamethasone group and 3.6% of patients in the lenalidomide plus dexamethasone group developed a second primary cancer; five patients in each group had non-invasive, cutaneous second primary cancer (e.g. basal or squamous cell carcinoma) [23].
Like all therapeutic proteins, daratumumab has the potential for immunogenicity [5]. In clinical trials of daratumumab as combination therapy, one patient receiving daratumumab developed neutralizing anti-drug antibodies [5, 7]. However, the assay used is known to have limitations in detecting anti-daratumumab antibodies in the presence of high concentrations of daratumumab [5, 7].
6 Dosage and Administration of Daratumumab
In the USA, daratumumab is indicated as monotherapy for the treatment of patients with multiple myeloma who have received ≥ 3 prior lines of therapy, including a PI and an IMiD, or who are double-refractory to a PI and an IMiD [5]. In the EU, daratumumab is indicated as monotherapy for the treatment of patients with relapsed and refractory multiple myeloma whose prior therapy included a PI and an IMiD and who have demonstrated disease progression on the last therapy [7]. Daratumumab is also approved in the EU, the USA and Japan for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, in patients with multiple myeloma who have received ≥ 1 prior therapy [5,6,7]. The efficacy and safety of daratumumab has not been established in paediatric patients (aged < 18 years) [5, 7].
The recommended dosage of daratumumab is 16 mg/kg administered as an intravenous infusion [5, 7]. In patients receiving monotherapy or combination therapy with lenalidomide, daratumumab should be administered weekly from weeks 1 to 8, every 2 weeks from weeks 9 to 24 and every 4 weeks from week 25 until disease progression. In patients receiving combination therapy with bortezomib, daratumumab should be administered weekly from weeks 1 to 9, every 3 weeks from weeks 10 to 24 and every 4 weeks from week 25 until disease progression [5, 7].
Blood typing and screening should be performed prior to starting daratumumab [5, 7]. Local prescribing information should be consulted for detailed information regarding regimen recommendations, the dosing schedule, preparation and administration procedures, pre- and post-infusion recommendations, serological testing, use in special patient populations, and warnings and precautions.
7 Place of Daratumumab in the Management of Relapsed or Refractory Multiple Myeloma
Clinical practice guidelines for the treatment of relapsed/refractory [36] or previously treated [37] multiple myeloma generally recommend regimens containing a PI and/or an IMiD. The US National Comprehensive Cancer Network guidelines now include daratumumab, in combination with bortezomib plus dexamethasone or lenalidomide plus dexamethasone, among the category 1 treatment options for patients with previously treated multiple myeloma [37]. These guidelines also recommend daratumumab monotherapy as a category 2A option for patients who have received ≥ 3 lines of prior therapy, including a PI and an IMiD, or who are double-refractory to a PI and an IMiD [37]. The most recent treatment recommendations from the European Society for Medical Oncology suggest that daratumumab triple combination therapy may become a new standard of care for patients with relapsed/refractory multiple myeloma [36].
Approval of daratumumab as monotherapy was based on data from the phase I/II GEN501 trial and the phase II SIRIUS trial (Sect. 4.1). A pooled analysis of data from both trials demonstrated that almost one-third of patients achieved an ORR with daratumumab, most of which were partial or very good partial responses. Patients who responded to daratumumab had a rapid, deep and durable (median 7.6 months) response, with some patients continuing to improve with continued treatment. Daratumumab also conferred an OS benefit, even in patients who achieved a minimal response or stable disease (Sect. 4.1). The survival benefit observed in these patients may be partly explained by the mechanisms of action of daratumumab (i.e. immune-mediated as well as immunomodulatory effects; Sect. 2) [19].
Approval of daratumumab for use in combination with bortezomib and dexamethasone, or lenalidomide and dexamethasone, was based on data from the phase III CASTOR and POLLUX trials (Sect. 4.2). In both trials, daratumumab triple combination therapy significantly prolonged median PFS and induced deep and durable responses compared with bortezomib plus dexamethasone (Sect. 4.2.1) or lenalidomide plus dexamethasone (Sect. 4.2.2). Of note, the significantly favourable effect of daratumumab-based therapy on PFS was observed regardless of age, time since last therapy, the number of previous lines of therapy, refractory status or cytogenetic risk status (Sects. 4.2.1 and 4.2.2). The clinical benefit of daratumumab was maintained over the longer term (Sects. 4.2.1.1 and 4.2.2.1); final OS data are awaited.
It should be noted that CASTOR and POLLUX differed in terms of trial design, and included patients with slightly different disease characteristics (Sect. 4.2). In CASTOR, bortezomib and dexamethasone were administered for a fixed duration (8 cycles), after which the trial essentially compared continuous daratumumab with no therapy. In POLLUX, all treatments were administered until disease progression, unacceptable toxicity, withdrawal of consent or death. CASTOR excluded patients with disease refractory to bortezomib, while POLLUX excluded patients with lenalidomide-refractory disease [22, 23]. In addition, more refractory patients were treated in CASTOR than in POLLUX (median of two vs. one prior lines of therapy) [22, 23]. In the absence of head-to-head comparisons, a number of factors may be considered when deciding which daratumumab-containing regimen to use in clinical practice. These include disease-related parameters associated with poor prognosis (e.g. ISS disease stage, cytogenetics), patient-related parameters (e.g. age, comorbidities, performance status) and treatment-related parameters (e.g. previous high-dose therapy plus ASCT, prior exposure and refractoriness to bortezomib and/or lenalidomide, number of lines of prior therapy) [38].
In CASTOR and POLLUX, daratumumab triple combination therapy was associated with significantly higher rates of MRD negativity than bortezomib plus dexamethasone (Sect. 4.2.1) or lenalidomide plus dexamethasone (Sect. 4.2.2), with MRD-negative patients having longer PFS than MRD-positive patients. The IMWG has issued revised response criteria in multiple myeloma, including new categories of MRD negativity [39]. These changes highlight the importance of MRD as a potential prognostic marker for PFS [40]. There has been some discussion in the literature regarding the use of MRD negativity as an endpoint for multiple myeloma trials, with some investigators advocating its widespread incorporation into clinical trials [41] and others highlighting the potential limitations of MRD analysis (e.g. assay sensitivity, patient acceptance, cost) [42]. While questions remain, incorporating MRD testing into clinical trials should help to define its possible role in guiding treatment decisions in clinical practice.
When daratumumab monotherapy was indirectly compared with physician’s choice [43, 44] or standard of care [45] in the real-world setting, OS [43,44,45] and PFS [44] outcomes favoured daratumumab, although the difference did not reach statistical significance for PFS (Czech population) [44].
In network meta-analyses comparing currently available regimens for relapsed/refractory multiple myeloma, combination therapy with daratumumab, lenalidomide and dexamethasone had the highest probability of being the best regimen in terms of PFS [46, 47], OS [46] and ORR [46], and a very low probability of being the worst regimen in terms of grade 3–4 toxicities [46]. Another network meta-analysis demonstrated that combination therapy with daratumumab, bortezomib and dexamethasone (in CASTOR) had a high (80–100%) probability of improving PFS and OS compared with panobinostat-, carfilzomib- and cyclophosphamide-based regimens containing dexamethasone ± bortezomib [48]. Similarly, combination therapy with daratumumab, lenalidomide and dexamethasone (in POLLUX) had a high (80–100%) probability of improving PFS and OS compared with carfilzomib-, elotuzumab- and ixazomib-based regimens containing lenalidomide and dexamethasone [48]. However, given the indirect nature of these comparisons, data should be interpreted with caution. Well-designed head-to-head trials assessing similar comparisons would be of interest.
Daratumumab was generally well tolerated when used as a single agent (Sect. 5.1), and had a generally manageable tolerability profile when used in combination with bortezomib plus dexamethasone or lenalidomide plus dexamethasone (Sect. 5.2). IRRs were the most common AEs. The majority of IRRs occurred during the first infusion and were grade 1 or 2 in severity, highlighting the safety of repeated daratumumab administration. The most common grade 3–4 AEs associated with daratumumab triple combination therapy were thrombocytopenia, neutropenia and anaemia (Sect. 5.2), which is consistent with the observation that daratumumab may increase neutropenia and thrombocytopenia induced by background therapy [5, 7].
The costs associated with chemotherapy constitute a substantial economic burden [49]. Combination therapy regimens used in multiple myeloma, particularly those containing newly approved agents such as daratumumab, can cost more than $US200,000 per year [49]. The National Institute for Health Care and Excellence (NICE) does not recommend daratumumab monotherapy as a cost-effective use of National Health Service resources, as a plausible incremental cost-effectiveness ratio for daratumumab could not be identified; however, the NICE guidance for daratumumab monotherapy in multiple myeloma is not yet finalized [50]. In a cost-effectiveness analysis, daratumumab-, carfilzomib-, elotuzumab- and ixazomib-based regimens containing lenalidomide and dexamethasone were associated with additional PFS life-years and quality-adjusted life-years (QALYs) gained over lenalidomide plus dexamethasone, but at an additional cost [51]. Of the four triple combination regimens, daratumumab plus lenalidomide and dexamethasone was associated with the greatest number of PFS life-years and QALYs gained at the lowest relative cost. The incremental cost-effectiveness ratio estimates for daratumumab plus lenalidomide and dexamethasone versus carfilzomib-, elotuzumab- and ixazomib-based triple combination regimens were $US23,035, $US166,655 and $US173,227 per PFS life-year, respectively [51]. Further robust pharmacoeconomic data are needed.
In conclusion, current evidence indicates that daratumumab is a valuable addition to the treatment options currently available for the management of relapsed or refractory multiple myeloma.
Data Selection Daratumumab: 203 records identified
Duplicates removed | 58 |
Excluded at initial screening (e.g. press releases; news reports; not relevant drug/indication) | 23 |
Excluded during initial selection (e.g. preclinical study; reviews; case reports; not randomized trial) | 27 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 44 |
Cited efficacy/tolerability articles | 21 |
Cited articles not efficacy/tolerability | 30 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Daratumumab, Darzalex, JNJ-54767414, myeloma, myelomatosis, myelomatoses. Records were limited to those in English language. Searches last updated 25 October 2017 | |
Change history
24 January 2018
The author has alerted us to the following error in Sect. 4.2.2.1, and the following correction should be noted:
References
Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, version 3.2017: NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(2):230–69.
Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–57.
Usmani S, Ahmadi T, Ng Y, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;02(21):1355–61.
Lonial S, Durie B, Palumbo A, et al. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30(3):526–35.
Janssen Biotech Inc. Darzalex (daratumumab) injection, for intravenous use: US prescribing information. 2016. http://www.janssenmd.com. Accessed 25 Oct 2017.
Genmab. Genmab announces approval of DARZALEX® (daratumumab) for relapsed or refractory multiple myeloma in Japan [media release]. 2017. http://ir.genmab.com.
European Medicines Agency. Darzalex, concentrate for solution for infusion: EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 25 Oct 2017.
de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–13.
Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–21.
Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Nijhof IS, Groen RWJ, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21(12):2802–10.
van der Veer MS, de Weers M, van Kessel B, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284–90.
Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70.
Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion (Paris). 2015;55(6 Pt 2):1545–54.
Clemens PL, Yan X, Lokhorst HM, et al. Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet. 2016;56(8):915–24.
Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–19.
Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60.
Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44.
Salomon-Perzynski A, Walter-Croneck A, Usnarska-Zubkiewicz L, et al. Real-world results of daratumumab monotherapy in heavily pretreated relapsed/refractory multiple myeloma in Poland: a prospective observational study of the Polish myeloma group [abstract no. E1262]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Plesner T, Arkenau H-T, Gimsing P, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128(14):1821–8.
Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66.
Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31.
Chanan-Khan AA, Lentzsch S, Quach H, et al. Daratumumab, bortezomib and dexamethasone versus bortezomib and dexamethasone alone for relapsed or refractory multiple myeloma based on prior treatment exposure: updated efficacy analysis of Castor [abstract no. 3313]. Blood. 2016;128(22).
Lentzsch S, Nooka A, Quach H, et al. Daratumumab, bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in RRMM based on prior lines and treatment exposure: CASTOR [abstract no. PS-238 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Mateos M-V, Estell J, Barreto W, et al. Efficacy of daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory myeloma based on prior lines of therapy: updated analysis of Castor [abstract no. 1150]. Blood. 2016;128(22).
Mateos M-V, Spencer A, Nooka A, et al. Daratumumab-based combination regimens in elderly (≥75 years) patients with relapsed or refractory multiple myeloma (RRMM): subgroup analysis of the phase 3 CASTOR and POLLUX studies [abstract no. P335 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
San-Miguel J, Weisel K, Cook G, et al. Efficacy by cytogenetic risk status for daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone in relapsed or refractory multiple myeloma [abstract no. S101]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Spencer A, Mark T, Spicka I, et al. Depth of response and MRD with daratumumab plus bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in RRMM: CASTOR [abstract no. PS-151 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Lentzsch S, Weisel K, Mateos M-V, et al. Daratumumab, bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in relapsed or refractory multiple myeloma (RRMM): efficacy and safety update (CASTOR) [abstract no. 8036 plus poster]. In: Annual meeting of the American Society of Clinical Oncology (ASCO); 2017.
Usmani SZ, Dimopoulos MA, Belch A, et al. Efficacy of daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma patients with 1 to 3 prior lines of therapy: updated analysis of Pollux [abstract no. 1151]. Blood. 2016;128(22).
Moreau P, Kaufman JL, Sutherland HJ, et al. Efficacy of daratumumab, lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone for relapsed or refractory multiple myeloma among patients with 1 to 3 prior lines of therapy based on previous treatment exposure: updated analysis of Pollux [abstract no. 489]. Blood. 2016;128(22).
Dimopoulos MA, Belch AR, White DJ, et al. Daratumumab, lenalidomide and dexamethasone (DRd) vs lenalidomide and dexamethasone (Rd) in RRMM based on prior lines and treatment exposure: POLLUX [abstract no. PS-207 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
San-Miguel J, Dimopoulos MA, Usmani S, et al. Depth of response and MRD with daratumumab plus lenalidomide and dexamethasone (DRd) vs lenalidomide and dexamethasone (Rd) in RRMM: POLLUX [abstract no. OP-028]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Dimopoulos M, Moreau P, Nahi H, et al. Efficacy and safety of daratumumab, lenalidomide, and dexamethasone (DRd) versus Rd alone in relapsed or refractory multiple myeloma (RRMM): updated analysis of POLLUX [abstract no. P334 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):52–61.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): multiple myeloma (version 2.2018). 2017. http://www.nccn.org/. Accessed 26 Oct 2017.
Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017;130(8):963–73.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46.
Anderson KC, Auclair D, Kelloff GJ, et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: current value and future applications. Clin Cancer Res. 2017;23(15):3980–93.
Anderson KC. Should minimal residual disease negativity be the end point of myeloma therapy? Blood Adv. 2017;1(8):517–21.
Sonneveld P. Should minimal residual disease negativity not be the end point of myeloma therapy? Blood Adv. 2017;1(8):522–5.
Usmani SZ, Diels J, Ito T, et al. Daratumumab monotherapy compared with historical control data in heavily pretreated and highly refractory patients with multiple myeloma: an adjusted treatment comparison. Am J Hematol. 2016;92:E146–52.
Hájek R, Jelinek T, Maisnar V, et al. Comparative effectiveness of daratumumab monotherapy versus a real-world historical control from the Czech Republic in heavily pretreated and highly refractory multiple myeloma patients [abstract no. 3332]. Blood. 2016;128(22).
Kumar SK, Durie BGM, Su Z, et al. Adjusted comparisons suggest daratumumab is associated with prolonged survival compared with standard of care therapies in patients with heavily pre-treated and highly refractory multiple myeloma [abstract no. 4517]. Blood. 2016;128(22).
Botta C, Ciliberto D, Rossi M, et al. Network meta-analysis of randomized trials in multiple myeloma: efficacy and safety in relapsed/refractory patients. Blood Adv. 2017;1(7):455–66.
van Beurden-Tan CHY, Franken MG, Blommestein HM, et al. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35(12):1312–9.
Dimopoulos M, Weisel K, Kaufman JL, et al. Efficacy of daratumumab-based regimens in patients with relapsed/refractory multiple myeloma—a systematic literature review and network meta-analysis [abstract no. E1281 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Rajkumar SV, Harousseau JL. Next-generation multiple myeloma treatment: a pharmacoeconomic perspective. Blood. 2016;128(24):2757–64.
National Institute for Health and Care Excellence. Appraisal consultation document: daratumumab monotherapy for treating relapsed and refractory multiple myeloma. 2017. http://www.nice.org.uk. Accessed 25 Oct 2017.
Alsaid N, McBride A, Agarwal AB, et al. Cost effectiveness of carfilzomib (CAR), ixazomib (IXA), elotuzumab (ELO), or daratumumab (DAR) with lenalidomide and dexamethasone (LEN+DEX) vs LEN+DEX in relapsed/refractory multiple myeloma (R/R MM) [abstract no. 8030]. In: Annual meeting of the American Society of Clinical Oncology (ASCO); 2017.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Hannah Blair is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/3AF8F06079DC09B5.
Additional information
The manuscript was reviewed by: S. Kumar, Hematology and Internal Medicine, Mayo Clinic, Rochester, MN, USA; H. Lokhorst, Department of Hematology, VU University Medical Center, Amsterdam, Netherlands; T. M. Mark, University of Colorado Cancer Center, Division of Hematology, Aurora, CO, USA; A. Oriol, Hospital Universitario Germans Trias I Pujol, Badalona, Spain.
Rights and permissions
About this article
Cite this article
Blair, H.A. Daratumumab: A Review in Relapsed and/or Refractory Multiple Myeloma. Drugs 77, 2013–2024 (2017). https://doi.org/10.1007/s40265-017-0837-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0837-7