Abstract
Intravenous daratumumab (DARZALEX®), a human monoclonal antibody targeting CD38, is approved in the EU and USA for use in combination with bortezomib, thalidomide and dexamethasone for the treatment of adults with newly diagnosed multiple myeloma (MM) who are eligible for autologous stem cell transplantation. A subcutaneous formulation of daratumumab has also been approved in the EU and USA (DARZALEX FASPRO™) for use in MM. In the pivotal phase III CASSIOPEIA trial in adults with newly diagnosed, transplant-eligible MM, the addition of intravenous daratumumab to bortezomib, thalidomide and dexamethasone significantly increased the proportion of patients with a stringent complete response and significantly prolonged progression-free survival; overall survival data are not yet mature. Some facets of health-related quality of life were improved by the addition of daratumumab. The addition of daratumumab had a minimal effect on overall toxicity and the most common grade ≥ 3 adverse events with daratumumab combination therapy were haematological (e.g. neutropenia, lymphopenia). The approval of daratumumab as combination therapy in patients with newly diagnosed, transplant-eligible MM expands the range of MM treatment settings in which daratumumab is an option and the availability of the subcutaneous formulation will likely be of benefit to patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First-in-class CD38 monoclonal antibody |
Prolongs progression-free survival and induces deeper responses when added to bortezomib, thalidomide and dexamethasone |
Manageable tolerability profile when used as combination therapy |
1 Introduction
Multiple myeloma (MM) is a plasma cell neoplasm that accounts for ≈ 10% of haematological malignancies [1]. While MM remains incurable, relentless evolution in the treatment landscape has contributed to progressively improving clinical outcomes and clinicians treating patients with newly diagnosed MM now have a vast array of drugs in their therapeutic arsenal [2,3,, 3]. Nevertheless, autologous stem cell transplantation (SCT) continues to be the mainstay of MM treatment ≈ 30 years after its introduction; in transplant-eligible patients with newly diagnosed MM, the standard of care is induction therapy with a triplet regimen (e.g. bortezomib, thalidomide and dexamethasone), followed by high-dose therapy (HDT) with autologous SCT [4]. In the EU, candidates for autologous SCT are generally patients aged < 65 years and fit patients aged < 70 years in good clinical condition [4]. Induction therapy prior to HDT/autologous SCT aims to reduce tumour burden, thus deepening the clinical response and increasing the likelihood of successful engraftment [1].
Monoclonal antibodies, which target specific antigens expressed on the surface of MM cells, represent a novel class of agents for the treatment of MM and are being investigated for use in induction therapy regimens. One potential drug target is CD38, a transmembrane glycoprotein that acts as both a receptor and an ectoenzyme [5]. CD38 is highly and uniformly expressed on MM cells, while also being expressed on normal lymphoid and myeloid cells [including natural killer (NK) cells, various B cell and T cell subpopulations, and myeloid-derived suppressor cells], on certain other haematological malignancies, and in some tissues of non-haematopoietic origin [5, 6]. Antibodies targeting CD38 are transforming the treatment of MM and constitute attractive candidates for inclusion in combination regimens, due to their distinct mechanisms of action, favourable safety profiles and marked anti-tumour activity [5].
Daratumumab (DARZALEX®), a first-in-class human IgG1κ monoclonal antibody targeting CD38, is now approved in the EU [7] and USA [8] for use in combination with bortezomib (a proteasome inhibitor), thalidomide (an immunomodulatory drug) and dexamethasone (a corticosteroid) for the treatment of adults with newly diagnosed MM who are eligible for autologous SCT. This follows previous approvals of daratumumab as monotherapy and combination therapy in patients with relapsed and/or refractory MM [9] and in combination with bortezomib, melphalan and prednisone or lenalidomide and dexamethasone in patients with newly diagnosed MM ineligible for autologous SCT [10]. While daratumumab was initially only available for intravenous infusion, a subcutaneous formulation is now approved in the EU for the same indications as intravenous daratumumab (including use in patients with newly diagnosed, transplant-eligible MM; Sect. 6) [7]. This approval was based on data from two clinical trials in patients with newly diagnosed or relapsed/refractory disease [7]. In the USA, subcutaneous daratumumab (DARZALEX FASPRO™) has been approved in the newly diagnosed, transplant-ineligible and relapsed/refractory MM treatment settings, but not in newly diagnosed, transplant-eligible MM [11]. This article reviews the clinical efficacy, safety and tolerability of daratumumab in patients with newly diagnosed, transplant-eligible MM, with a brief overview of its pharmacological properties.
2 Pharmacological Properties of Daratumumab
The pharmacological properties of daratumumab administered as monotherapy and in combination therapies have been reviewed previously [9, 10, 12]. In brief, daratumumab binds to CD38 with high affinity, potently inhibiting CD38-expressing myeloma cells via a well-characterized mechanism of action (Fig. 1). This includes direct on-tumour activity through several CD38 immune-mediated actions, apoptosis, and modulation of CD38 enzymatic activity, as well as immunomodulatory effects [6, 13,14,15,16,16]. While the direct anti-tumour effects of daratumumab may explain rapid anti-myeloma responses, the systemic modulation of the immune system might contribute to the durable responses and improved patient survival observed with daratumumab therapy [13, 17]. Daratumumab also reduced total and activated NK cells in the peripheral blood and bone marrow of patients with MM, although neither baseline NK cell levels nor count reductions influenced the efficacy or safety of daratumumab [18].
Mechanisms of action of daratumumab, [12]
In patients with MM, clinical responses to daratumumab display marked heterogeneity [19, 20]. Levels of CD38 expression and complement inhibitory proteins on myeloma cells contribute to this heterogeneity [19, 20]. Increased CD38 expression rendered patient-derived myeloma cells more susceptible to daratumumab-mediated antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity [20], while increased expression of the complement-inhibitory proteins CD55 and CD59 was associated with the emergence of resistance to daratumumab [19].
Daratumumab was demonstrated to have additive or synergistic anti-myeloma activity when combined with various other anti-myeloma agents in preclinical or clinical studies [21]. This potentially occurs through daratumumab-induced reductions in CD38 expression, sensitizing myeloma cells to the effects of other drugs [21]. Adding daratumumab to bortezomib and dexamethasone [22] or lenalidomide and dexamethasone [23] significantly (p < 0.001) prolonged progression-free survival (PFS) and increased the overall response rate relative to bortezomib and dexamethasone or lenalidomide and dexamethasone alone in patients with relapsed and/or refractory MM.
Through binding to CD38 on red blood cells (RBCs), daratumumab may result in positive indirect antiglobulin test (indirect Coombs test) in recipients for up to 6 months after the last infusion and detection of antibodies to minor antigens may be masked in serum [7, 8]. Prior to initiating daratumumab therapy, patients should therefore be typed and screened [7, 8]. There are various strategies that can be used to mitigate daratumumab interference with compatibility testing (e.g. treating reagent RBCs with dithiothreitol [24]) [5, 7, 8]. Determination of ABO and Rh blood types are not affected by daratumumab [7, 8]. For planned blood transfusions in the EU, blood transfusion centres should be informed of the interference of daratumumab with indirect antiglobulin tests [7]. In the USA, blood transfusion centres and blood banks should be notified that a patient has received daratumumab [8]. If an emergency transfusion is necessary, non-cross-matched ABO/RhD-compatible RBCs can be given according to local blood bank practices [7, 8].
Daratumumab may interfere with serum protein electrophoresis and immunofixation tests used to monitor disease monoclonal immunoglobulins (M-protein), producing false positive test results in some patients with IgG kappa myeloma protein; this may impact the assessment of complete response and disease progression [7, 8]. In patients with persistent very good partial response and suspected daratumumab interference, clinicians should consider using a validated daratumumab-specific immunofixation assay to inform their assessment of clinical response [7, 8, 25].
In population pharmacokinetic analyses, the pharmacokinetics of daratumumab in combination regimens were similar to the pharmacokinetics of daratumumab monotherapy [26]. This apparent lack of impact of background therapies on daratumumab pharmacokinetics was as expected, given the clearance mechanisms of daratumumab and the background small-molecule therapies do not overlap [26].
Daratumumab pharmacokinetics are typical of an IgG1 monoclonal antibody [27]. Following intravenous administration, daratumumab exhibited time- and concentration-dependent, non-linear pharmacokinetics consistent with target-mediated drug disposition [27, 28]. After an initial infusion of daratumumab 1–24 mg/kg, daratumumab peak serum concentration (Cmax) increased roughly in proportion to dose, while increases in area under the concentration–time curve (AUC) were greater than dose-proportional [7, 27]. After weekly infusions of daratumumab 1–24 mg/kg, both daratumumab Cmax and AUC increased in a greater than dose-proportional manner [7, 27].
Daratumumab is primarily confined to the vascular system (volume of distribution of 4.4 L when administered as combination therapy [8]); extravascular distribution is limited [27]. Clearance decreased with increasing dose (suggesting a saturation of target-mediated clearance at higher doses) and with repeated doses at the same dose level [7, 27]. Tapered administration of daratumumab (Sect. 5) allows for rapid saturation of target-mediated clearance during the first 8 weeks of infusions and the maintenance of target saturation thereafter [27]. In combination therapy, daratumumab had a mean estimated terminal half-life (associated with linear clearance) of ≈ 15–23 days [7].
Various patient and disease characteristics [e.g. renal impairment, hepatic impairment, age, sex, race, albumin level, Eastern Cooperative Oncology Group (ECOG) performance status, type of myeloma (IgG vs non-IgG)] had no clinically important effects on daratumumab exposure, irrespective of whether daratumumab was administered as monotherapy or in combination with other drugs; no dose adjustments based on these characteristics are required [7, 8, 26]. Daratumumab clearance and volume of distribution increased with patient body weight; weight-based dosing of intravenous daratumumab is appropriate (Sect. 5) [7, 8].
Subcutaneous daratumumab 1800 mg co-formulated with 30,000 units hyaluronidase was noninferior to intravenous daratumumab 16 mg/kg with respect to the pharmacokinetic co-primary endpoint of maximum trough concentration (geometric means ratio 107.93%; 90% CI 95.74–121.67) in the phase III COLUMBA trial in patients with relapsed or refractory MM [29].
3 Therapeutic Efficacy of Daratumumab
The efficacy of intravenous daratumumab in combination with bortezomib, thalidomide and dexamethasone in patients with autologous SCT-eligible newly diagnosed MM was demonstrated in the randomized, open-label, active-controlled, phase III CASSIOPEIA trial [30]. The active comparator in this trial was bortezomib, thalidomide and dexamethasone [30].
CASSIOPEIA enrolled adults aged 18–65 years with newly diagnosed, documented MM who were eligible for HDT and autologous SCT [30]. For enrollment, patients were also required to have an ECOG performance status of 0–2, an absolute neutrophil count ≥ 1 × 109/L, a hemoglobin level ≥ 7.5 g/dL, a platelet count ≥ 70 × 109/L (if < 50% of bone marrow nucleated cells were plasma cells, otherwise a platelet count > 50 × 109/L), a creatinine clearance ≥ 40 mL/min, a corrected serum calcium level ≤ 14 mg/dL and adequate hepatic function. Key exclusion criteria included having had prior systemic therapy or autologous SCT for any plasma cell dyscrasia or having grade ≥ 2 peripheral neuropathy or neuropathic pain [30].
The CASSIOPEIA trial consisted of two parts [30]. In Part 1 (Fig. 2), patients were randomized (for the first time) to receive daratumumab combination therapy (n = 543) or the active comparator (n = 542) as pre-transplant induction treatment and post-transplant consolidation treatment. Randomization was stratified by variables including site affiliation, International Staging System (ISS) disease stage (I, II or III) and cytogenetic risk status [standard risk or high risk, with the latter defined by the presence of del17p and/or t(4;14) cytogenetic abnormalities]. In the absence of cytogenetic results, patients were stratified as standard risk and classified as such in analyses. Patients received up to four 28-day induction treatment cycles prior to their transplant and two 28-day consolidation cycles after their transplant (see Table 1 for dose regimens). Pre-infusion medications (e.g. paracetamol, antihistamines) were administered prior to daratumumab. After the final induction cycle, stem cell mobilization with cyclophosphamide 3 g/m2 and granulocyte colony-stimulating factor was conducted. Based on mobilization response, peripheral blood stem cells were harvested and, following conditioning with intravenous melphalan 200 mg/m2, patients proceeded to undergo autologous SCT. Consolidation cycles commenced after hematopoietic reconstitution (and ≥ 30 days after SCT). Patients who achieved a partial response or better at day 100 after transplantation entered Part 2 of the study, in which they were randomized (for the second time) to either daratumumab maintenance therapy or observation. This article focuses on the primary and final analysis of CASSIOPEIA Part 1; CASSIOPEIA Part 2 is ongoing [30].
Design of Part 1 of the CASSIOPEIA clinical trial in adults with transplant-eligible newly diagnosed multiple myeloma [30]. Primary endpoint results are reported in the animated figure (available online). VTd bortezomib, thalidomide and dexamethasone
CASSIOPEIA Clinical Trial Design (MP4 14631 kb)
The primary endpoint in CASSIOPEIA Part 1 was the proportion of patients achieving a stringent complete response follwing consolidation treatment, assessed 100 days after autologous SCT (or immediately following consolidation treatment if > 100 days after autologous SCT) and analyzed centrally according to International Myeloma Working Group criteria [30]. Efficacy analyses were conducted in the intention-to-treat population (i.e. all patients randomized in Part 1) [30].
At baseline, demographic and clinical characteristics were generally well-balanced between the treatment groups [30]. Patients had a median age of 58 years and a median time since MM diagnosis of 0.9 months; 40%, 45% and 15% of patients were at ISS disease stages I, II and III, respectively, and the majority of patients (90%) had an ECOG performance status of 0 or 1. With regard to cytogenetic profiles, 15.5% of patients were considered high risk [30].
During stem cell mobilization, 22% of patients in the daratumumab combination therapy group and 8% of patients in the active comparator group received plerixafor [30]. The median numbers of CD34 + cells collected and transplanted were 6.3 × 106/kg and 3.3 × 106/kg, respectively, with daratumumab combination therapy versus 8.9 × 106/kg and 4.3 × 106/kg, respectively, with the active comparator [30]. Despite this apparent difference in ease of stem cell collection, there were no marked differences between the treatment arms in the proportion of patients who underwent autologous SCT (91.2% and 90.0% with daratumumab combination therapy and the active comparator [31]) or in the hematopoietic reconstitution rate for patients who received transplants (99.8% and 99.6% [31]) [30]. Most patients (85% and 81% of daratumumab combination therapy and active comparator groups, respectively) had completed four induction cycles and both consolidation cycles at data cut-off (June 19, 2018; median follow-up 18.8 months) [30].
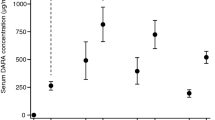
The proportion of patients achieving a stringent complete response after consolidation was significantly greater with daratumumab combination therapy than with the active comparator (primary endpoint; Table 1) [30]. In both treatment groups, the proportion of patients with a stringent complete response increased over the course of the study [30]. Prespecified subgroup analyses for the primary endpoint favoured daratumumab combination therapy over the active comparator in most subgroups, with exceptions including patients with a high-risk cytogenetic profile (odds ratio 0.83; 95% CI 0.42–1.66) or ISS disease stage III (odds ratio 1.07; 95% CI 0.54–2.12) [30, 32]. In addition, significantly higher proportions of daratumumab combination therapy recipients than active comparator recipients achieved a complete response or better (Table 1), a very good partial response or better (83% vs 78%; p = 0.024) and a negative status for minimal residual disease (threshold 1 tumor cell/105 white cells) after consolidation, as assessed either by multiparametric flow cytometry (Table 1) or next-generation sequencing (57% vs 37%; p < 0.0001) [30]. In prespecified subgroup analyses, the effect of daratumumab combination therapy versus the active comparator on minimal residual disease negativity was consistent across subgroups, including in patients with a high-risk cytogenetic profile or ISS disease stage III [30, 33]. In post hoc analyses based on flow cytometry, the proportions of patients with both minimal residual disease negativity and either a complete response or better (34% vs 20%) or a very good partial response or better (62% vs 43%) were significantly (p < 0.0001) greater in the daratumumab combination therapy group than in the active comparator group [30] (Fig. 3).
Most common any-grade AEs (incidence ≥ 20% in either treatment group) and grade 3 or 4 AEs (incidence ≥ 10% in either treatment group) in CASSIOPEIA Part 1 [30]. AEs adverse events, D-VTd daratumumab in combination with bortezomib, thalidomide and dexamethasone, VTd bortezomib, thalidomide and dexamethasone
Daratumumab combination therapy significantly improved PFS from first randomization (based on inverse probability weighting) relative to the active comparator (Table 1) [30]. Events of disease progression or death occurred in 45 daratumumab combination therapy recipients and in 91 active comparator recipients; at data cut-off, median PFS had not been reached in either group (Table 1). At 18 months, the probability of PFS was 93% (95% CI 90–95) with daratumumab combination therapy and 85% (95% CI 81–88) with the active comparator [30]. Prespecified subgroup analyses of PFS indicated that the benefit of daratumumab combination therapy over the active comparator was generally consistent across subgroups (all hazard ratios < 1) [30, 32]. Median overall survival (OS) from first randomization regardless of second randomization had not been reached with either treatment at data cut-off (hazard ratio 0.43; 95% CI 0.23–0.80), with 14 deaths on study with daratumumab combination therapy and 32 with the active comparator; OS data are immature and follow-up is ongoing [30].
In an analysis using data from the CASSIOPET companion study to CASSIOPEIA, in which patients treated in CASSIOPEIA completed positron emission tomography and computed tomography (PET/CT) scans at baseline and post-consolidation (n = 184 PET-evaluable post-consolidation, excluding patients who were PET negative at baseline), the proportion of patients with post-consolidation PET/CT and minimal residual disease (by multiparametric flow cytometry) double negativity was significantly greater with daratumumab combination therapy than with the active comparator [66.7% vs 47.5%; odds ratio 2.21 (95% CI 1.20–4.07); p = 0.0105] [34].
With respect to patient-reported outcomes assessing health-related quality of life (HR-QoL) in CASSIOPEIA, daratumumab combination therapy offered some benefits over the active comparator [35]. While there were no significant differences between the treatment groups in change from baseline to post-consolidation in HR-QoL as assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30) global health status and most functional and symptom subscales, there were significantly (p ≤ 0.0416) greater improvements in pain and emotional functioning subscale scores and significantly (p = 0.0358) less decline in cognitive functioning subscale scores with daratumumab combination therapy than with the active comparator. Improvements in EuroQol 5-dimensional descriptive system utility score and visual analog scale improvements did not significantly differ between the treatment groups [35].
4 Tolerability of Daratumumab
Intravenous daratumumab added to bortezomib, thalidomide and dexamethasone had a manageable tolerability profile in adults with transplant-eligible, newly diagnosed MM in CASSIOPEIA Part 1 (Sect. 3) [30]. In the safety population (n = 536 and 538 receiving daratumumab combination therapy and the active comparator, respectively), the most common any-grade adverse events (AEs; reported in ≥ 20% of patients in either treatment group) were peripheral sensory neuropathy, constipation, asthenia, nausea, peripheral edema, neutropenia, pyrexia, paresthesia and thrombocytopenia (Fig. 3). The grade 3 or 4 AEs with the highest incidences (≥ 10% of patients in either treatment group) were neutropenia, lymphopenia, stomatitis and thrombocytopenia (Fig. 3) [30]. In patients receiving daratumumab combination therapy, complete blood cell counts should be monitored periodically according to prescribing information for the background therapies; patients with neutropenia should be monitored for signs of infection [7, 8]. A delay in daratumumab dose administration may be necessary to allow blood cell counts to recover (no dose reduction is recommended); supportive care with transfusions or growth factors should be considered [7, 8].
Serious AEs occurred in 47% of patients in each treatment group during CASSIOPEIA Part 1, with the most common of these (reported in ≥ 3% of patients in either group) being neutropenia (4% of patients receiving daratumumab combination therapy vs 1% receiving the active comparator), pneumonia (4% vs 2%), pyrexia (3% vs 4%) and pulmonary embolism (1% vs 4%) [30]. The proportion of patients discontinuing treatment due to treatment-emergent AEs was comparable between groups (7% of patients receiving daratumumab combination therapy vs 8% receiving the active comparator). Treatment-emergent AEs led to death in one patient receiving daratumumab combination therapy and in nine patients receiving the active comparator [30].
With respect to AEs of special interest, infections of any grade occurred in 65% receiving daratumumab combination therapy and 57% of patients receiving the active comparator; grade 3 or 4 infections occurred in 22% and 20%, respectively [30]. Treatment-emergent infection AEs resulted in six patients discontinuing daratumumab (1%). Second primary malignancies occurred infrequently with either treatment (2% of patients in each group) [30].
Cases of hepatitis B reactivation (some fatal) have been reported in patients treated with daratumumab [7]. In the EU, all patients should be screened for hepatitis B before treatment with daratumumab is initiated. Those with evidence of positive hepatitis B serology should be monitored for signs of hepatitis B reactivation during treatment and for ≥ 6 months after discontinuation. If reactivation develops, daratumumab should be suspended and appropriate treatment initiated [7].
Serious infusion-related reactions (including anaphylactic reactions) can occur with daratumumab [7, 8]. In CASSIOPEIA Part 1, daratumumab-related infusion reactions occurred in 35% of patients treated with daratumumab combination therapy [30]. These infusion reactions were most common during the first infusion (occurring in 27% of patients), with 2% of patients experiencing infusion reactions during their second infusion and 12% during subsequent infusions (mainly during the first infusion post-autologous SCT; 11% of 466 patients). Most infusion reactions associated with daratumumab combination therapy were of mild severity; grade 3 and 4 reactions occurred in 3% and < 1% of patients, respectively [30]. To reduce the risk of infusion-related reactions, patients should receive premedication (antihistamines, antipyretics and corticosteroids) prior to daratumumab infusions [7, 8]. Patients should be monitored throughout the infusion of daratumumab. If a reaction of any severity occurs, the infusion should be interrupted and the reaction appropriately managed [7, 8]; patients should be monitored post-infusion whilst symptoms persist [7]. Following a grade 1–3 infusion-related reaction, the infusion should be restarted at a reduced infusion rate [7, 8]. If an anaphylactic reaction or life-threatening infusion reaction occurs, daratumumab should be permanently discontinued and emergency care should be instituted. Following daratumumab infusions, oral corticosteroids should be administered to reduce the risk of delayed infusion-related reactions. Additional post-infusion medications should be considered in patients with a history of chronic obstructive pulmonary disease [7, 8].
Subcutaneous daratumumab 1800 mg co-formulated with 30,000 units hyaluronidase had a safety profile consistent with that of intravenous daratumumab 16 mg/kg when the formulations were compared as monotherapy in patients with relapsed or refractory MM (phase III COLUMBA trial) [29] and in combination with various regimens in patients with transplant-eligible newly diagnosed MM (use in combination with bortezomib, thalidomide and dexamethasone not investigated), transplant-ineligible newly diagnosed MM or relapsed or refractory MM (phase II PLEIADES trial) [36]. Infusion-related reactions were less common with the subcutaneous formulation, however [29, 36]; for example, in COLUMBA, significantly fewer subcutaneous daratumumab recipients than intravenous daratumumab recipients experienced an infusion-related reaction [13% (33/260) vs 34% (89/258); p < 0.0001], with grade 3 infusion-related reactions occurring in 2% of subcutaneous daratumumab recipients and 5% of intravenous daratumumab recipients [29]. While no infusion-related reactions with subcutaneous daratumumab resulted in treatment discontinuation or dose interruption, infusion-related reactions with intravenous daratumumab led to treatment discontinuation in 1% of patients and dose interruptions for 31% patients [29].
5 Dosage and Administration of Daratumumab
In the EU [7] and USA [8], daratumumab (in combination with bortezomib, thalidomide and dexamethasone; 4-week cycle dosing regimens) is indicated for the treatment of adult patients with newly diagnosed MM who are eligible for autologous SCT. As an intravenous infusion, the recommended dosage is 16 mg/kg body weight administered as induction therapy weekly from weeks 1 to 8 (a total of eight doses) and every 2 weeks from weeks 9 to 16 (a total of four doses), and, after high dose chemotherapy and autologous SCT, as consolidation therapy every 2 weeks from weeks 1 to 8 (a total of four doses) [7, 8]. As a subcutaneous injection, the recommended dosage is 1800 mg daratumumab and 30,000 units hyaluronidase (one 15 mL vial) administered into the abdomen over ≈ 3–5 min; the administration schedule is as described for the intravenous formulation [11]. Consult local prescribing information for detailed information regarding preparation and administration procedures, dosing schedule, management of infusion reactions, warnings and precautions, and use in special populations.
6 Place of Daratumumab in the Management of Newly Diagnosed MM in the Transplant-Eligible Setting
Clinical practice guidelines from the European Society for Medical Oncology (ESMO) recommend four to six courses of induction therapy with a triplet regimen such as bortezomib, thalidomide and dexamethasone in patients with transplant-eligible symptomatic MM [4]. Following autologous SCT, ESMO suggests there is currently insufficient evidence to support the systematic application of consolidation therapy. Instead, maintenance therapy with lenalidomide is recommended [4]. It should be noted that these guidelines were published prior to the approval of daratumumab as combination therapy in this setting. The most recent NCCN guidelines similarly recommend triplet regimens as the preferred regimens for primary therapy in transplant candidates, but state that other regimens such as daratumumab in combination with bortezomib, thalidomide and dexamethasone may be useful in certain circumstances [37]. Lenalidomide is the preferred regimen for maintenance therapy [37].
Approval of daratumumab as combination therapy in patients with newly-diagnosed, transplant-eligible MM was based on data from CASSIOPEIA Part 1 (Sect. 3). In CASSIOPEIA Part 1, the addition of daratumumab to bortezomib, thalidomide and dexamethasone significantly improved the proportion of patients achieving a stringent complete response after consolidation therapy and significantly prolonged PFS (with median PFS not reached after a median follow-up of 18.8 months) (Sect. 3). Of note, the addition of daratumumab also increased the proportion of patients with minimal residual disease-negativity (Table 1; Sect. 3), an increasingly sensitive measure of deep response that is emerging as a strong prognostic factor for both PFS and OS [38]. The relative benefits of daratumumab combination therapy versus the active comparator were generally consistent across prespecified subgroups (Sect. 3); while patients with poor prognosis (i.e. ISS disease stage III or a high-risk cytogenetic profile) were an exception with respect to stringent complete response, an improved rate of minimal residual disease-negativity nevertheless applied in these patients. HR-QoL was not negatively impacted by the additional drug and in fact, some benefits (less deterioration in cognitive functioning, improved pain and emotional functioning) were observed with daratumumab combination therapy (Sect. 3). Mature OS data from CASSIOPEIA are not yet available (Sect. 3). These data are awaited with interest and will help to further elucidate the role of daratumumab in transplant-eligible patients.
The addition of daratumumab to bortezomib, thalidomide and dexamethasone had a minimal effect on overall toxicity in patients with newly diagnosed, transplant-eligible MM (Sect. 4). Peripheral sensory neuropathy was the most common AE in each CASSIOPEIA treatment group, consistent with the pre-existing body of research on bortezomib- and thalidomide-induced peripheral neuropathy [39]. Daratumumab was associated with increased incidences of grade 3–4 haematological AEs (e.g. neutropenia, lymphopenia, thrombocytopenia; Sect. 4) [30]; dose delays and supportive care with transfusions or growth factors may be required to manage haematological AEs in daratumumab recipients (Sect. 4). Despite this, the proportion of patients who discontinued treatment due to treatment-emergent AEs was similar between treatment arms and the addition of daratumumab did not notably increase rates of grade 3–4 infections. While daratumumab-related infusion reactions occurred in more than one-third of daratumumab recipients during CASSIOPEIA Part 1, these were mostly of mild or moderate severity and typically occurred during the first infusion (Sect. 4) [30]. Successful transplantation was not affected by the addition of daratumumab, despite differences in plerixafor use and stem cell yield (Sect. 3) [30].
Head-to-head comparisons of daratumumab combined with bortezomib, thalidomide and dexamethasone versus other regimens used in the transplant-eligible setting (aside from bortezomib, thalidomide and dexamethasone) are currently lacking. In the absence of direct comparisons, matching-adjusted indirect comparisons suggest that daratumumab in combination with bortezomib, thalidomide and dexamethasone may have improved efficacy relative to other regimens (doublet and triplet) in patients with newly diagnosed, transplant-eligible MM [40,41,42,42], although these results must be interpreted with due caution. The addition of daratumumab to a similar triplet regimen (a proteasome inhibitor, an immunomodulatory agent and dexamethasone) recently improved depth of response relative to the triplet regimen alone in patients with newly diagnosed, transplant-eligible MM in a phase II trial [43]; a phase III trial of this daratumumab combination is underway.
Pharmacoeconomic concerns are particularly pertinent in MM, as regimens combining expensive new drugs are necessary and the disease course is often prolonged [44]. Adding a targeted therapy such as daratumumab to a triplet regimen can increase treatment costs considerably [44]. Consequently, robust cost-effectiveness analyses comparing regimens are required and will be important in determining the uptake of daratumumab.
Previously only available for intravenous administration, daratumumab is now available as a subcutaneous formulation in the EU and USA (Sect. 1) [11]. While subcutaneous daratumumab is not approved for use in patients with newly diagnosed, transplant-eligible MM in the USA [11], the EU approval covers use in all indications for which intravenous daratumumab is approved (including in patients with newly diagnosed, transplant-eligible MM; Sect. 1) [7]. Subcutaneous daratumumab was non-inferior to intravenous daratumumab with respect to pharmacokinetic (Sect. 2) and efficacy parameters in patients with relapsed and/or refractory MM [29]. Subcutaneous administration does, however, appear to confer improved safety; although the safety profile of subcutaneous daratumumab was generally similar to that of intravenous daratumumab in various patient populations (including patients with newly diagnosed, transplant-eligible MM, albeit not investigated as a component of the approved combination therapy), the subcutaneous formulation was associated with considerably lower rates of infusion-related reactions (Sect. 4) [29, 36]. Additionally, subcutaneous daratumumab can be administered over ≈ 3–5 min (compared with a recommended time of ≈ 7 h for the initial intravenous infusion and 3–4 h for subsequent infusions [7, 8, 29]), which may also reduce the burden on patients and healthcare resources. Indeed, real-world evidence suggests that the subcutaneous administration of biologics in oncology reduces drug preparation and administration time, thus saving provider and facility time and lowering costs relative to the intravenous administration of the same drug [45]. Furthermore, patients tend to report a preference for subcutaneous administration over intravenous administration, as well as improvements in quality of life with the former relative to the latter [45].
Evidence thus far indicates that daratumumab is an effective addition to bortezomib, thalidomide and dexamethasone in patients with newly diagnosed, transplant-eligible MM, with a generally manageable tolerability profile. The approval of daratumumab as combination therapy in patients with newly diagnosed, transplant-eligible MM expands the range of MM treatment settings in which daratumumab is an option and the availability of the subcutaneous formulation will likely be of benefit to patients.
Data Selection Daratumumab: 179 records identified
Duplicates removed | 19 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 19 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 96 |
Cited efficacy/tolerability articles | 8 |
Cited articles not efficacy/tolerability | 37 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were daratumumab, Darzalex, Daratumumab-Vtd, multiple myeloma, transplant-eligible. Records were limited to those in English language. Searches last updated 3 August 2020 | |
Change history
17 December 2020
Correction in reviewer name.
References
Al Hamed R, Bazarbachi AH, Malard F, et al. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9:44.
Fonseca R, Abouzaid S, Bonafede M, et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017;31(9):1915–21.
Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Ther Adv Hematol. 2018;9(5):123–33.
Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):52–61.
van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Janssen-Cilag International NV. DARZALEX (daratumumab): EU summary of product charateristics. 2020. https://ec.europa.eu/. Accessed 3 Aug 2020.
Janssen Biotech. DARZALEX (daratumumab): US prescribing information 2020. https://www.accessdata.fda.gov/. Accessed 3 Aug 2020.
Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77(18):2013–24.
Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–54.
Janssen Biotech. DARZALEX FASPRO™: US prescribing information. 2020. https://www.accessdata.fda.gov/. Accessed 3 Aug 2020.
McKeage K, Lyseng-Williamson KA. Daratumumab in multiple myeloma: a guide to its use as monotherapy in the EU. Drugs Ther Perspect. 2016;32:463–9.
Adams HC, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A. 2019;95(3):279–89.
de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–13.
Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–21.
Kitadate A, Kobayashi H, Abe Y, et al. Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica. 2020;105:e37–40.
Casneuf T, Xu XS, Adams HC 3rd, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–14.
Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70.
Nijhof IS, Groen RW, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015;29(10):2039–49.
Plesner T, Krejcik J. Daratumumab for the treatment of multiple myeloma. Front Immunol. 2018;9:1228.
Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66.
Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31.
Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion (Paris). 2015;55(6 Pt 2):1545–54.
McCudden C, Axel AE, Slaets D, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med. 2016;54(6):1095–104.
Xu XS, Dimopoulos MA, Sonneveld P, et al. Pharmacokinetics and exposure-response analyses of daratumumab in combination therapy regimens for patients with multiple myeloma. Adv Ther. 2018;35(11):1859–72.
Clemens PL, Yan X, Lokhorst HM, et al. Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet. 2017;56(8):915–24.
Xu XS, Yan X, Puchalski T, et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin Pharmacol Ther. 2017;101(6):721–4.
Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomized, phase 3 trial. Lancet Haematol. 2020;7(5):e370–e380380.
Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38.
Hulin C, Moreau P, Attal M, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/ dexamethasone (D-VTd): phase 3 CASSIOPEIA study [abstract no. PF598 and poster]. HemaSphere. 2019;3(Suppl 1):252.
Sonneveld P, Attal M, Perrot A, et al. Daratumumab plus bortezomib, thalidomide, and dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma (NDMM): subgroup analysis of high-risk patients (pts) in CASSIOPEIA [abstract no. OAB-003]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e2–e3.
Avet-Loiseau H, Moreau P, van der Velden VHJ, et al. Efficacy of daratumumab + bortezomib/thalidomide/dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma based on minimal residual disease status: analysis of CASSIOPEIA [abstract no. S874]. HemaSphere. 2019;3(Suppl 1):391–2.
Moreau P, Zweegman S, Perrot A, et al. Evaluation of the prognostic value of positron emission tomography-computed tomography (PET-CT) at diagnosis and follow-up in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) patients treated in the phase 3 CASSIOPEIA study: results of the CASSIOPET companion study [abstract]. Blood. 2019;134(Suppl 1).
Roussel M, Moreau P, Attal M, et al. Improvement in health-related quality of life for newly diagnosed multiple myeloma transplant-eligible patients treated with daratumumab, bortezomib, thalidomide, and dexamethasone: CASSIOPEIA study [abstract no. PS1377 and poster]. HemaSphere. 2019;3(Suppl 1):630.
Chari A, San-Miguel J, McCarthy H, et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy: PLEIADES study update. Blood. 2019;134(Suppl 1):3152.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): multiple myeloma (version 4.2020). https://www.nccn.org. Accessed 3 Aug 2020.
Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–64.
Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608.
Moreau P, Attal M, Facon T, et al. A matching-adjusted indirect comparison (MAIC) of bortezomib-thalidomide-dexamethasone (VTd) and daratumumab plus VTd (D-VTd) versus bortezomib-dexamethasone (Vd) in patients with newly diagnosed multiple myeloma (NDMM) who are transplant eligible (TE) [abstract no. SP-008]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e200-e1.
Moreau P, Attal M, Facon T, et al. A matching-adjusted indirect comparison (MAIC) of daratumumab-bortezomib-thalidomide-dexamethasone (D-VTd) versus bortezomib-lenalidomide-dexamethasone (VRd) in patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant eligible [abstract no. SP-007]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e199-e200.
Sonneveld P, Dejoie T, Zweegman S, et al. A matching-adjusted indirect comparison (MAIC) of bortezomib-thalidomide-dexamethasone (VTd) and daratumumab plus VTd (D-VTd) versus bortezomib-cyclophosphamide-dexamethasone (VCd) in patients (pts) with newly diagnosed multiple myeloma (NDMM) who are transplant eligible (TE) [abstract no. SP-009]. Clin Lymphoma Myeloma Leuk. 2019;19(Suppl 10):e201-e2.
Voorhees PM, Kaufman JL, Laubach JP, et al. Daratumumab, lenalidomide, bortezomib & dexamethasone for transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Blood. 2020. https://doi.org/10.1182/blood.2020005288.
Rajkumar SV, Harousseau JL. Next-generation multiple myeloma treatment: a pharmacoeconomic perspective. Blood. 2016;128(24):2757–64.
Anderson KC, Landgren O, Arend RC, et al. Humanistic and economic impact of subcutaneous versus intravenous administration of oncology biologics. Future Oncol. 2019;15(28):3267–81.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yvette N. Lamb is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Ethics approval, Consent to participate and consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12678944.
The manuscript was reviewed by: N. Callander, University of Wisconsin Carbone Cancer Center, Madison, WI, USA; C. Cerchione, Hematology Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; S. Knop, Medizinische Klinik und Poliklinik II, Universitätsklinikum Würzburg, Würzburg, Germany; S. J. Kumar, Hematology and Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Daratumumab: A Review in Combination Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma. Drugs 80, 1455–1464 (2020). https://doi.org/10.1007/s40265-020-01385-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01385-x