Abstract
Purpose
Combined therapy is considered when monotherapy fails to control blood pressure (BP). This study evaluated the effects of combined treatment with telmisartan (an angiotensin receptor blocker, ARB) and perindopril (an angiotensin-converting enzyme inhibitor, ACEI) on BP and cardiovascular damage.
Methods
Spontaneously hypertensive rats (SHRs) were treated with telmisartan and perindopril at usual (high, average) or low (1/4 of the usual dose) doses, alone or in combination. BP and heart rate (HR) were measured using a telemetry system. Ligation-induced myocardial ischemia/reperfusion-injured SHRs were used to measure the infarct area and endothelial nitric oxide synthase (eNOS) expression in the ligated vessel area in response to combination treatment. The effects of the two drugs, alone or in combination, on neointima hyperplasia inhibition in cuff-placed C57BL/6 mice were evaluated.
Results
Combining the two drugs at a fixed dose resulted in the most significant reduction in BP; however, even the low-dose combination resulted in, without reflex tachycardia, a much greater reduction than the usual dose of perindopril and a similar reduction to the usual dose of telmisartan. The usual dose of each drug alone, but not in combination, reduced the infarct area. The drugs increased eNOS expression individually and in combination; however, there was no synergistic effect between the combined and alone groups.
Conclusion
The low-dose combination of telmisartan and perindopril may help effectively lower BP with no additive effect on other cardiovascular outcomes and may be a viable clinical strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood pressure (BP) is usually expressed as the ratio of the pressure exerted by the blood on the arterial walls when the heart contracts to the pressure when the heart relaxes (Oparil et al. 2018). Hypertension refers to a continuous increase in arterial BP across the body. According to most commonly used guidelines, hypertension is defined as a systolic BP (SBP) of 140 mmHg or higher and a diastolic BP (DBP) of 90 mmHg or higher (Bohm et al. 2018; Oparil et al. 2018; Son et al. 2018; Flint et al. 2019; Beaney et al. 2020). Globally, a high BP is a life-threatening cause of stroke, ischemic heart disease, other vascular diseases, and kidney disease (Lida et al. 2003; Messerli et al. 2007; Lackland and Weber 2015; Collaboration 2021).
The global incidence of hypertension is increasing every year and is projected to continue to increase, possibly due to increasing prevalence in emerging economies and the aging of the global population (Justin et al. 2022). International recommendations on hypertension management are being published continuously. The definition of prehypertension varies across societies. The American Heart Association/American College of Cardiology (AHA/ACC) considers BP between 130/80 mmHg as prehypertensive. In comparison, the European Society of Cardiology (ESC) defines BP between 130/80 mmHg and 140/90 mmHg as prehypertensive. The International Society of Hypertension (ISH) definition is generally similar to that of the European Society of Hypertension (ESH) (Kjeldsen et al. 2014; Justin et al. 2022). Patients with hypertension should have a healthy life, regardless of drug treatment. In patients with stage 1 hypertension, BP can be controlled with non-drug treatments such as weight control, exercise, sodium restriction, alcohol abstinence, and maintain a vegetarian diet (Kaplan 1984; Samadian et al. 2016; Chobanian 2017). If stage 1 hypertension cannot be controlled to less than 140 mmHg systolic or 90 mmHg diastolic, lifestyle modifications and drug therapy can be initiated (Chobanian 2017).
The renin-angiotensin-aldosterone system (RAAS) plays a vital role in the occurrence and persistence of hypertension, and treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) is recommended first (Banerjee et al. 2022; Gallo et al. 2022). ACEIs inhibit angiotensin II type 1 receptor (AT1R)- and angiotensin II type 2 receptor (AT2R)-mediated effects by inhibiting angiotensin II formation. AT1R mediates vasoconstriction, cell growth, sodium and water retention, and sympathetic activation, while AT2R exerts vasodilatory and antiproliferative effects (Dasgupta and Zhang 2011; Forrester et al. 2018; Gallo et al. 2022).
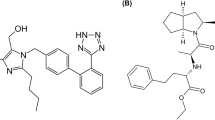
Combination therapy is recommended for pharmacological intervention to improve compliance and persistence. This may include diuretics (thiazides or thiazide-like), ACEIs, ARBs, and long-acting dihydropyridine calcium channel blockers (CCBs) (Kalra et al. 2010; Campbell et al. 2022). Telmisartan is a non-peptide ARB that selectively inhibits AT1R (McClellan and Markham 1998; Lai et al. 2020). Perindopril, an ACEI, reportedly offers a long-lasting BP-lowering effect, vascular protection (improvement in endothelial function and reduction in wall stiffness), and reduction in BP variability (Ghiadoni 2011; Hodzic et al. 2020). This study combined telmisartan, an ARB, and perindopril, an ACEI, to verify their synergistic effects on lowering BP and preventing cardiovascular complications in animals.
Materials and methods
Materials
Telmisartan and perindopril were purchased from Masung Co. (Seongnam, Korea). Normal saline was purchased from Daihan Pharm. Co. (Seoul, Korea). Sodium pentobarbital was purchased from Hanlim Pharm. Co. (Seoul, Korea). Formaldehyde was purchased from Samchun Pure Chemical Co. (Seoul, Korea). Polyethylene tubing-50 (PE-50) was purchased from Becton, Dickinson & Co. (Franklin Lakes, NJ, USA). Mouse anti-bromodeoxyuridine (BrdU) monoclonal antibody was purchased from Chemicon International, Inc. (Temecula, CA, USA). Anti-mouse IgG antibody was purchased from Vector Laboratories (Burlingame, CA, USA).
Animal models
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All the animal experiments were approved by the Chungnam National University Animal Care and Use Committee (2009-02-19). Male spontaneously hypertensive rats (SHRs) aged 7–8 weeks (200–220 g) and male C57BL/6 mice aged 5 weeks (25–28 g) were purchased from Orient Bio, Inc. (Seongnam, Korea). The animals were raised under a 12 h/12 h dark/light cycle at constant temperature (22 ± 2 ℃) and humidity (50 ± 5%).
Experimental design
Drug doses were calculated based on the average human equivalent dose of each drug (Table 1) (Nair and Jacob 2016; Park et al. 2020). To evaluate changes in BP, a telemetry system was applied to each SHR administered according to the dosing protocol (Table 2), as described previously (Shin et al. 2009; Park et al. 2020). The BP and heart rate (HR) were monitored immediately after drug or vehicle administration. The subsequent drug was administered with a sufficiently recovered SBP (approximately 200 mmHg) at each HR. Table 3 shows the effects of cuff-induced vascular remodeling in C57BL/6 mice.
Measurement of BP and HR
Changes in BP and HR were measured using a telemetry system (Data Sciences International, New Brighton, MN, USA), as described previously (Shin et al. 2009; Lee et al. 2010). The transmitter was surgically inserted into the abdominal artery of SHR. After two weeks of recovery, SBP, mean arterial pressure (MAP), and HR were measured before drug administration (baseline). BP measurement was performed in SHRs using a crossover dosing protocol; all SHRs (n = 8) used in the experiment received all drugs once each, according to the dosing protocol as described previously (Park et al. 2020). Telemetric hemodynamic data were collected at 10-s intervals every 5 min. Change in SBP were recorded at 3–4, 7–8, 11–12, and 23–24 h after drug administration.
Measurement of myocardial ischemia/reperfusion (MI/R) injury
To examine the cardioprotective effects, we used SHRs that had completed a BP-lowering experiment to focus on increased cardiac comorbidities caused by elevated BP. The combined effects of telmisartan and perindopril on cardiovascular injury were evaluated using a ligation-induced MI/R model as described previously (Park et al. 2014, 2020). The SHRs were anesthetized with pentobarbital (60 mg/kg, intraperitoneal injection), and a rodent ventilator (SAR 830/P Ventilator; CWE, Inc., Ardmore, PA, USA) was inserted into the trachea of each rat. Mechanical ventilation was performed at a tidal volume of 1 mL and a respiratory rate of 60 breaths/min. After opening the chest and exposing the heart through a left thoracotomy in the 5th and 6th intercostal space, a suture thread (5/0 silk) was placed around the left anterior descending coronary artery (LAD). After stabilization for 20 min, the vehicle or drug was injected into each experimental animal through the femoral vein (intravenous bolus). After 15 min, the suture thread placed around the LAD was completely tightened, and ischemia was induced for 30 min. Reperfusion was allowed for 2.5 h by releasing the sutures. After reperfusion, the LAD was closed again, and 2 mL of 1% Evans blue (Sigma-Aldrich, Korea) was administered to the femoral vein through the catheter. To analyze the area at risk of infarction in the left ventricle (AAR/LV), images of a 2-mm-thick heart slice (from the left ventricle just below the ligation site) were observed. To assess the infarct zone (IZ), the same heart slice was incubated with 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, Korea) in phosphate buffer (pH 7.4) at 37 ℃ for 15 min and then fixed overnight in 10% formalin solution. The AAR/LV and IZ/AAR ratios were calculated.
Measurement of endothelial nitric oxide synthase (eNOS)
To evaluate the combined effect of telmisartan and perindopril on eNOS expression in vascular tissue, eNOS expression was measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions and expressed as a percentage (Park et al. 2020).
Construction of cuff-induced vascular remodeling
To evaluate the combined effect of telmisartan and perindopril on vascular intima thickening, a cuff was placed in the left femoral artery of C57BL/6 mice, as described previously (Yi et al. 2010). A polyethylene tube (2-mm long PE-50 tube, 0.58 mm inner diameter) was placed around the left femoral artery. Additionally, the mice were administered telmisartan and perindopril for 2 weeks. The mice were euthanized using an excess dose of pentobarbital, and the cuffed femoral artery was removed and fixed in 4% formalin for 12 h. After constructing the paraffin blocks, sections were cut to a thickness of 5 mm. For histological analysis, the femoral arteries were stained with hematoxylin & eosin, and combined Masson’s elastin. The ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to calculate the intimal and medial areas of the cuffed artery.
Measurement of BrdU incorporation
The BrdU incorporation in the left femoral artery was detected using anti-BrdU antibodies (BrdU Cell Proliferation Kit, Amersham, UK) (Yi et al. 2010; Park et al. 2020). The cell proliferation in the intimal and medial layers was generated by calculating the ratio of BrdU-positive cell nuclei to total nuclei.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Student’s t test or one-way analysis of variance (ANOVA) with Dunnett’s test was performed to determine statistical significance using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Statistical significance was set at p < 0.05 difference.
Results
The combination effect of telmisartan and perindopril on BP and HR in SHR
To evaluate the combined effect of telmisartan and perindopril on hypertension, SBP, MAP, and HR were measured in telemetered SHRs. The P-L (low-dose perindopril), P-H (average- dose perindopril), T-L (low-dose telmisartan), and T-H (average-dose telmisartan) groups had significantly decreased SBP (Fig. 1a) and MAP (Fig. 1b) compared with the V1 (normal saline) or V2 (0.15 N NaOH) groups. However, no significant differences were observed between the P-H and P-L groups. Interestingly, the PT-L (low-dose combination of telmisartan and perindopril) group showed a higher BP-lowering effect than the P-L and PT-H (average-dose combination of telmisartan and perindopril) groups. Moreover, the BP-lowering effect of the PT-L group was similar to that of the T-H group (Fig. 1a, b). No significant differences were found between the T-H and PT-H groups or between the T-L and PT-L groups. Changes in SBP were the highest at 7–8 h and showed a similar trend between the PT-H and PT-L groups (Fig. 1c).
Effect of single or combined treatment with telmisartan and perindopril on systolic blood pressure (SBP), mean arterial pressure (MAP), and heart rate (HR) in telemetered spontaneously hypertensive rats (SHRs). (a). Effects of single or combined administration of telmisartan and perindopril on SBP. ***p < 0.001 vs. V1 or V2 group, ###p < 0.001 vs. P-H group, $$p < 0.01 vs. P-L group. (b). Effects of telmisartan and perindopril alone or in combination on MAP. *** p < 0.001 vs. V1 or V2 group, ###p < 0.001 vs. P-H group, $$$p < 0.001 vs. P-L group. (c). Changes in SBP after 24 h of drug administration. (d). Effects of telmisartan and perindopril alone or in combination on HR. Measurements of SBP, MAP, and HR was described under the Materials and methods section. V1 (0.9% NaCl, vehicle for perindopril), V2 (0.15 N NaOH, vehicle for telmisartan), P-H (high-dose perindopril-treated), T-H (high-dose telmisartan-treated), PT-H (high-dose combination-treated), P-L (low-dose perindopril-treated), T-L (low-dose telmisartan-treated), PT-L (low-dose combination-treated)
In general, administering BP-lowering agents such as nitrates, α1-adrenoceptor antagonists, and dihydropyridine CCBs increases HR to compensate for the drop in BP, which is known as reflex tachycardia (O’Rourke et al. 2007). The results shown in Fig. 1d presented that none of the drugs used in this experiment induced reflex tachycardia after either single or combined administration, except for the P-H group.
The combination effect of telmisartan and perindopril on MI/R injury in coronary ligation model
The T-H and P-H groups showed no significant change in the AAR/LV ratio compared with the V1 and V2 groups, respectively, and the PT-H group also showed no significant change compared with the T-H or P-H groups (Fig. 2a). In addition, the IZ/AAR ratio after T-H or P-H treatment was significantly reduced; however, the PT-H group showed no significant reduction compared with the drug-alone treatment group. The expression of eNOS in the coronary vascular tissues was significantly higher in the P-H, T-H, and PT-H groups than in the V1 and V2 groups (Fig. 2b). However, there was no significant increase in the PT-H group compared to monotherapy with each drug (P-H or T-H group).
Effects of single or combined treatment with telmisartan and perindopril on myocardial ischemia/reperfusion (MI/R) injury in spontaneously hypertensive rats (SHRs). (a). Effects of telmisartan and perindopril alone or in combination on the area at risk of infarction in the left ventricle (AAR/LV) and the ratio of the infarct zone (IZ) in the AAR. *p < 0.05 and ***p < 0.001 vs. V1 or V2 group. (b). Effects of telmisartan and perindopril alone or in combination on endothelial nitric oxide synthase (eNOS) expression in coronary arterial tissues including occlude sites in SHRs. Construction of MI/R injury model and measurement of eNOS were described under the Materials and methods section. *p < 0.05 vs. V1 group, ***p < 0.001 vs. V1 or V2 group. V1 (0.9% NaCl, vehicle for perindopril), V2 (0.15 N NaOH, vehicle for telmisartan), P-H (high-dose perindopril-treated), T-H (high-dose telmisartan-treated), PT-H (high-dose combination-treated)
The combination effect of telmisartan and perindopril on vascular remodeling in a cuff-induced injury model
Cuff placement induced an increase in the area of the intima and media layers compared to the control group (normal saline), and monotherapy with each drug induced a decrease in the intima area; however, the combination of the two drugs showed only a tendency to decrease without a significant reduction (Fig. 3a, b). Consistent with the above results, the BrdU index increased after cuff placement in both the intima and media layers (Fig. 3c, d). The increased BrdU index showed a tendency to decrease in both the monotherapy and combination therapy groups; however, only the index from the combined treatment group showed statistical significance in the intima layer (Fig. 3c). These findings suggest that the decrease in the BrdU index in the combination treatment group did not show a significant synergistic effect compared to the single-administration group. The BrdU index in the media layer showed a significant decrease in both the drug-alone and combination groups; however, there was no significant difference among the groups (Fig. 3d).
Effects of single or combined treatment with telmisartan and perindopril on cuff-induced neointimal formation and DNA synthesis in C57BL/6 mice. The intimal (a) and medial (b) layers were stained with hematoxylin & eosin (H&E) and combined Masson’s elastin (CME) to determine the thickness, and histological analysis was performed. DNA synthesis in the intimal (c) and medial (d) layers of the femoral artery was measured using a bromodeoxyuridine (BrdU) incorporation assay. The tissue staining and BrdU assay were described under the Materials and methods section. *p < 0.05, **p < 0.01 vs. cuff-placement, #p < 0.05 vs. telmisartan or perindopril alone
Discussion
In 2021, the World Health Organization (WHO) updated the guidelines for the pharmacological treatment of adult hypertension. Pharmacological treatment was initiated if the SBP was 140 or more than 90 mmHg. As first-line drugs, thiazide and thiazide-like agents, ACEIs, ARBs, and long-acting dihydropyridine CCBs are the initial treatments for patients (Campbell et al. 2022). Two or more antihypertensive agents with different mechanisms are required in patients with hypertension that cannot be controlled with a single drug. ACEIs (or ARBs)/CCBs, ACEIs (or ARBs)/ thiazides (or thiazide-like diuretics), CCBs/thiazides (or thiazide-like diuretics), CCBs/β-blockers, β-blockers/thiazides (or thiazide-like diuretics), and ACEIs (or ARBs)/CCBs/thiazides (or thiazide-like diuretics) are acceptable combinations of antihypertensive drugs (Palatini 2005; Guerrero-García and Rubio-Guerra 2018).
ACEIs are recommended as the first-line drug in most guidelines, and ARBs are considered an alternative for ACEI intolerance (Caldeira et al. 2012; Morales et al. 2021). This study evaluated the antihypertensive and cardiovascular protective effects of the combination of telmisartan (an ARB) and perindopril (an ACEI). In this study, when a low dose (1/4 of the usual dose) of telmisartan and perindopril was co-administered, significant antihypertensive effects similar to those of the usual dose of telmisartan were observed. However, when the normal doses of the two drugs were co-administered, no additional preventive effects on cardiovascular damage were observed. In patients with vascular disease or high-risk diabetes, the combination of telmisartan and ramipril (an ACEI) results in more adverse events without increased benefits (Investigators et al. 2008). Previous data from our research group reported that fixed-dose combination therapy with losartan (an ARB) and ramipril could prevent myocardial infarction and vascular remodeling with a more significant BP-lowering effect (Park et al. 2020). We also reported that telmisartan and ramipril combination therapy could effectively treat hypertension and its complications of hypertensive disorders (Lee et al. 2022). Subsequent reports showed that ramipril plus telmisartan did not increase the incidence of stroke, other major cardiovascular diseases, or kidney disease in diabetic patients (Mann et al. 2013). Therefore, although the combined administration of ARBs and ACEIs is not yet recommended in clinical practice, it is vital to establish a combination strategy through well-controlled studies based on the characteristics of each drug.
Although this study showed no therapeutic benefit of the combination of telmisartan and perindopril on cardiovascular events other than BP-lowering, one encouraging finding was that the combination of a quarter of the usual dose of each drug delivered a BP-lowering effect superior to the usual dose of perindopril and comparable to the usual dose of telmisartan. Furthermore, reflex tachycardia caused by the administration of BP-lowering drugs was not observed with this low-dose combination. Previous reports from our group have shown that reflex tachycardia is not observed in SHRs treated with either high- or low-dose telmisartan (Lee et al. 2010, 2022; Jo et al. 2021). In the present study, perindopril produced mild reflex tachycardia only at high (average) concentrations; no significant change in HR was observed at lower doses. Other research groups have shown that perindopril caused little change in HR (Ferrari et al. 2005), while telmisartan led to a change in HR with antihypertensive effects (Maeda et al. 2010). Therefore, the results of this study suggest that a combination of telmisartan and perindopril can be used for BP-lowering purposes at low doses to minimize the adverse drug reactions (ADRs) that may occur with the use of the usual doses of each drug as much as possible.
Conclusion
In summary, when telmisartan and perindopril were administered to SHRs in combination at either the usual (high, average) dose or a low dose (1/4 of the usual dose), the low-dose combination produced superior BP-lowering effects compared to the usual dose of perindopril alone and similar BP-lowering effects to the usual dose of telmisartan alone. In MI/R injury-induced SHRs, the combined administration of the two drugs at regular doses did not result in an improvement in AAR/LV and IZ/AAR and failed to show synergism in increasing eNOS expression in coronary tissues compared to either drug individually. In cuff-induced neointima hyperplasia C57BL/6 mice, the combination of the two drugs failed to show synergistic effects on the reduction of intima and media areas and BrdU index compared to each drug individually. Therefore, combining the two drugs is ineffective in preventing or treating complications of hypertension; however, when administered in combination at low doses to lower BP, it is considered suitable for effectively lowering BP without causing reflex tachycardia.
References
Banerjee D, Winocour P, Chowdhury TA, De P, Wahba M, Montero R, Fogarty D, Frankel AH, Karalliedde J, Mark PB, Patel DC, Pokrajac A, Sharif A, Zac-Varghese S, Bain S, Dasgupta I, Association of British Clinical D, The Renal A (2022) Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrol 23:9
Beaney T, Schutte AE, Stergiou GS, Borghi C, Burger D, Charchar F, Cro S, Diaz A, Damasceno A, Espeche W, Jose AP, Khan N, Kokubo Y, Maheshwari A, Marin MJ, More A, Neupane D, Nilsson P, Patil M, Prabhakaran D, Ramirez A, Rodriguez P, Schlaich M, Steckelings UM, Tomaszewski M, Unger T, Wainford R, Wang J, Williams B, Poulter NR, MMM Investigators (2020) May Measurement Month 2019: the global blood pressure screening campaign of the International Society of Hypertension. Hypertension 76:333–341
Bohm M, Schumacher H, Teo KK, Lonn E, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder R, Weber M, Sliwa K, Williams B, Yusuf S (2018) Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J 39:3105–3114
Caldeira D, David C, Sampaio C (2012) Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs 12:263–277
Campbell NRC, Paccot Burnens M, Whelton PK, Angell SY, Jaffe MG, Cohn J, Espinosa Brito A, Irazola V, Brettler JW, Roccella EJ, Maldonado Figueredo JI, Rosende A, Ordunez P (2022) 2021 World Health Organization guideline on pharmacological treatment of hypertension: policy implications for the region of the Americas. Lancet Reg Health Am 9:None
Chobanian AV (2017) Guidelines for the management of hypertension. Med Clin North Am 101:219–227
Collaboration NCDRF (2021) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398:957–980
Dasgupta C, Zhang L (2011) Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today 16:22–34
Ferrari R, Pasanisi G, Notarstefano P, Campo G, Gardini E, Ceconi C (2005) Specific properties and effect of perindopril in controlling the renin-angiotensin system. Am J Hypertens 18:142S–154S
Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL (2019) Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med 381:243–251
Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S (2018) Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98:1627–1738
Gallo G, Volpe M, Rubattu S (2022) Angiotensin receptor blockers in the management of hypertension: a real-world perspective and current recommendations. Vasc Health Risk Manag 18:507–515
Ghiadoni L (2011) Perindopril for the treatment of hypertension. Expert Opin Pharmacother 12:1633–1642
Guerrero-García C, Rubio-Guerra AF (2018) Combination therapy in the treatment of hypertension. Drugs Context 7:212531
Hodzic E, Pecar E, Dzubur A, Smajic E, Hondo Z, Delic D, Rustempasic E (2020) Efficacy and safety of perindopril in patients with essential hypertension. Mater Sociomed 32:4–9
Investigators ONTARGET, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358:1547–1559
Jo JH, Lee DH, Han JH, Lee M, Jang KW, Myung CS (2021) Effects of combination treatment with cilnidipine and telmisartan on hypertension, cardiovascular injury, and high blood glucose. J Pharm Investig 51:337–346
Justin J, Fayol A, Bruno RM, Khettab H, Boutouyrie P (2022) International guidelines for hypertension: Resemblance, divergence and inconsistencies. J Clin Med 11:1975
Kalra S, Kalra B, Agrawal N (2010) Combination therapy in hypertension: an update. Diabetol Metab Syndr 2:44
Kaplan NM (1984) Use of non-drug therapy in treating hypertension. Am J Med 77:96–101
Kjeldsen S, Feldman RD, Lisheng L, Mourad JJ, Chiang CE, Zhang W, Wu Z, Li W, Williams B (2014) Updated national and international hypertension guidelines: a review of current recommendations. Drugs 74:2033–2051
Lackland DT, Weber MA (2015) Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol 31:569–571
Lai MC, Wu SN, Huang CW (2020) Telmisartan, an antagonist of angiotensin II receptors, accentuates voltage-gated Na+ currents and hippocampal neuronal excitability. Front Neurosci 14:902
Lee JJ, Shin CY, Park HJ, Zhang WY, Kim Y, Kim IS, Lee KH, Myung CS (2010) Drug synergism of antihypertensive action in combination of telmisartan with lercanidipine in spontaneous hypertensive rats. Arch Pharm Res 33:1411–1418
Lee DH, Kim MT, Lee HW, Han JH, Myung CS (2022) The combined effects of telmisartan and ramipril on hypertension and cardiovascular injury. J Pharm Investig 52:443–451
Lida M, Ueda K, Okayama A, Kodama K, Sawai K, Shibata S, Tanaka S, Keijnkai T, Horibe H, Minowa M, Yanagawa H, Hashimoto T, Nippon Data 80 Research G (2003) Impact of elevated blood pressure on mortality from all causes, cardiovascular diseases, heart disease and stroke among japanese: 14 year follow-up of randomly selected population from japanese - Nippon data 80. J Hum Hypertens 17:851–857
Maeda S, Nishizaki M, Yamawake N, Ashikaga T, Ihara K, Murai T, Fujii H, Sakurada H, Hiraoka M, Isobe M (2010) Effect of high-dose telmisartan on the prevention of recurrent atrial fibrillation in hypertensive patients. J Atr Fibrillation 3:289
Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, Sleight P, Teo KK, Yusuf S, ONTARGET Investigators (2013) Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 31:414–421
McClellan KJ, Markham A (1998) Telmisartan Drugs 56:1039–1044 discussion 1045 – 1036
Messerli FH, Williams B, Ritz E (2007) Essential hypertension. Lancet 370:591–603
Morales DR, Lipworth BJ, Donnan PT, Wang H (2021) Intolerance to angiotensin converting enzyme inhibitors in asthma and the general ppopulation: a UK population-based cohort study. J Allergy Clin Immunol Pract 9:3431–3439 e3434
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
O’Rourke ST (2007) Antianginal actions of β-adrenoceptor antagonists. Am J Pharm Edu 71:95
Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cifkova R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK (2018) Hypertension. Nat Rev Dis Primers 4:18014
Palatini P (2005) Combination therapy in the management of hypertension: focus on angiotensin receptor blockers combined with diuretics. J Clin Hypertens (Greenwich) 7:96–101
Park ES, Kang DH, Yang MK, Kang JC, Jang YC, Park JS, Kim SK, Shin HS (2014) Cordycepin, 3’-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovasc Toxicol 14:1–9
Park HS, Lee DH, Han JH, Jung SH, Lee M, Jang KW, Myung CS (2020) The effects of combined treatment of losartan and ramipril on hypertension and related complications. J Pharm Investig 50:573–581
Samadian F, Dalili N, Jamalian A (2016) Lifestyle modifications to prevent and control hypertension. Iran J Kidney Dis 10:237–263
Shin CY, Choi WS, Yi I, Nan MH, Myung CS (2009) Synergistic decrease in blood pressure by captopril combined with losartan in spontaneous hypertensive rats. Arch Pharm Res 32:955–962
Son JS, Choi S, Kim K, Kim SM, Choi D, Lee G, Jeong SM, Park SY, Kim YY, Yun JM, Park SM (2018) Association of blood pressure classification in korean young adults according to the 2017 American College of Cardiology/American Heart Association Guidelines with subsequent Cardiovascular Disease events. JAMA 320:1783–1792
Yi I, Lee JJ, Park JS, Zhang WY, Kim IS, Kim Y, Shin CY, Kim HS, Myung CS (2010) Enhanced effect of losartan and rosuvastatin on neointima hyperplasia. Arch Pharm Res 33:593–600
Acknowledgements
This work was supported by BK21 FOUR Program by Chungnam National University Research Grant, 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (D.-H. Lee, Y.-E. Choi, S.P. Lee, H.-W. Lee, Y.W. Sim, J.-S. Park, and C.-S. Myung) declare that they have no conflict of interest.
Research involving human and animal rights
This work complies with all ethical standards. All animal experiments were approved by the Chungnam National University Animal Care and Use Committee (2009-2-19).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, DH., Choi, YE., Lee, S.P. et al. Effects of telmisartan and perindopril combination on hypertension and cardiovascular damage. J. Pharm. Investig. 53, 563–570 (2023). https://doi.org/10.1007/s40005-023-00624-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00624-z