Abstract
Poly-ADP-ribose polymerase (PARP) inhibitors have been one of the most exciting developments in the treatment of ovarian cancer in recent years. Demonstration of anti-cancer activity has led to the European Medicines Agency (EMA) approval of the PARP inhibitor (PARPi) olaparib as maintenance therapy in women with BRCA-mutated (BRCAm) ovarian cancer with platinum-sensitive recurrence following response to platinum therapy and the US Food and Drug Administration (US FDA) approval of olaparib in relapsed germline BRCA-mutated (gBRCAm) ovarian cancer in women who have received at least three prior chemotherapy treatments, both occurring in 2014. Additional trials are underway or awaiting final analysis with olaparib, other PARPis, and PARPi combinations to further elucidate the activity of these drugs in various clinical settings. This review will focus on the current clinical experience and ongoing trials with PARPis in ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
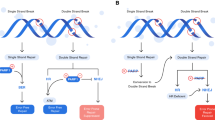
Poly-ADP-ribose polymerase (PARP) is a family of enzymes composed of 17 members [1]. PARP-1 is the best characterized member of this family and plays a critical role in the repair of single-strand breaks (SSB) by base excision repair (BER) and has also been implicated in other roles in DNA repair. In cells that are deficient in double-strand break (DSB) repair due to defects in homologous recombination (HR), inhibition of PARP and SSB repair results in synthetic lethality, as first described in two landmark papers in 2005 [2, 3]. As both BRCA1 and BRCA2 play key roles in HR, development of PARPis in ovarian cancer initially focused on germline BRCA-mutated (gBRCA-mutated) tumors. However, findings from The Cancer Genome Atlas (TCGA) project demonstrating that up to 50 % of high-grade serous ovarian cancers (HGSOCs) may have some defect in the HR pathway [4•] have subsequently suggested that a broader population of ovarian cancers may be responsive to the activity of PARPis.
While inhibition of the enzymatic function of PARP was initially postulated to be the primary mechanism by which PARPis mediated their activity, subsequent research has suggested that PARPis may mediate their effects through a number of different mechanisms; several manuscripts have reviewed the mechanisms of action of PARPis [5–7]. One hypothesis includes the possibility of “PARP trapping,” whereby PARP-1 that has been inactivated by the PARPi is “trapped” to the site of DNA damage, preventing further DNA repair at the site [8]. As different PARPis have varying potency in their ability to enzymatically inhibit PARP and to “trap” PARP, the mechanism of action by which PARPis achieve their effect may influence which of the various agents in this drug class prove to be most effective. Other mechanisms of PARPi action have been proposed, including promotion of increased non-homologous end joining [9] and impairment of BRCA1 recruitment to the site of DNA damage [6].
Clinical exploration of the activity of PARPis in ovarian cancer followed closely after the discovery of synthetic lethality of these agents with BRCA deficiency, and a timeline of PARPi development is shown in Fig. 1. Monotherapy trials of PARPis in the BRCA-deficient setting have been further augmented by studies exploring the activity of PARPis as maintenance therapy or in combination with chemotherapy or other targeted therapies, in patients with and without known BRCA mutations. While the findings of these studies have started to shape our application of PARPis in the clinical setting, findings from several key trials are still pending that may further clarify where these agents will be best utilized in the treatment of ovarian cancer.
PARPis as Monotherapy for Relapsed Ovarian Cancer
BRCA-Mutated Tumors
The activity of PARPis as monotherapy was first reported by Fong et al. in a phase 1 trial of olaparib, where 9 of 19 evaluable patients with BRCA-mutated ovarian, breast, or prostate cancer had a complete or partial response [10] (Fig. 1). In this trial, the dose and schedule of olaparib were increased from 10 mg daily for 2 out of every 3 weeks to 600 mg twice daily on a continuous basis. Dose-limiting toxicities of somnolence and thrombocytopenia were observed in the 600 mg twice daily dose, and 400 mg twice daily of the capsule formation of olaparib was deemed the maximally tolerated dose (MTD). Subsequently, an expansion cohort in this trial enrolled 50 women with gBRCA-mutated ovarian cancer and reported a 40 % response rate in this population [11]. A phase 2 study confirmed these findings of activity in ovarian cancer patients with a gBRCA mutation, where two sequential cohorts of women were enrolled to receive olaparib in the capsule formulation at either 400 mg twice daily or 100 mg twice daily [12]. Of note, women in this study had received a median of 3 prior lines of therapy. Response rates of 33 % were reported in the 400 mg twice daily cohort, while the response rate in the 100 mg twice daily cohort was 13 %. In a dedicated ovarian subset of a separate phase 2 study of olaparib monotherapy using the capsule formulation at 400 mg twice daily in patients with gBRCA mutation, the response rate was 31.1 % in 193 ovarian cancer patients [13••]. In the 137 women in this cohort who had received three or more prior lines of chemotherapy and who had measurable disease at baseline, the overall response rate was 34 %, with a median duration of response of 7.9 months [14]. The response rate for platinum-sensitive cancers was 46 % (18 of 39), while for platinum-resistant cancers, it was 30 % (24 of 81). Duration of response in both platinum-sensitive and platinum-resistant cancers was similar (8.2 and 8.0 months, respectively). These results served as the basis for the US FDA approval of olaparib in 2014 (see Fig. 1).
A phase 2 trial of another PARPi, veliparib, as monotherapy in 50 women with relapsed gBRCAm ovarian cancer who had received three or fewer prior lines of chemotherapy also demonstrated some activity, with an overall response rate of 26 %, including response rates of 20 % (6 of 30) in platinum-resistant and 35 % (7 of 20) in platinum-sensitive patients [15]. The PARPis niraparib and rucaparib have also demonstrated single-agent activity in gBRCAm ovarian cancer in phase 1 dose finding studies, while the PARPi talazoparib is currently being studied in BRCA-mutated advanced solid tumors (NCT01989546), as well as in gBRCAm ovarian cancer that has progressed through treatment with any of the other PARPis (NCT02326844). In a phase 1 study of niraparib which tested doses from 30 to 400 mg daily and found the maximum tolerated dose to be 300 mg daily, 40 % (8 of 20) of patients with gBRCA mutations and measurable disease had a confirmed Response Evaluation Criteria In Solid Tumors (RECIST) and CA125 GCIG partial response [16]. Rucaparib was tested in a separate phase 1 study using doses from 40 mg up to 500 mg once per day continuously as well as 240 to 840 mg BID, and the recommended phase 2 dose of single-agent rucaparib was determined to be 600 mg BID [17]. In this study, 100 % (3 of 3) patients with platinum-sensitive and 75 % (6 of 8) of patients with platinum-resistant gBRCAm ovarian cancer who received doses of rucaparib of 360 mg twice daily or higher experienced disease control at 24 weeks.
Based upon the observed activity of PARPi monotherapy in gBRCAm ovarian cancers, studies have also been designed to evaluate their activity in this setting in comparison with standard chemotherapy (Table 1 lists these and other selected randomized phase II studies testing PARPis). In an open-label phase 2 study of 97 women with ovarian cancer and a gBRCA mutation by Kaye et al., olaparib, dosed in the capsule formulation at either 200 mg twice daily or 400 mg twice daily, demonstrated similar median progression-free survival (PFS) and overall response rates (ORR) to pegylated liposomal doxorubicin (PLD) [18]. Women in the study had ovarian cancer that had recurred within 12 months of prior platinum therapy, no prior anthracyclines, and were randomized in a 1:1:1 fashion to one of the three arms. ORR and PFS to olaparib 200 mg twice daily, olaparib 400 mg twice daily, and PLD at 50 mg/m2 were 25, 31, and 18 % and 6.5, 8.8, and 7.1 months, respectively. Differences between the arms were not statistically significant. A separate phase 3 study called SOLO3 is currently ongoing that compares olaparib monotherapy to single-agent non-platinum-based chemotherapy in women with recurrent somatic or gBRCA-mutated platinum-sensitive ovarian cancer who have received at least two prior platinum-based therapies (see Table 2).
BRCA-Non-Mutated Tumors
Growing research in ovarian cancer suggests that responsiveness to PARPis is not limited to BRCA-mutated cancers alone. Findings from TCGA suggest that up to 50 % of HGSOCs have defects in HR that might render them vulnerable to the effects of PARPis [4•]. In a separate study, Pennington and colleagues found that 31 % of a panel of 390 ovarian cancers had a deleterious germline or somatic mutation in one or more of 13 HR genes tested [19]. Of note, in this study, the rate of HR mutations was similar between HGSOC and ovarian cancers of non-serous (including clear cell, endometrioid, and carcinosarcoma) histologies, with rates of 28 and 31 %, respectively. As sensitivity to PARPis has been demonstrated in the setting of deficiency in multiple HR genes aside from BRCA1 and BRCA2 in vitro, these molecular findings suggest that a non-BRCA-mutated or wild-type (wt) BRCA subset of ovarian cancers may also respond robustly to therapy with PARPis.
Clinically, this hypothesis was supported by the results of an early phase 2 trial of olaparib monotherapy in women with or without BRCA mutations and breast or ovarian cancer [20]. In the subset of 64 women treated on this trial with ovarian cancer, a response rate of 41 % (7 of 17) was observed in women with gBRCA mutation, while women without a gBRCA mutation had a response rate of 24 % (11 of 46). Of note, all women in this study underwent germline BRCA testing. Similarly, the phase 1 study of niraparib reported that 2 out of 3 patients with platinum-sensitive and 3 out of 19 patients with platinum-resistant sporadic HGSOC demonstrated response to niraparib monotherapy by RECIST or GCIG CA125 criteria [16]. However, in further developing PARPi therapy for women without BRCA mutation, one challenge has been in identifying a biomarker that suggests the presence of HR deficiency. Various such mechanisms have been proposed, including mutational profiling of a targeted panel of HR or other DNA repair genes [19], identifying gene expression signatures of “BRCAness” [21], or using the pattern of genome-wide chromosomal alterations that can occur in the setting of HR deficiency [22–24].
Results were reported recently on part 1 of an ongoing phase 2 trial of monotherapy with the PARPi rucaparib in women with relapsed platinum-sensitive ovarian cancer (ARIEL2) [25]. In this trial, 206 women with high-grade serous or endometrioid ovarian cancer were enrolled, with enrollment of known gBRCA mutation carriers capped at 15 patients. All patients were required to have platinum-sensitive relapsed measurable disease and had received at least one prior platinum-based therapy. A pre-treatment biopsy and archival tumor collection were mandated on all patients. Using BRCA sequencing and a measurement of genomic loss of heterozygosity (LOH) on the pre-treatment biopsy, patients were categorized into one of three categories: tBRCAmt (presence of a BRCA mutation within the tumor), tBRCA-like (no BRCA mutation within the tumor, but a genomic LOH score that was felt to reflect the presence of HR deficiency), and biomarker negative (no BRCA mutation within the tumor and a low genomic LOH score). Overall, 20 % of patients were found to be tBRCAmt, 40 % tBRCA-like, and 34 % biomarker negative. A remaining 6 % of patients could not be classified. In the primary endpoint of PFS, the hazard ratio for tBRCAmt versus biomarker negative was 0.22 (12.8 vs 5.3 months, p < 0.0001) while that for tBRCA-like versus biomarker negative was 0.67 (5.7 vs 5.3 months, p = 0.045). Interestingly, while the response rate for tBRCA-like tumors was less than that for tBRCAmt tumors (36 vs 75 %), the duration of response was more similar (8.2 vs 9.5 months). This may reflect the fact that while genomic LOH can capture the prior presence of HR deficiency, it is a permanent change to the DNA and does not reverse even if the HR defect is no longer present. Of note, in biomarker-negative patients, the response rate was 16 %, with a duration of response of 5.5 months.
Ongoing studies of PARPi monotherapy in non-selected women with ovarian cancer continue to seek to define a biomarker signature that can identify those patients who are most likely to respond. Part 2 of the ARIEL2 study will apply the genomic LOH signature described above to ovarian cancer patients who have received at least three prior chemotherapy treatment regimens. Similarly, an ongoing trial of niraparib monotherapy in women who have received at least three prior lines of chemotherapy for ovarian cancer (QUADRA) as well as the NOVA study described below will plan to analyze the activity of niraparib by evaluating HR deficiency (HRD) status using the Myriad HRD test (developed by Myriad Genetics). This test calculates an HRD score from three components reflecting different types of tumor rearrangements. The results of the QUADRA study and part 2 of the ARIEL study will provide greater information regarding the use of PARPi monotherapy in women with relapsed non-BRCA-mutated ovarian cancer.
PARPis as Maintenance Therapy for Ovarian Cancer
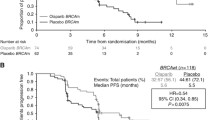
A key appeal of PARPis has been their relatively low side effect profile, especially in comparison to traditional chemotherapies. This low side effect profile has also made them appealing agents to consider as maintenance therapy following a response to standard chemotherapy. A randomized, double-blind, placebo-controlled phase 2 study of maintenance olaparib therapy following response to platinum therapy in women with platinum-sensitive ovarian cancer who had received at least two prior platinum-based regimens demonstrated a significant PFS benefit in women receiving olaparib maintenance therapy [26••]. In this study, 265 women were randomized to receive either olaparib in capsule formulation at 400 mg twice daily or placebo following either partial or complete response to their most recent chemotherapy regimen. PFS was 8.4 months following randomization in women receiving olaparib compared to 4.8 months in women receiving placebo (hazard ratio 0.35, p < 0.001). In a pre-planned retrospective analysis to determine the effects of olaparib on PFS based on BRCA mutation status of the cancer, patients with a known germline or tumor BRCA mutation (74 patients receiving olaparib; 62 patients receiving placebo), the median PFS was 11.2 months in the olaparib group and 4.3 months in the placebo group (hazard ration 0.18, p < 0.0001) [27••]. While overall survival at 58 % maturity did not significantly differ between the groups (hazard ratio 0.88, p = 0.44 in entire study population; 0.73, p = 0.19 in BRCA-mutated patients), these results suggest that maintenance therapy with PARPi may provide clinical benefit, especially to those patients with BRCA-mutated tumors. Two phase 3 studies of maintenance olaparib in women with BRCA-mutated ovarian cancer, either following initial therapy with surgery and platinum-based chemotherapy (SOLO1) or in the recurrent platinum-sensitive setting following response to platinum-based chemotherapy (SOLO2), have completed accrual and are awaiting final analysis (Table 2).
Other ongoing or completed trials are also examining the role of PARPis as maintenance therapy in non-BRCA-mutated ovarian cancers. The NOVA trial (see Table 2), which randomized women with platinum-sensitive HGSOC or BRCA-mutated ovarian cancer to receive either niraparib or placebo maintenance following response to platinum-based chemotherapy, will evaluate outcome analyses in patients without a BRCA mutation using HRD status as an accompanying biomarker. The NOVA trial has completed accrual, and results are anticipated in the near future. Similarly, ARIEL3, which is still accruing patients, randomizes women with platinum-sensitive high-grade ovarian cancer to receive either rucaparib or placebo maintenance following response to prior platinum therapy and will include secondary outcome analyses utilizing the genomic LOH biomarker applied in the ARIEL2 trial.
PARPis in Combination with Chemotherapy
Another approach that has been explored is whether PARPis could be combined with chemotherapy to achieve greater clinical effect. As certain chemotherapy agents potentiate DNA damage, the effects of PARPis in this setting could conceivably be enhanced. Challenges to combining PARPis with chemotherapy have included increased hematologic toxicities. For example, in a phase 1/1b study combining carboplatin with olaparib in BRCA-mutated breast or ovarian cancer, continuous dosing of olaparib capsules at 200 mg twice daily and every 3-week carboplatin at AUC3 resulted in grade 3 thrombocytopenia lasting greater than 7 days in 2 of 2 ovarian cancer patients [28]. Ultimately, dosing could be achieved with carboplatin AUC5 every 3 weeks with olaparib 400 mg twice daily on days 1 through 7 of the cycle.
An open-label randomized phase 2 trial compared the combination of carboplatin, paclitaxel, and olaparib to carboplatin and paclitaxel alone in women with recurrent platinum-sensitive HGSOC who had received up to three prior courses of platinum-based chemotherapy [29] (Table 1). In women receiving the combination and olaparib, carboplatin was dosed at an AUC4, paclitaxel at 175 mg/m2, and olaparib at 200 mg twice daily in the capsule formulation on days 1 through 10 of each 21-day cycle. Women receiving carboplatin and paclitaxel only were dosed at carboplatin AUC6 and paclitaxel 175 mg/m2 every 21 days. Women receiving combination therapy continued on olaparib maintenance at 400 mg twice daily following completion of chemotherapy. One hundred sixty-two women were eligible and treated on the study; 41 had a known BRCA mutation. PFS was significantly longer in the combination compared to the chemotherapy-alone arm (12.2 vs 9.6 months, hazard ratio 0.51, p = 0.0012), with the effect most pronounced in those patients with a known BRCA mutation (not reached vs 9.7 months, hazard ratio 0.21, p = 0.0015). As this trial included olaparib both in combination with chemotherapy and as subsequent maintenance therapy, it is difficult to distinguish what degree of the observed effect can be attributed to the combination with chemotherapy, as opposed to maintenance therapy alone.
Veliparib has been combined with oral cyclophosphamide in a randomized phase II study in patients with recurrent gBRCA-mutated ovarian cancer; cyclophosphamide alone (50 mg once daily) was compared to cyclophosphamide with veliparib (60 mg once daily) given on a continuous basis. Adding veliparib at this 60-mg daily dose did not improve either the response rate or the median PFS compared to oral cyclophosphamide alone [30]. A multi-arm phase 1 trial has also explored the combination of veliparib together with one of three chemotherapy regimens in women with newly diagnosed advanced-stage ovarian cancer [31]. The three chemotherapy regimens examined included (1)carboplatin AUC6 and paclitaxel 175 mg/m2 on day 1 of a 21-day cycle; (2) carboplatin AUC6 on day 1 and paclitaxel 80 mg/m2 on days 1, 8, and 15 of a 21-day cycle; and (3) paclitaxel IV 135 mg/m2 on day 1, cisplatin 75 mg/m2 IP on day 1 or 2, and paclitaxel 60 mg/m2 IP on day 8 of a 21-day cycle. All patients received bevacizumab starting with cycle 2 of therapy. The study found the recommended phase 2 dosing for continuous veliparib dosing to be 150 mg twice daily in combination with all of the chemotherapy regimens. Based upon the findings from this phase 1 study, a placebo-controlled phase 3 study (GOG-3005) comparing carboplatin and paclitaxel chemotherapy to carboplatin, paclitaxel, and veliparib therapy and to carboplatin, paclitaxel, and veliparib therapy followed by veliparib maintenance therapy has begun accrual in women with newly diagnosed advanced-stage HGSOC (Table 2).
PARPis in Combination with Other Therapies
Combining PARPis with other non-chemotherapy treatments has also been proposed as a method to either expand the population in which PARPis may demonstrate clinical relevance or prevent the development of PARPi resistance. Several strategies of combination PARPi therapy have been explored, while others are being actively developed.
A randomized open-label phase 2 trial compared the combination of olaparib together with the anti-angiogenic agent cediranib [32••] (Table 2). Cediranib is an oral tyrosine kinase inhibitor with activity against VEGFR1, 2, and 3 and has also been documented to have single-agent activity in recurrent ovarian cancer [33, 34]. Ninety women with recurrent platinum-sensitive ovarian cancer were enrolled to this trial; 47 had a known germline BRCA mutation. The combination was dosed at cediranib 30 mg and olaparib 200 mg twice daily in the capsule formulation, based upon results from a prior phase 1 study [35], while olaparib monotherapy was dosed at 400 mg twice daily. PFS on the cediranib/olaparib combination was 17.7 months compared to 9.0 months on olaparib monotherapy (hazard ratio 0.42, p = 0.005). Interestingly, while women with a known BRCA mutation derived a non-statistically significant benefit from the combination (median PFS 19.4 vs 16.5 months; hazard ratio 0.55, p = 0.16), those whose BRCA status was wild type or unknown appeared to derive more marked benefit from the combination (median PFS 16.5 vs 5.7 months, hazard ratio 0.32, p = 0.008). One explanation for the results seen in these subset analyses was that the effects of an anti-angiogenic such as cediranib may induce a more HR-deficient state in HR-proficient cells by downregulation of HR proteins such as RAD51 and BRCA1 [36–38]. Two trials exploring the activity of the cediranib/olaparib combination in women with recurrent ovarian cancer in either the platinum-sensitive (NRG-GY004) or platinum-resistant (NRG-GY005) setting are now under way, and a third trial that will explore the role of the cediranib/olaparib combination as maintenance therapy following platinum-based chemotherapy is in development (ICON9). A separate phase 1/2 trial (AVANOVA) currently enrolling patients is also exploring the effects of combining an anti-angiogenic agent with a PARPi utilizing the combination of bevacizumab and niraparib.
A second PARPi combination strategy builds upon pre-clinical data in mouse models of BRCA-deficient and triple-negative breast cancer, which suggested that duration of response to PARPis could be enhanced by combining PARPis with phospho-inositol-3 kinase (PI3K) inhibitors [39, 40]. A phase 1 trial combining olaparib together with the PI3K inhibitor BKM120 demonstrated a response rate of 26 % (12 of 46) in ovarian cancer and 21 % (5 of 24) in triple-negative breast cancer patients [41]. A study of olaparib together with the PI3K inhibitor BYL719 in ovarian and triple-negative breast cancer patients is also ongoing. Another potential PARPi combination of interest in ovarian cancer is the combination of PARPis together with immune checkpoint agents. A recent study found that BRCA-mutated HGSOCs demonstrated significantly higher predicted neoantigens, increased CD3+ and CD8+ tumor-infiltrating lymphocytes, and elevated expression of PD-1 and PD-L1, in comparison to HR-proficient tumors [42]. Other pre-clinical studies have reported immunoregulatory effects of PARPi [43] and synergy between immunomodulatory agents and PARPi [44].
Other strategies for combining PARPi with agents that might induce HRD in HR-proficient cells have also been described [5]. These strategies can include combining a PARPi together with a CDK1 inhibitor, which can decrease BRCA1 phosphorylation and thereby inhibit BRCA function [45], with a HDAC inhibitor, which can cause downregulation of HR [46], or potentially with HSP90 inhibitors, which may also regulate HR protein expression [47]. Should these combination strategies prove successful, they could potentially significantly broaden the population of ovarian cancer patients in whom PARPis will demonstrate clinical activity.
Conclusion
The discovery and development of PARPis in ovarian cancer has proven to be a major breakthrough in the search for novel targeted therapies in this disease. With olaparib approved in the USA as monotherapy in women with gBRCA-mutated recurrent ovarian cancer who have received at least three prior lines of chemotherapy and in Europe as maintenance therapy in women with BRCA-mutated recurrent platinum-sensitive ovarian cancer after response to a platinum therapy, PARPis have already proven to be a valuable addition to our current armamentarium against ovarian cancer.
While the activity of PARPis in women with known BRCA mutations has been demonstrated, ongoing trials seek to clarify when is the ideal time in the course of ovarian cancer therapy for these agents to be used. Given their low toxicity profile and PFS benefit observed in a phase 2 trial, maintenance therapy is an appealing potential strategy; however, the awaited results from the SOLO1 and SOLO2 trials for women with BRCA-mutated ovarian cancer and the NOVA and ARIEL3 trials for women without a known BRCA mutation will be necessary before we understand whether PARPis should be considered as maintenance therapy. Similarly, results from the ARIEL2 and QUADRA trials will help us to understand whether a BRCA wild-type, biomarker-positive population for HRD can be identified where PARPi monotherapy will be active. Finally, the combination of PARPis with other agents, whether chemotherapy or other targeted therapies, is an area of active investigation and interest and may broaden the population in which this exciting class of therapies may ultimately be used in ovarian cancer.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26(8):882–93. eng.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. eng.
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. eng.
Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. Pubmed Central PMCID: 3163504. This publication describes findings from analyzing messenger RNA expression, microRNA expression, promoter methylation, and DNA copy number in 489 HGSOCs and exon sequencing in 316 of these samples. Importantly, it describes that ∼50% of HGSOC have a molecular alteration in genes involved in HR and may therefore have increased susceptibility to PARP inhibition.
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–54. Pubmed Central PMCID: 4631624.
Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–406. Pubmed Central PMCID: 4517072.
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–99. Pubmed Central PMCID: 3528345.
Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108(8):3406–11. Pubmed Central PMCID: 3044391.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. eng.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9.
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51. eng.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50. This publication describes the activity of olaparib monotherapy in patients with germline BRCA1/2 mutations, including ovarian cancer, where the response rate was 31%. The results of this trial served as the basis for US FDA approval of olaparib in 2014.
Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203.
Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–91. Pubmed Central PMCID: 4447525.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92.
Kristeleit R, Shapira-Frommer R, Burris H, Patel MR, Lorusso P, Oza A, et al. Phase 1/2 study of oral rucaparib: updated phase 1 and preliminary phase 2 results. Ann Oncol. 2014;25 suppl 4:iv305–iv26. Abstract, ESMO 2014.
Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9.
Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–75. Pubmed Central PMCID: 3944197.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. eng.
Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28(22):3555–61. Pubmed Central PMCID: 2917311.
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776–82. Pubmed Central PMCID: 3493866.
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2(4):366–75. Pubmed Central PMCID: 3806629.
Popova T, Manie E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72(21):5454–62.
Kristeleit R, Swisher EM, Oza A, Coleman RL, Scott C, Konecny G, et al. Final results of ARIEL2 (Part 1): a phase 2 trial to prospectively identify ovarian cancer (OC) responders to rucaparib using tumor genetic analysis. Presented at ECCO 2015. 2015.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. This randomized Phase 2 trial observed significant improvement in PFS study-wide and in pre-defined subgroups of platinum-sensitive ovarian cancer who received olaparib maintenance following platinum therapy compared to placebo.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852. This pre-planned subset analysis of BRCA-mutated (tumor or somatic) tumors within the randomized Phase 2 trial described in Ledermann et al. in 2012 (previous reference) reported marked improvement in PFS (11.2 mos vs. 4.3 mos) for women receiving olaparib maintenance therapy versus placebo. These findings form the basis for the EMA approval of olaparib in 2014.
Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106(6):dju089. Pubmed Central PMCID: 4049120.
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97.
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21(7):1574–82. Pubmed Central PMCID: 4383665.
Bell-McGuinn K, Brady WE, Schilder RJ, Fracasso PM, Moore KN, Walker JL, et al. A phase I study of continuous veliparib in combination with IV carboplatin/paclitaxel or IV/IP paclitaxel/cisplatin and bevacizumab in newly diagnosed patients with previously untreated epithelial ovarian, fallopian tube, or primary peritoneal cancer: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33(suppl; abstr 5507):Presented at ASCO 2015.
Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14. Pubmed Central PMCID: 4294183. This Phase 2 study demonstrated significant improvement in PFS and response rates in women with recurrent platinum-sensitive ovarian cancer receiving a combination of cediranib and olaparib compared to olaparib alone, with demonstrated activity of the combination in patients both with and without a known BRCA mutation. This trial suggests that combining PARPi’s with other targeted agents may result in synergistic effects and broaden the population of ovarian cancer patients in whom PARPi-based therapies might be pursued.
Hirte H, Lheureux S, Fleming GF, Sugimoto A, Morgan R, Biagi J, et al. A phase 2 study of cediranib in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: a trial of the Princess Margaret, Chicago and California Phase II Consortia. Gynecol Oncol. 2015;138(1):55–61.
Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33):5601–6. eng.
Liu JF, Tolaney SM, Birrer M, Fleming GF, Buss MK, Dahlberg SE, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49(14):2972–8. Pubmed Central PMCID: 3956307.
Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65(24):11597–604. eng.
Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24(19):8504–18. eng.
Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia. 2014;16(4):343–53. e2.
Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2(11):1036–47.
Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmana J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2(11):1048–63. Pubmed Central PMCID: 3733368.
Matulonis U, Wulf GM, Barry WT, Birrer M, Westin S, Spagnoletti T, et al. Phase I of oral BKM120 or BYL719 and olaparib for high-grade serous ovarian cancer or triple-negative breast cancer: final results of the BKM120 plus olaparib cohort. Cancer Res. 2015;75(Abst CT324):Presented at AACR 2015.
Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016 Feb 9 doi:10.18632/oncotarget.7277.
Huang J, Wang L, Cong Z, Amoozgar Z, Kiner E, Xing D, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(-/-) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463(4):551–6.
Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immun Res. 2015;3(11):1257–68.
Johnson N, Li YC, Walton ZE, Cheng KA, Li D, Rodig SJ, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17(7):875–82. Pubmed Central PMCID: 3272302.
Konstantinopoulos PA, Wilson AJ, Saskowski J, Wass E, Khabele D. Suberoylanilide hydroxamic acid (SAHA) enhances olaparib activity by targeting homologous recombination DNA repair in ovarian cancer. Gynecol Oncol. 2014;133(3):599–606. Pubmed Central PMCID: 4347923.
Choi YE, Battelli C, Watson J, Liu J, Curtis J, Morse AN, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5(9):2678–87. Pubmed Central PMCID: 4058036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joyce Liu has received compensation from AstraZeneca and Genentech/Roche for service as a consultant.
Ursula A. Matulonis has received compensation from Genentech/Roche, Pfizer, AstraZeneca, and Merck for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Liu, J.F., Matulonis, U.A. What Is the Place of PARP Inhibitors in Ovarian Cancer Treatment?. Curr Oncol Rep 18, 29 (2016). https://doi.org/10.1007/s11912-016-0515-z
Published:
DOI: https://doi.org/10.1007/s11912-016-0515-z