Abstract
Background
Between 5% and 20% of patients treated with angiotensin-converting enzyme inhibitors (ACE inhibitors) develop intolerance. Angiotensin II type 1 receptor antagonists (angiotensin receptor blockers [ARBs]) can be used as an alternative treatment.
Objective
In this study we aimed to evaluate the tolerability of ARBs in patients with intolerance to ACE inhibitors.
Data Sources
The electronic databases PubMed, MEDLINE/EMBASE via Dialog, CENTRAL, and ISI Web of Knowledge were searched.
Study Selection
Randomized controlled trials (RCTs) evaluating ARBs in patients with intolerance to ACE inhibitors were selected.
Data Synthesis
Risk ratio (RR) and 95% confidence intervals (CIs) were estimated assuming the random effects method. We found 11 RCTs comparing ARBs with ACE inhibitors, diuretics, or placebo, and one RCT comparing high-dose versus low-dose ARB.
Results
ARBs had fewer cough events versus ACE inhibitors (RR 0.37; 95% CI 0.28, 0.48). ARBs had drug discontinuation (RR 0.99; 95% CI 0.84, 1.17) and cough risk (RR 1.01; 95% CI 0.74, 1.39) rates similar to placebo. Angioedema risk with ARBs was also similar to placebo (RR 1.62; 95% CI 0.17, 15.79). Compared with placebo, hypotension (RR 2.63; 95% CI 1.77, 3.92), renal dysfunction (RR 2.07; 95% CI 1.45, 2.95) and hyperkalemia (RR 3.37; 95% CI 1.60, 7.11) were more frequent with ARBs.
Conclusions
ACE inhibitor rechallenge should be discouraged in patients with previous intolerance to ACE inhibitors due to a higher risk of cough. ARBs had cough and angioedema incidences similar to placebo. Despite a significantly higher incidence of hypotension, renal dysfunction and hyperkalemia, discontinuation of ARBs was similar to placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
The renin-angiotensin-aldosterone system (RAAS) plays an important role in the development and progression of cardiovascular diseases.[1] Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor antagonists (angiotensin receptor blockers [ARBs]) are used to block the RAAS and to prevent cardiovascular events.[1–3]
About 5–20% of patients treated with ACE inhibitors have intolerance to these drugs, frequently due to dry cough, angioedema, hypotension, hyperkalemia, or renal dysfunction.[4–7]
In ACE inhibitor-intolerant patients, ARBs can be prescribed to maintain RAAS blockade in order to decrease cardiovascular risk.[8] ARBs are safe drugs with a general safety profile at least similar to that of ACE inhibitors, as the VALIANT (Valsartan in Acute Myocardial Infarction Trial), OPTIMAAL (Optimal Therapy in Myocardial Infarction with the Angiotensin II Antagonist Losartan), and ONTARGET (the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) trials have shown.[9–11] Some adverse effects result from RAAS blockade and patients intolerant to ACE inhibitors may be more likely to experience these adverse effects. In the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) program, the incidence of candesartan discontinuation in patients intolerant to ACE inhibitors in the CHARM-Alternative trial was 21.5% compared with 17.8% in the CHARM-Preserved population, which included tolerant and intolerant patients, respectively.[12,13]
The literature lacks a systematic review and discussion about these issues in this specific population. As more extended information on safety and tolerability of ARBs in these patients is required, we aimed to add powered and more precise data to the actual literature on this topic. For this purpose we systematically reviewed the safety profile of ARBs in patients with intolerance to ACE inhibitors through analysis of randomized controlled trials (RCTs).
2. Methods
2.1 Eligibility Criteria
We searched for RCTs evaluating ARBs in patients with intolerance to ACE inhibitors. Controls were allowed to be other ARBs, different ARB dose, another active drug, or placebo.
We accepted each study definition of intolerance to ACE inhibitors regardless of specific type of manifestation. We did not establish any limits regarding language or follow-up. We focused on clinical and laboratory adverse events of interest.
Analysed outcomes were discontinuation due to adverse events, cough, angioedema/anaphylaxis, hypotension, renal dysfunction, and hyperkalemia. The relapse of the adverse event relative to the baseline manifestation of ACE inhibitor intolerance was also studied.
2.2 Information Sources and Search Method
The following databases were searched to retrieve studies: PubMed, MEDLINE/EMBASE via Dialog, CENTRAL, and ISI Web of Knowledge. The search was initiated in October 2010, and the last search to update the review with new trials was performed in March 2011. References of obtained studies were also searched for any missing trials. See Supplemental Digital Content 1, for details of the search method, links.adisonline.com.
2.3 Studies and Data Selection
The title and abstract of obtained trial citations were screened by two investigators. Full-text assessment of potentially eligible studies determined inclusion in the systematic review and meta-analysis in accordance with outlined criteria.
We extracted detailed data about analyzed interventions, characteristics of the patients, reasons for intolerance, follow-up and primary outcome, and quantitative data related to selected outcomes. Data entry into software was double-checked. All disagreements were resolved by consensus.
The reported methodological quality was assessed using the Jadad score.[14]
2.4 Quantitative Data Synthesis
RevMan software version 5.0.23 (Copenhagen, Nordic Cochrane Centre, Cochrane Collaboration) was used to calculate the risk ratio (RR). Results of individual studies and pooled analysis were expressed using 95% confidence intervals (CIs). When cells with a value of zero were present in one arm, RevMan automatically added 0.5 to each cell to perform calculations.
Meta-analyses were based on the random effects model due to the different characteristics of study populations with regard to reason for intolerance. Statistical heterogeneity was considered when I2 > 50%.
We planned to analyze outcomes according to different ARBs, ARB dose, and diseases evaluated in the included trials, particularly hypertension and heart failure.
The chi-squared (χ2) test with p-value interaction <0.05 was used to explore differences in the effects of different ARBs or different ARB dosages.
3. Results
3.1 Search
After title and abstract screening of citations obtained in PubMed, MEDLINE/EMBASE via Dialog, CENTRAL, and ISI Web of Knowledge, 27 studies were selected for full-text assessment, after which 15 studies were rejected. Two of the rejected studies were retrospective analyses of patients with previous angioedema due to ACE inhibitors, one evaluated different ACE inhibitors in patients with ACE inhibitor-induced cough, and 12 evaluated other drugs to suppress manifestations of intolerance to ACE inhibitors such as non-steroidal anti-inflammatory drugs, theophylline, capsaicin, baclofen, iron, or sodium cromoglycate.
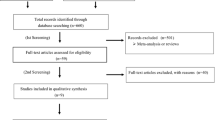
Twelve studies were eligible to include in the systematic review.[12,15–27] The data from one RCT were published in three articles.[15–17] Figure 1 illustrates the phases of study selection.
Flowchart showing study selection. = angiotensin-converting enzyme; ARB = angiotensin II type 1 receptor antagonist (angiotensin receptor blocker); NSAID = non-steroidal anti-inflammatory drug. References: Benz et al.[18]; Chan et al.[19]; Paster et al.[20]; Rake et al.[23]; Tanser et al.[22]; SPICE[25]; TRANSCEND[26]; Val-HeFT[24]; CHARM-Alternative.[12]
One included RCT, the HEAAL (Heart Failure End Point Evaluation of Angiotensin II Antagonist Losartan) study, evaluated different doses of the same ARB, losartan,[27] and the remaining 11 RCTs evaluated an ARB against other drugs/placebo in patients with intolerance to ACE inhibitors.[12,15–26] We did not find any study comparing different ARBs in this population.
3.2 Characteristics of the Studies
Seven RCTs had data for ARBs versus ACE inhibitor comparison,[15–23] and ten RCTs were used to compare ARBs with controls that were not directly related to the RAAS such as diuretics or placebo.[12,15–26] Table I summarizes the main characteristics of the trials.
Due to heterogeneity in the patients included in the trials and the use of different ARBs with different dosages, clinical heterogeneity was assumed and we performed a meta-analysis using a random effects model irrespective of the I2 value.
3.3 ARB versus ACE Inhibitor/Diuretic/Placebo
A comparison of ARBs versus ACE inhibitors included 564 patients (281 vs 283 respectively). Studies that compared ARBs with placebo or active drugs without direct effect on the RAAS evaluated 8845 patients: 4433 in the ARBs arm and 4412 patients in the control arm.
With the exception of Val-HeFT (Valsartan Heart Failure Trial), all trials enrolled patients with confirmed previous intolerance to an ACE inhibitor. The Val-HeFT substudy included patients who were not previously taking ACE inhibitors. This trial was the first to propose an ARB for cardiovascular protection in this specific population.[24] A sensitivity analysis excluding the Val-HeFT subgroup was carried out.
Population size and follow-up varied between studies. The smallest trial enrolled 84 patients and had a follow-up of 12 weeks.[19] Benz et al.[18] and Rake et al.[23] conducted trials with the shortest follow-up: 6 weeks. TRANSCEND (Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease) was the largest and the most extensive trial, involving 5296 patients with high cardiovascular risk who were followed over a mean of 56 months.[26] Across all trials, the patients’ mean ages ranged between 53.6 and 73 years. Some studies only enrolled patients with ACE inhibitor-induced cough, but in studies that admitted patients with all causes of intolerance, cough was the most common with event rates above 60%. All studies were designed to assess cough as the primary outcome with the exception of the placebo-controlled trials Val-HeFT, SPICE (Study of Patients Intolerant of Converting Enzyme Inhibitors),[25] CHARM-Alternative, and TRANSCEND.
Trials evaluating ARB versus ACE inhibitor only supplied data of withdrawals and cough. No other data were obtained for this comparison.
Reported methodological quality assessed by the Jadad scale ranged between 3 and 4 points. All trials had at least 3 points on the Jadad scale because they had randomized and blinded designs, and reported patient dropout reasons or loss to follow-up when these occurred. Trials rated at 4 points reported adequate randomization methods.
Table II shows the summary of pooled analysis and event rates according to treatments. None of the outcomes evaluated presented statistical heterogeneity.
3.3.1 Discontinuation Due to Adverse Events
Meta-analysis showed a non-significant trend towards a lower rate of ARB withdrawal due to adverse events compared with ACE inhibitors. The pooled risk ratio was 0.47 (95% CI 0.18, 1.23) [figure 2]. Most of the studies in this comparison had at least a zero-count cell or a single event in a treatment arm. Therefore, as sample sizes for both treatments were not imbalanced, we performed a meta-analysis using Peto’s odds ratio (OR) estimate in order to obtain a less biased result.[28] The pooled OR was statistically significant: 0.42 (95% CI 0.20, 0.88). Discontinuation events were similar in patients treated with ARBs, placebo (RR 0.99; 95% CI 0.84, 1.17), diuretics (RR 1.50; 95% CI 0.26, 8.52), or both placebo and diuretics (RR 0.99; 95% CI 0.85, 1.15). Figure 2 shows the forest plot of the meta-analysis.
3.3.2 Cough
Cough was the only outcome with available data in all trials. The RR of cough was 0.37 (95% CI 0.28, 0.48), favoring ARBs instead of ACE inhibitors. The incidence of cough was 67% with ACE inhibitors and, in the same trials, 24% with ARBs. Just 2.3 patients were needed to treat with ARBs instead of ACE inhibitors to prevent a drug withdrawal due to cough. This meta-analysis also showed that the cough risk was similar in patients taking an ARB instead of placebo or a diuretic. The pooled RR was 1.01 (95% CI 0.74, 1.39) for ARBs versus placebo, RR 1.01 (95% CI 0.76, 1.34) for ARBs versus diuretics, and RR 1.01 (95% CI 0.76, 1.34) for ARBs versus placebo or diuretics. The incidence of cough was 1.7% for placebo/diuretics and 1.9% with ARBs. Figure 3 shows the forest plot.
3.3.3 Angioedema
The risk of angioedema was analyzed exclusively by placebo-controlled trials. We analyzed 8320 patients with intolerance to ACE inhibitors and angioedema was a rare event, with incidences of 0.12% for ARBs and 0.07% for placebo. The RR was 1.62 (95% CI 0.17, 15.79) [figure 4] and Peto’s OR was 1.66 (95% CI 0.41, 6.63).
Patients treated with ARBs had an angioedema risk similar to those taking placebo.
3.3.4 Hypotension
Data from four studies with 8590 patients were pooled for hypotension analysis. All these trials were placebo-controlled studies. Only one study showed significant results favoring placebo.[12]
Meta-analysis demonstrated that, in comparison with placebo or diuretics, the use of ARBs in patients with previous ACE inhibitor intolerance was associated with an increased incidence of hypotensive episodes (2.3% vs 0.8%). The RR was 2.63 (95% CI 1.77, 3.92) [figure 4].
3.3.5 Renal Dysfunction
In this outcome only one of the three included trials had statistically significant results favoring placebo.[12] Pooled analysis of three placebo-controlled trials with 8224 individuals showed that ARBs may cause renal dysfunction in these patients with a RR of 2.07 (95% CI 1.45, 2.95) [figure 4]. The incidence was 2.2% for ARBs and 1.1% for placebo.
3.3.6 Hyperkalemia
In an analysis of hyperkalemia from CHARM-Alternative and TRANSCEND, both trials showed significantly lower events in the placebo arm compared with an ARB. Meta-analysis showed that ARBs are associated with a higher incidence of hyperkalemia compared with placebo The RR was 3.37 (95% CI 1.60, 7.11) [figure 4]. The rate of events was 3.3% for ARBs and 1.1% for placebo.
3.3.7 Relapse of Same Adverse Event
Table II shows the RRs of relapse of events with regard to previous cough, angioedema, hypotension, and renal dysfunction.
Relapse of cough was significantly lower in patients treated with ARBs versus ACE inhibitors (RR 0.37; 95% CI 0.28, 0.48) and the incidence was 24.2% with ARBs versus 66.8% with ACE inhibitors. Renal dysfunction relapse was significantly higher in patients taking an ARB compared with placebo (RR 1.83; 95% CI 1.01, 3.34).
In the remaining outcomes no significant relapse differences were found between ARB and control groups (figure 5).
3.4 Subgroup/Sensitivity Analysis
3.4.1 Arterial Hypertension
Comparisons between ARBs and ACE inhibitors only included trials with hypertensive patients and results presented for discontinuation due to adverse events and cough outcomes under this comparison are the same as shown above, with a significantly higher risk for cough in this group of patients with ACE inhibitors and a trend towards drug discontinuation. ARBs and placebo/diuretics share a similar risk of drug withdrawal, cough, and cough relapse in hypertensive patients. These data are detailed in table III.
Sensitivity analysis[18]
3.4.2 Heart Failure
Studies that enrolled patients with heart failure were all placebo-controlled and included only patients with systolic dysfunction. Meta-analysis pooling these patients showed that drug discontinuation, cough, and angioedema were similar between ARBs and placebo. Hypotension (RR 3.32; 95% CI 2.03, 5.45), renal dysfunction (RR 2.15; 95% CI 1.41, 3.28), and hyperkalemia (RR 6.35; 95% CI 1.88, 21.38) were significantly more frequent with ARBs. Relapse of intolerance events was based on data from the CHARM-Alternative trial and renal dysfunction relapse was more frequent with ARBs versus placebo. Detailed data are displayed in table III.
3.4.3 Val-HeFT
Inclusion of the Val-HeFT subgroup of patients not taking an ACE inhibitor was only possible in the following outcomes: discontinuation due to adverse events, cough, angioedema, and hypotension. Sensitivity analysis excluding this trial did not change the significance of analyzed harmful effects (table III).
3.4.4 Analysis According to Different ARBs and ARB Dosage
We did not find any differences in these outcomes between different ARBs (table IV).
Analysis of ARB dosage effect was only possible in four outcomes: discontinuation due to adverse events, cough, hypotension, and renal dysfunction (table V).
Valsartan and candesartan were tested with different doses in different trials. A valsartan 80 mg daily dose was not different from valsartan 160 mg with regard to drug withdrawals. Similarly, there was no difference between candesartan 8 mg, 16 mg, and 32 mg with regard to drug withdrawal. Candesartan 32 mg did not have a significantly different incidence of cough, hypotension, or renal dysfunction compared with lower dosages.
3.4.5 Pre-Specified Outcome
Tolerability of ARBs with ACE inhibitor-intolerant patients was evaluated as a primary outcome in some studies: seven evaluated cough[17–23] and one study assessed tolerability as the proportion of patients completing 3 months of treatment.[25]
All previous estimates for ARBs versus ACE inhibitor comparison only included studies primarily evaluating the tolerability.
A sensitivity analysis was performed for ARBs versus placebo/diuretics. Risks of ARB drug discontinuation (RR 1.21; 95% CI 0.72, 2.05) and cough (RR 1.08; 95% CI 0.78, 1.48) were similar to those of placebo or diuretics.
3.5 High-Dose ARB versus Low-Dose ARB
The randomized controlled trial HEAAL evaluated high-dose ARB (losartan 150 mg) versus low-dose ARB (losartan 50 mg) in 3846 patients with heart failure over 4.7 years: 1927 patients in the high-dose arm and 1919 in the low-dose arm. The Jadad score for this study was 5.
Outcomes and results are represented in figure 6.
High-dose losartan did not reduce the HEAAL study primary composite outcome all-cause mortality and hospitalization for heart failure. High-dose losartan also did not reduce individually all-cause mortality or cardiovascular mortality. The benefits of high- versus low-dose losartan were in heart failure hospitalizsations. Adverse events were significantly more frequent with high-dose losartan, particularly hypotension, renal dysfunction, and hyperkalemia, but drug discontinuation was similar between the two intervention arms.[27]
4. Discussion
In this review we focused on patients with intolerance to ACE inhibitors treated with ARBs.
ARB treatment was likely to halve the relative risk of withdrawal and had a 67% relative risk reduction in the incidence of cough compared with ACE inhibitors. A placebo-like tolerability was also observed regarding drug discontinuation, cough, and angioedema/anaphylaxis outcomes. This means that the cardiovascular protection of ARBs can be preserved without an increased risk of withdrawal or the referred adverse events.
Compared with ACE inhibitors, ARBs significantly decreased the risk of drug withdrawal when more adjusted meta-analysis was performed using Peto’s method. The relative risk of treatment discontinuation of an ARB was similar to placebo/diuretics.
A Cochrane systematic review evaluated ARBs versus placebo for primary hypertension in an undifferentiated population. In contrast to our results, the RR of withdrawals due to adverse events with ARBs was about one-third less than with placebo.[29] We studied a special population of patients that may be more prone to adverse events and drug discontinuation. In addition, the present review included placebo-controlled studies with longer follow-up than those in the Cochrane review. This allowed us to determine more accurately estimates for tolerability, concluding that the incidence of withdrawals with ARBs in ACE inhibitor-intolerant patients was similar to that seen with placebo.
Despite this favorable ARB tolerability profile, absolute risks of drug withdrawal were heterogeneous. Studies comparing ARBs with ACE inhibitors had 4.3% of event rates compared with 20.2% in those compared with placebo/diuretics. Study follow-up may have contributed to this difference because longer placebo-controlled trials had more withdrawals than short-term ACE inhibitor studies.
The incidence of cough was significantly lower with ARBs than with ACE inhibitors. This adverse event showed a reproducibility of 66.8% with an ACE inhibitor, which is consistent with the 62.7% reported in a previous study.[30] Recurrence of cough was less frequent in patients treated with ARBs, but it is important to notice that about a quarter of patients treated with ARBs will re-experience cough. Surprisingly, the incidence of cough was higher in studies with a shorter follow-up. This may be explained because these trials were designed to evaluate cough relapse in patients with previous cough with an ACE inhibitor. Most of the studies used questionnaires developed to assess this adverse event and did not mention efforts to exclude other causes of cough, with the exception of the Benz et al. study.[18]
Increased bradykinin levels were thought to be related to ACE inhibitor-induced cough because as opposed to ARBs, ACE inhibitors are kininase inhibitors. However, the use of an ACE inhibitor or ARB leads to similar bradykinin levels in the general population.[31,32] ACE inhibitor-induced cough is likely to be multifactorial and idiosyncratic. It may be associated with substance P, increased levels of kinins due to functional genetic polymorphisms of ACE, or bradykinin-receptor polymorphisms in prone patients.[4,33,34] Thus, it was expected that ACE inhibitors would have a significantly higher incidence of cough compared with ARBs in this specific population.
An angioedema event rate of 0.12% in patients with ACE inhibitor intolerance treated with ARBs was similar to the incidence of angioedema in the placebo arm. In patients with previous angioedema, the event rate was 4.1%, which was low compared with data from a previous meta-analysis that showed a 9.2% risk of probable angioedema.[35] In the same meta-analysis, the rate of confirmed cases of angioedema was 3.5%.[35] The referred incidence partially determined by observational data was similar to that obtained in our study. In the CHARM-Alternative study, three patients under ARB treatment had an angioedema episode; however, only one patient stopped candesartan.[12] This idiosyncratic event may be severe in some cases and can be lethal. The understanding of its pathophysiology is important for the development of effective drugs. The role of bradykinin in the pathogenesis of this adverse event seems substantial. Icatibant, an injected bradykinin antagonist that does not interact with angiotensin II and substance P receptors, showed promising results in symptom relief and avoidance of invasive procedures such as tracheotomy and intubation.[36,37]
Hypotension, renal dysfunction, and hyperkalemia events were superior in the ARB arms compared with placebo. Reduced hemodynamic and endocrine effects of angiotensin II are the probable causes of these adverse events. ARB use may be accompanied by small increases of creatinine but renal hemodynamics may be improved with long-term therapy and, excluding the most severe cases, discontinuation of ARBs should be discouraged.[38,39] However, hypotension and inadequately decreased resistance of the vascular bed in the absence of a homeostatic function of angiotensin II can lead to renal dysfunction in patients taking ARBs.[40] Our review shows that patients with intolerance to ACE inhibitors are not protected from these adverse effects when treated with ARBs. Hyperkalemia is also an adverse effect that these patients taking ARBs are significantly more susceptible to experience compared with placebo. Interference with the RAAS may result in functional hypoaldosteronism leading to hyperkalemia.[41]
Data analysed based on previous specific manifestations of intolerance suggest that patients with previous renal dysfunction are likely to re-experience the same adverse event. Renal dysfunction relapse occurred in 19.6% of patients treated with ARBs. The remaining outcomes did not show significant differences between ARBs and other non-ACE inhibitor comparators. Data were scarce and most of the weighted results were based on the CHARM-Alternative study.[12] In patients with previous cough there was a higher risk of cough relapse if they continued to take ACE inhibitors instead of ARBs.
Previous studies showed that the cumulative RAAS blockade with both ACE inhibitors and ARBs together leads to an increased risk of drug discontinuation, hypotension, renal dysfunction, and hyperkalemia in patients with left ventricular dysfunction.[42,43] In our study, sensitivity analysis pooling patients with heart failure due to systolic dysfunction had similar results to referred studies but, in contrast to them, our meta-analysis did not show a statistically significant risk of drug withdrawal.
No significant differences were found between different ARBs, and between different doses of the same ARB in drug withdrawal, cough, hypotension, or renal dysfunction.
The HEAAL study was a trial designed to determine whether the ARB dose could influence survival or cardiovascular events. This trial enrolled patients with established heart failure. No survival benefit was obtained with high-dose losartan but it reduced heart failure hospitalizations compared with low-dose losartan. Despite the higher number of adverse events with high-dose losartan, most of them did not lead to drug withdrawal, with a similar rate of drug discontinuation between groups. The main adverse events in decreasing order of incidence were: renal dysfunction, hypotension, hyperkalemia, and angioedema. Cough was not reported. The cause of angioedema is multifactorial and seems to be idiosyncratic,[44,45] but this trial suggests that the ARB dose may influence its occurrence because six cases were reported in the 150 mg arm compared with none in the 50 mg arm. This was not statistically significant but due to the severity of this adverse event a clinically important difference could not be excluded and this topic deserves further study. The other referred events were all dose dependent and significantly more frequent in the losartan 150 mg arm.
4.1 Limitations
This review includes a meta-analysis of randomized controlled trials. Results were pooled from reported outcomes and not from individual patient data, which is a potential source of bias in this type of analysis. The definition of each adverse event in individual studies varied, and this limitation should be taken into account.
Data presented here were pooled from different treatment groups with a broad spectrum of baseline characteristics, different proportions of causes of previous ACE inhibitor intolerance, heterogeneous effect measures, and different time-line outcomes. We used random effects meta-analysis; nevertheless this multiplicity has limitations that need to be considered.[46]
Trials designed to retrieve the incidence of cough in patients with ACE inhibitor intolerance reported a higher incidence of this outcome, reflecting differences in methodologies to assess cough that can limit our conclusions.
In the SPICE study, results for hypotension and renal dysfunction may be underestimated because only patients who had discontinued drugs due to the mentioned reasons were reported.[25]
5. Conclusions
In patients with ACE inhibitor intolerance, ARBs are well tolerated, with discontinuation rates, incidence of cough, and angioedema risk similar to those with placebo and diuretics. However, the risk of hypotension, renal dysfunction, or hyperkalemia is significantly higher compared with placebo. Patients with previous intolerance due to renal dysfunction are particularly susceptible to recurrence: about a fifth of patients with previous renal dysfunction will also have this adverse event with ARBs.
In patients with previous ACE inhibitor-induced cough, drug rechallenge should be discouraged because about two-thirds of these patients will cough again with an ACE inhibitor compared with only about a quarter treated with an ARB.
High-dose losartan had more adverse events but a similar discontinuation rate compared with the lower dose.
References
Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 2007; 13: 9–20
Pitt B. RAAS inhibition/blockade in patients with cardiovascular disease: implications of recent large-scale randomised trials for clinical practice. Heart 2009; 95: 1205–8
Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet 2007; 369: 1208–19
Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: a review of the literature and pathophysiology. Ann Intern Med 1992; 117: 234–42
Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med 2010; 123: 1016–30
Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med 2004; 19: 684–91
Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10: 499–509
Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119: e391–479
Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–906
Dickstein K, Kjekshus J, OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–60
ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–59
Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362: 772–6
Yusuf S, Pfeffer MA, Swedberg K, et al., CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362: 777–81
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12
Lacourcière Y, Brunner H, Irwin R, et al. Effects of modulators of the renin-angiotensin-aldosterone system on cough. Losartan Cough Study Group. J Hypertens 1994; 12: 1387–93
Ramsay LE, Yeo WW. ACE inhibitors, angiotensin II antagonists and cough. The Losartan Cough Study Group. J Hum Hypertens 1995; 9 Suppl. 5: S51–4
Ramsay LE, Yeo WW, Losartan Cough Study Group. Double-blind comparison of losartan, lisinopril and hydrochlorothiazide in hypertensive patients with a previous angiotensin converting enzyme inhibitor-associated cough. J Hypertens Suppl 1995; 13: S73–6
Benz J, Oshrain C, Henry D, et al. Valsartan, a new angiotensin II receptor antagonist: a double-blind study comparing the incidence of cough with lisinopril and hydrochlorothiazide. J Clin Pharmacol 1997; 37: 101–7
Chan P, Tomlinson B, Huang TY, et al. Double-blind comparison of losartan, lisinopril, and metolazone in elderly hypertensive patients with previous angiotensin-converting enzyme inhibitor-induced cough. J Clin Pharmacol 1997; 37: 253–7
Paster RZ, Snavely DB, Sweet AR, et al. Use of losartan in the treatment of hypertensive patients with a history of cough induced by angiotensin-converting enzyme inhibitors. Clin Ther 1998; 20: 978–89
Lacourciére Y. The incidence of cough: a comparison of lisinopril, placebo and telmisartan, a novel angiotensin II antagonist. Telmisartan Cough Study Group. Int J Clin Pract 1999; 53: 99–103
Tanser PH, Campbell LM, Carranza J, et al. Candesartan cilexetil is not associated with cough in hypertensive patients with enalapril-induced cough. Multicentre Cough Study Group. Am J Hypertens 2000; 13: 214–8
Rake EC, Breeze E, Fletcher AE. Quality of life and cough on antihypertensive treatment: a randomised trial of eprosartan, enalapril and placebo. J Hum Hypertens 2001; 15: 863–7
Maggioni AP, Anand I, Gottlieb SO, et al., Val-HeFT Investigators (Valsartan Heart Failure Trial). Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 2002; 40: 1414–21
Granger CB, Ertl G, Kuch J, et al. Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. Am Heart J 2000; 139: 609–17
Telmisartan Randomised Assessment Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372: 1174–83
Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet 2009; 374: 1840–8
Bradburn MJ, Deeks JJ, Berlin JA, et al. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007; 26: 53–77
Heran BS, Wong MM, Heran IK, et al. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev 2008; CD003822
Charlon V, Dollow S, Fidel J, et al. Reproducibility of angiotensin converting enzyme inhibitor induced cough: a double-blind randomised study. Br J Clin Pharmacol 1995; 39: 125–9
Tomiyama H, Motobe K, Zaydun G, et al. Insulin sensitivity and endothelial function in hypertension: a comparison of temocapril and candesartan. Am J Hypertens 2005; 18: 178–82
Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation 2005; 111: 315–20
Grilo A, Sáez-Rosas MP, Santos-Morano J, et al. Identification of genetic factors associated with susceptibility to angiotensin-converting enzyme inhibitors-induced cough. Pharmacogenet Genomics 2011; 21: 10–7
Mukae S, Itoh S, Aoki S, et al. Association of polymorphisms of the renin-angiotensin system and bradykinin B2 receptor with ACE-inhibitor-related cough. J Hum Hypertens 2002; 16: 857–63
Haymore BR, Yoon J, Mikita CP, et al. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: a meta-analysis. Ann Allergy Asthma Immunol 2008; 101: 495–9
Bas M, Adams V, Suvorava T, et al. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62: 842–56
Bas M, Greve J, Stelter K, et al. Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: a case series. Ann Emerg Med 2010; 56: 278–82
Winkelmayer WC, Zhang Z, Shahinfar S, et al. Efficacy and safety of angiotensin II receptor blockade in elderly patients with diabetes. Diabetes Care 2006; 29: 2210–7
Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 2000; 160: 685–93
Matys T, Pawlak R, Kucharewicz I, et al. Hypotensive effect of angiotensin II after AT1-receptor blockade with losartan. J Physiol Pharmacol 2000; 51: 161–6
Sica DA, Gehr TW, Yancy C. Hyperkalemia, congestive heart failure, and aldosterone receptor antagonism. Congest Heart Fail 2003; 9: 224–9
Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. J Card Fail 2008; 14: 181–8
Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med 2007 1; 67: 1930–6
Brown NJ, Ray WA, Snowden M, et al. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60: 8–13
Bas M, Hoffmann TK, Tiemann B, et al. Potential genetic risk factors in angiotensin-converting enzyme-inhibitor-induced angio-oedema. Br J Clin Pharmacol 2010; 69: 179–86
Bender R, Bunce C, Clarke M, et al. Attention should be given to multiplicity issues in systematic reviews. J Clin Epidemiol 2008; 61: 857–65
Acknowledgments
The authors have no conflicts of interest that are directly related to this review. No funding was received for the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caldeira, D., David, C. & Sampaio, C. Tolerability of Angiotensin-Receptor Blockers in Patients with Intolerance to Angiotensin-Converting Enzyme Inhibitors. Am J Cardiovasc Drugs 12, 263–277 (2012). https://doi.org/10.1007/BF03261835
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261835