Abstract
Pamiparib (PARTRUVIX™; BeiGene Ltd.) is a selective poly (ADP-ribose) polymerase 1 and 2 (PARP1 and PARP2) inhibitor being developed for the treatment of various cancers. Based on the results from the pivotal phase II portion of a phase I/II trial (NCT03333915) pamiparib was recently approved in China for the treatment of germline BRCA mutation-associated recurrent advanced ovarian, fallopian tube or primary peritoneal cancer previously treated with two or more lines of chemotherapy. This article summarizes the milestones in the development of pamiparib leading to this first approval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.14759040. |
A PARP inhibitor is being developed by BeiGene Ltd. for the treatment of various cancers |
Received its first approval on 30 April 2021 in China |
Approved for use in germline BRCA mutation-associated recurrent advanced ovarian, fallopian tube or primary peritoneal cancer |
1 Introduction
Pamiparib (PARTRUVIX™) is a selective inhibitor of poly (ADP-ribose) polymerase 1 and 2 (PARP1 and 2) being developed by BeiGene Ltd. (BeiGene) for the treatment of various cancers. PARP 1 and 2 are involved in the repair of damaged DNA as part of the DNA damage response (DDR). PARP1, which accounts for 90% of PARP activity, is activated by binding to DNA break sites and recruits various DDR-related proteins to repair damaged DNA, primarily via base excision repair (BER). PARP 2 and 3 also have a role in DNA repair with the former accounting for 5–10% of total PARP activity [1]. Pamiparib selectively binds to PARP 1 and 2 and prevents PARP-mediated DNA repair. This leads to an accumulation of DNA strand breaks, inducing genomic instability and eventually causing apoptosis. Pamiparib is approved in China for the treatment of ovarian cancer, fallopian tube cancer and peritoneal cancer [2]. The recommended dose is 60 mg twice daily until disease progression or unacceptable adverse reactions [3].

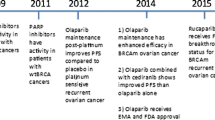
Key milestones in the development of pamiparib, focusing on its use in the treatment of ovarian cancer. NDA new drug application
2 Scientific Summary
2.1 Pharmacodynamics
Pamiparib had IC50 values of 1.3 and 0.92 nM for PARP-1 and -2, respectively, but was less active or inactive against other PARP isoforms (IC50 68 to > 100,000 nM), and trapped PARP1-DNA-complexes with an EC50 of 13 nM [4]. An X-ray cocrystal structure of pamiparib within PARP-1 revealed the drug binds to similar sites to other PARP inhibitors (e.g., niraparib and olaparib). Pamiparib had an EC50 of 10.6 nM against MDA-MB-436 human breast cancer cells carrying the BRCA1 mutation, 53.8 nM against UWB1.289 human ovarian cancer cells carrying the BRCA1 mutation and 42.5 nM against HCC1395 carrying the BRCA1/2 mutation, but did not have activity against MDA-MB-231 and SK-BR-3 breast cancer cells that did not have BRCA mutations (EC50s > 10,000 nM) [5].
In a murine model it was established that a pamiparib dose > 5.45 mg/ml is required to induce ≥ 80% PAR inhibition, corresponding to an intra-tumour concentration of ≈ 0.8 µmol/kg. Administration of a single 5.45 mg/ml dose of pamiparib produced > 80% inhibition of PARylation for 12 h, dropping to 53% after 24 h, indicating twice daily dosing is required to maintain efficacy in this model [4].
In vivo in a murine model of human BRCA1 mutant MDA-MB-436 breast cancer, oral pamiparib 1.6–6.3 mg/kg twice daily for 28 days induced tumour regression on day 29 and was associated with objective response in all animals. Two months after treatment was stopped tumour relapse was observed only in mice treated with pamiparib at the lowest dose (1.6 mg/kg) [4].
2.2 Pharmacokinetics
Plasma exposure was dose proportional after administration of pamiparib 2.5–120 mg twice daily. Geometric mean Cmax and AUC0-12 were 2275.1 ng/ml and 16,841.5 h·ng/ml, respectively, after administration of a single 60 mg dose of the drug to Chinese patients. Steady-state Cmax and AUC0-12 after multiple doses were 5251.5 ng/ml and 48802.4 h·ng/ml, respectively. Pamiparib is rapidly absorbed after oral administration, with a tmax of 1–2 h. Administration after a high-fat breakfast delayed absorption, increasing tmax to 7 h, with AUC0-inf and Cmax reduced by 12% and 41%, respectively. This was not considered clinically significant, however, and the drug may be taken without regard to food intake [3].
Pamiparib is 95.7% bound to human plasma protein and has an apparent volume of distribution of ≈ 37 L after administration at the recommended dose [3].

Chemical structure of pamiparib
Mean cumulative excretion was 84.7% after administration of 60 mg [14C]-pamiparib to patients with solid tumours (n = 4). The drug was mostly metabolised via N-oxidation and oxidation of the pyrrolidine ring, primarily to a dehydrogenated oxidative product. Most radioactivity (57.8%) was recovered from urine, with 26.9% recovered from faeces. Only a small amount of [14C]-pamiparib was recovered as unchanged drug (2.11% and 1.11% in faeces and urine, respectively) [6].
Coadministration with the strong CYP3A inducer rifampin reduced pamiparib plasma exposure by 38–43%, however, coadministration with the strong CYP3A inhibitor itraconazole did not affect pamiparib plasma exposure [7].
2.3 Therapeutic Trials
2.3.1 Ovarian Cancer
Promising antitumor activity was observed in patients with platinum-sensitive/resistant advanced ovarian cancer in an ongoing phase I/II study (NCT03333915) in Chinese patients with advanced solid tumors with known/suspected BRCA1/2 mutation. Ninety patients with platinum-sensitive and 23 with platinum-resistant advanced ovarian cancer were enrolled and treated with pamiparib at the recommended dose. In the platinum-sensitive cohort the confirmed objective response rate assessed by an independent review committee (ORRIRC) per RECIST v1.1 (primary endpoint) was 68.3% and median duration of response was 13.8 months. In the platinum-resistant cohort the confirmed ORRIRC was 31.6%, median duration of response was 11.1 months, median progression-free survival was 6.2 months and median overall survival was 13.6 months [3, 8]. The dose escalation phase of this trial enrolled Chinese patients with advanced non-mucinous high-grade ovarian or fallopian tube cancer (n = 9) or triple-negative breast cancer (n = 6) whose disease either progressed despite standard therapy or had no standard therapy. Germline BRCA (gBRCA) mutation was present in five patients with ovarian cancer and two with breast cancer with four and four, respectively having gBRCA wild type. Patients were assigned to pamiparib 20 (n = 4), 40 (n = 4), or 60 mg (n = 7) twice daily. At data cut off (30 September 2019) after median follow-up of 3.5 months (range 1.2–32.7 months) two patients with ovarian cancer (one with gBRCA mutant and one with wild type) had a confirmed partial response and six had stable disease (four with gBRCA mutant and two with wild type). Median progression-free survival was 5.6 months. At data cut off 14 patients had stopped treatment with pamiparib and one patient with high-grade ovarian cancer remained on treatment. Most (n = 8) patients discontinued treatment because of disease progression [9].
Pamiparib demonstrated antitumor activity in a phase I dose-escalation/expansion study in patients with advanced solid tumours including ovarian cancer (NCT02361723). Of 97 patients enrolled, 48 (including 30 with ovarian cancer) received pamiparib at the recommended dose. The confirmed objective response rate per RECIST v1.1 criteria in patients with ovarian cancer who were evaluable for efficacy (≥ 1 post-baseline tumour assessment) was 39% (22 of 57) comprising 4 complete and 18 partial responses. The median duration of response was 12.3 months (range 1.3–40.8 months) [10].
2.3.2 Glioblastoma
Pamiparib plus radiation therapy and/or temozolomide as treatment for newly diagnosed or recurrent/refractory glioblastoma multiforme is being evaluated in a phase Ib/II dose-escalation trial (NCT03150862). Patients with newly diagnosed glioblastoma multiforme received pamiparib over 2, 4 or 6 weeks in combination with radiation therapy over 6–7 weeks. Patients with recurrent/refractory glioblastoma multiforme received pamiparib continuously plus temozolomide on days 1–21 of a 28-day cycle. At data cut off (14 September 2018), 18 patients with newly diagnosed glioblastoma multiforme had been enrolled with a median follow-up duration of 19 weeks (range 2–54 weeks). Two of 15 patients who were evaluable per modified response assessment in neuro-oncology (mRANO) criteria had partial response and six had stable disease. Fifteen patients with recurrent/refractory glioblastoma multiforme were enrolled at data cutoff with a median follow-up duration of 12.9 weeks (range 0.3–31.4 weeks). Among 10 patients evaluable per mRANO criteria there were two partial responses and three patients had stable disease [11].
2.3.3 Other Solid Tumours
Pamiparib in combination with tislelizumab was associated with antitumour efficacy and clinical benefit in patients with advanced solid tumours (including 34 with ovarian, fallopian tube or peritoneal cancer) in a phase Ib dose-escalation study (NCT02660034). Patients received pamiparib 20, (n = 12) 40 (n = 12) or 60 mg (n = 6) twice daily plus IV tislelizumab 2 mg/kg once every 3 weeks, or pamiparib 40 (n = 13) or 60 mg (n = 6) twice daily plus IV tislelizumab 200 mg once every 3 weeks. The objective response rate according to RECIST 1.1 criteria was 20% (n = 10) including two (4%) complete responses and eight (16%) partial responses. The two patients with complete response received pamiparib 40 mg twice daily plus tislelizumab 2 mg/kg and had BRCA wild type ovarian cancer. Partial responses were observed in patients with ovarian, fallopian tube or peritoneal cancer (n = 7) and breast cancer (n = 1). Stable disease was observed in 16 (32%) patients. Median progression-free survival and overall survival were 92 and 388 days, respectively [12].
Pamiparib plus low dose temozolomide had promising antitumor efficacy in an interim analysis of an ongoing phase Ib dose escalation/expansion study (NCT03150810) in patients with advanced solid tumours. During the dose escalation phase, patients received pamiparib on days 1–28 and low dose oral temozolomide at escalating once daily doses on days 1–7, 1–14, or 1–28 of each 28-day cycle. In the dose-expansion phase, which included patients with gastric cancer and SCLC previously treated with 1–2 lines of chemotherapy, pamiparib was given on days 1–28 and low dose oral temozolomide 60 mg once daily on days 1–7. Biomarker assessments included determination of DDR mutational status (SNV/CNV homozygous loss) of 16 core DDR genes in circulating tumour DNA and genomic instability score (GIS) status according to the Myriad myChoice® HRD test. At data cut off (10 April 2020) 66 and 48 patients had been enrolled in the dose escalation and dose expansion phases, respectively, with median follow-up of 8.5 months (range 0.3–26.5 months). Eleven of 36 patients (31%) analysed for GIS were GIS positive. In this subgroup the overall response and disease control rates were 82 and 91%, respectively, across multiple tumour types. In patients who were BRCA-mutated/GIS positive (n = 5) the overall response and disease control rates were 100%. In BRCA-wild type/GIS positive patients (n = 6) the overall response and disease control rates were 67 and 83%, respectively. In patients who were GIS negative (n = 3) the overall response and disease control rates were 12 and 52%, respectively. Twenty-seven of 104 patients (26%) analysed for DDR mutational status were DDR-positive; the overall response and disease control rates in this group were 26 and 52%. In DDR negative patients, overall response and disease control rates were 14 and 67%, respectively [13].
2.4 Adverse Events
Tolerability data for pamiparib are available from three clinical trials (BGB-290-102 [NCT03333915], BGB-290-AU-002 [NCT02361723] and BGB-290-201 [NCT03575065]), involving a total of 317 patients (including 185 with advanced ovarian cancer) with a median duration of exposure to pamiparib of 5.5 months (range: 0.1–57.1 months). Adverse reactions occurring with an incidence of ≥ 10% in this population included anaemia, nausea, leukopenia, neutropenia, vomiting, fatigue, thrombocytopenia, loss of appetite, diarrhoea, abdominal pain, elevated aspartate aminotransferase (AST) levels, elevated alanine aminotransferase (ALT) levels, elevated blood bilirubin levels, and lymphopenia [3].
The incidence of adverse reactions of grade 3 and above was 55.8%, with those occurring with an incidence of ≥ 1% including anaemia, neutropenia, leukopenia, thrombocytopenia, lymphopenia, vomiting, fatigue, diarrhoea, nausea and elevated AST levels. The incidence of drug-related serious adverse events was 21.5% with those occurring with an incidence of ≥ 1% including anaemia and leukopenia [3].
Adverse reactions occurring with an incidence of ≥ 10% in patients with ovarian cancer (n = 113) participating the phase II component of BGB-290-102 (median duration of treatment 8.3 months [range 0.1–26.0 months]) included anaemia, leukopenia, nausea, neutropenia, vomiting, thrombocytopenia, loss of appetite, fatigue, abdominal pain, elevated ALT levels, diarrhoea, elevated AST levels, lymphopenia, elevated γ-glutamyltransferase levels, upper respiratory tract infection, elevated blood bilirubin levels, malaise, weight loss, and dizziness. The incidence of grade 3 and above adverse reactions was 71.7%, with those occurring at an incidence of ≥ 1% including anaemia, neutropenia, leukopenia, thrombocytopenia, lymphopenia, vomiting, diarrhoea, elevated γ-glutamyltransferase levels, hypokalaemia, abdominal pain, fatigue, upper respiratory tract infection, pancytopenia and elevated blood pressure. Anaemia was the only adverse reaction that led to permanent discontinuation of pamiparib in more than one patient (incidence 4.4%). Adverse reactions occurring with an incidence of ≥ 5% included anaemia (grades 1–4 90.3%, grades 3–4 42.5%), leukopenia (79.6%, 30.1%), neutropenia (68.1%, 37.2%), thrombocytopenia (45.1%, 8.8%), lymphopenia (22.1%, 7.1%), nausea (68.1%, 0.9%), vomiting (49.6%, 4.4%), abdominal pain (25.7%, 1.8%), diarrhoea (22.1%, 3.5%), constipation (8.0%, 0.0%), weight loss (11.5%, 0.0%), fatigue (29.2%, 1.8%), feeling unwell (14.2%, 0.0%), fever (5.3%, 0.9%), loss of appetite (31.0%, 0.0%), hyperglycaemia (7.1%, 0.9%), hypokalaemia (7.1%, 2.7%), hyperuricaemia (6.2%, 0.0%), upper respiratory tract infection (11.5%, 1.8%), nasopharyngitis (6.2%, 0.0%), dizziness (11.5%, 0.9%), headache (5.3%, 0.0%), difficulty breathing (5.3%, 0.0%), tachycardia (8.8%, 0.0%) and backache (6.2%, 0.0%) [3].
Laboratory abnormalities occurring with an incidence of ≥ 5% included increased AST levels (grades 1–4 23.0%, grades 3–4 0.9%), increased ALT levels (22.1%, 0.9%), increased γ-glutamyltransferase levels (17.7%, 3.5%), elevated bilirubin levels (15.9%, 0.0%), increased blood creatinine levels (8.8%, 0.0%), elevated alkaline phosphatase levels (7.1%, 0.0%) and elevated blood lactate dehydrogenase levels (5.3%, 0.0%) [3].
2.5 Companion Diagnostic
The myChoice® HRD and BRACAnalysis CDx® companion diagnostic tests developed by Myriad Genetics are being used to support the development of pamiparib [14].
2.6 Ongoing Clinical Trials
The pivotal phase I/II trial in patients with advanced ovarian cancer, fallopian tube cancer and primary peritoneal cancer, or advanced triple negative breast cancer described above (NCT03333915) is ongoing. The phase I/II study evaluating the efficacy of pamiparib in combination with temozolomide described above (NCT03150810) remains in the recruitment phase. A further study evaluating this combination is pending in patients with renal carcinoma (NCT04603365) and two studies are recruiting patients with glioblastoma/glioma (NCT03914742, NCT03749187). A phase III trial comparing the efficacy and tolerability of pamiparib with placebo as maintenance treatment for platinum-sensitive recurrent ovarian cancer is underway in China (NCT03519230). In addition, a multinational phase III trial is comparing pamiparib with placebo in patients with advanced or inoperable gastric cancer (NCT03427814). Phase II studies evaluating pamiparib as treatment for ovarian cancer or carcinosarcoma with BRCA1/2 gene mutations (NCT03933761) and HER2-negative breast cancer with BRCA mutation (NCT03575065) are recruiting patients.
3 Current Status
Pamiparib received its first approval on 30 April 2021 for the treatment of gBRCA mutation-associated recurrent advanced ovarian, fallopian tube or primary peritoneal cancer that has been treated with ≥ 2 prior lines of chemotherapy in China [2].
References
Wu Z, Cui P, Tao H, et al. The synergistic effect of PARP inhibitors and immune checkpoint inhibitors. Clin Med Insights Oncol. 2021. https://doi.org/10.1177/1179554921996288.
Businesswire. China NMPA approves PARP inhibitor pamiparib for patients with previously treated advanced ovarian cancer [media release]. May 7 2021. https://www.businesswire.com.
BeiGene. Pamiparib: prescribing information. 2021.
Xiong Y, Guo Y, Liu Y, et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia. 2020;22(9):431–40.
Wang H, Ren B, Liu Y, et al. Discovery of pamiparib (BGB-290), a potent and selective poly (ADP-ribose) polymerase (PARP) inhibitor in clinical development. J Med Chem. 2020;63(24):15541–63.
Mu S, Palmer D, Fitzgerald R, et al. Human mass balance and metabolite profiling of [14C]-pamiparib, a poly (ADP-ribose) polymerase inhibitor, in patients with advanced cancer. Clin Pharmacol Drug Dev. 2021. https://doi.org/10.1002/cpdd.943.
Mu S, Lin C, Skrzypczyk-Ostaszewicz A, et al. The pharmacokinetics of pamiparib in the presence of a strong CYP3A inhibitor (itraconazole) and strong CYP3A inducer (rifampin) in patients with solid tumors: an open-label, parallel-group phase 1 study. Cancer Chemother Pharmacol. 2021. https://doi.org/10.1007/s00280-021-04253-x.
Wu X, Zhu J, Wang J, et al. Phase II study of pamiparib in Chinese patients (pts) with advanced ovarian cancer (aOC) [abstract no. 820P and Poster]. Ann Oncol. 2020;31 (Suppl 4):S619–20.
Xu B, Yin Y, Dong M, et al. Pamiparib dose escalation in Chinese patients with non-mucinous high-grade ovarian cancer or advanced triple-negative breast cancer. Cancer Med. 2021;10(1):109–18.
Voskoboynik M, Mileshkin L, Gan H, et al. Safety, antitumor activity, and pharmacokinetics (PK) of pamiparib (BGB-290), a PARP1/2 inhibitor, in patients (pts) with advanced solid tumours: updated phase I dose-escalation/expansion results [abstract no. 452PD]. Ann Oncol. 2019;30(Suppl 5):v167.
BeiGene. BeiGene announces preliminary clinical data on PARP inhibitor pamiparib presented at annual scientific meeting and education day of the Society for Neuro-Oncology [media release]. 16 Nov 2018. http://www.beigene.com.
Friedlander M, Meniawy T, Markman B, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(9):1306–15.
Stradella A, Johnson M, Goel S, et al. Clinical benefit in biomarker-positive patients (pts) with locally advanced or metastatic solid tumours treated with the PARP1/2 inhibitor pamiparib in combination with low-dose (LD) temozolomide (TMZ) [abstract no. 530MO]. Ann Oncol. 2020;31(Suppl 4):S465–6.
Myriad Genetics. Myriad Genetics and BeiGene sign agreement to develop companion diagnostics for use with BeiGene’s novel PARP Inhibitor, BGB-290 [media release]. 06 Apr 2017. https://myriad.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Pamiparib: First Approval. Drugs 81, 1343–1348 (2021). https://doi.org/10.1007/s40265-021-01552-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01552-8