Abstract
Purpose
Polypharmacy has been an important strategy for the management of hypertension. The aim of this study was to investigate the effect of combined treatment of losartan with ramipril on hypertension, myocardial infarction and vascular remodeling in disease animal models.
Methods
In 8-telemetered spontaneous hypertensive rats (SHRs) orally treated with various doses of drugs or vehicle by cross-over protocol, systolic blood pressure (SBP), mean arterial pressure (MAP) and heart rate (HR) were measured. The infarct size was measured in rats subjected to 30 min occlusion of the left anterior descending coronary artery (LAD) followed by 150 min reperfusion in myocardial ischemia/reperfusion (MI/R) rats injected drugs or vehicle once through femoral vein 15 min before inducing ischemia. The neointima formation and DNA synthesis in intima and media layer of the blood vessels were measured in cuff-induced vascular injury mice orally treated with drugs for 2 weeks.
Results
As compared with single treatment, the combination therapy of both drugs significantly decreased SBP and MAP without significant reflex tachycardia. The combined treatment of both drugs significantly increased the expression of endothelial nitric oxide synthase (eNOS) in coronary arterial tissues including area at occlusion. The combination therapy significantly decreased the thickness of vascular media layer and the number of bromodeoxyuridine (BrdU)-positive cells in both intima and media.
Conclusion
The present data suggest that the combination therapy with fixed doses of losartan and ramipril may exert greater BP-lowering effects with protection against myocardial infarction and vascular remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For hypertensive patients with over 140/90 mmHg (systolic/diastolic blood pressure), a variety of drugs was used for the management of high blood pressure (BP) (Wermelt et al. 2017). Anti-hypertensive drugs are categorized by their mechanisms including angiotensin-converting enzyme inhibitors (ACEIs) (Chen et al. 2018), angiotensin receptor blockers (ARBs) (Hill and Vaidya 2019), β-blockers (Mann 2017), calcium channel blockers (CCBs) (Lin and Ma 2018), diuretics (Roush et al. 2016) and vasodilators (McComb et al. 2016). These drugs can be selected according to the patient's concomitant disease states including diabetes, heart failure and kidney disease (Azar et al. 2019). If a single drug failed to control high BP, combination therapy with different modes of action would be helpful to lower BP (Avdeev et al. 2018; Guerrero et al. 2018). Thus, polypharmacy can be an effective strategy to adequately manage high BP.
Angiotensin II is a strong vasoconstrictor, inducing increase in BP (Te Riet et al. 2015). Ramipril belongs to the ACEIs, which inhibits the formation of angiotensin II, resulting in decrease in BP (de Leeuw et al. 1987; Becker et al. 1987). Losartan is a member of ARBs, which blocks the binding of angiotensin II to angiotensin II receptor type 1 (AT1) receptor, reducing BP (Tedesco et al. 1998). These drugs are known to have several adverse effects including chest pain, diarrhea, and muscle cramps with ARBs (Pongsuthana and Chutpongtada 2016), and thirst, cough, abdominal pain, headache, and hyperkalemia with ACEIs (Overlack 1996; Parish et al. 1992).
Both losartan and ramipril are known to have beneficial action to prevent hypertension-related complications such as heart failure and kidney disease (Schoolwerth et al. 2001; Crozier et al. 1995). Angiotensin II is known to induce vascular remodeling, which causes cardiovascular diseases such as atherosclerosis, stroke, and heart failure (Montezano et al. 2014; Heeneman et al. 2007). Thus, the combined treatment of losartan with ramipril may be synergistically beneficial for protecting heart disease and vascular remodeling as well as lowering BP. This compelled to perform experiment to investigate the protective effect of combination therapy on hypertension, myocardial infarction and vascular remodeling using disease animal models.
Materials and methods
Materials
Losartan (Fig. 1a) and ramipril (Fig. 1b) were purchased from Masung Co. (Seongnam, Korea). Normal saline was purchased from Daihan Co. (Seoul, Korea), formaldehyde from Samchun Co. (Yeosoo, Korea) and polyethylene tubing-50 (PE-50) from Becton, Dickinson & Co. (Franklin Lakes, NJ, USA). Pentobarbital sodium was purchased from Hanlim (Seoul, Korea). Mouse anti-bromodeoxyuridine (BrdU) monoclonal antibody was purchased from Chemicon International, Inc. (Seongnam, Korea), and anti-mouse IgG was purchased from Vector (Burlingame, CA, USA). All other reagents were of analytical grade. Slide glass and cover glass were purchased from Paul Marienfeld GmbH Company & Co. (Lauda-Köenigshofen, Germany).
Animal
Spontaneous hypertensive rats (SHRs) at 7-week-old age (200–220 g, Charles River Laboratories Japan, Inc.) and C57BL/6 mice at 5-week-old age (25–28 g, Charles River Laboratories Japan, Inc.) were purchased from Orient Bio, Inc. (Seongnam, Korea). All the animal research protocols were conducted in accordance with the Guide for the National Institutes of Health Guide for the Care and Use of Laboratory Animals, approved by Chungnam National University Animal Care and Use Committee (2009-2-19). Animals were raised in the conditioned room where the 12 h dark–light cycle, temperature (22 ± 2 °C), and humidity (50 ± 5%) were automatically maintained. After one-week acclimatization period, animals were used for experiments.
Dosing and experimental protocols for animal experiments
Based on human doses for losartan (100 mg) and ramipril (5 mg) for 60 kg of adult, doses for animals were calculated as follows:
as the correcting factor (Km) is estimated by dividing the average body weight (kg) of species to its body surface area (m2) (Nair et al. 2016). The values of Km for human, rat and mouse were 37, 6 and 3, respectively (Nair et al. 2016). The average doses of losartan and ramipril for animals as clinically relevant to human equivalent doses were summarized in Table 1. As low doses of both drugs, the half doses of both drugs, 5.15 mg/kg for losartan and 0.25 mg/kg for ramipril, were used in SHRs. The experimental rats were randomly divided into seven dosing protocols as follows (Table 2):
-
Protocol a: vehicle (0.15 N NaOH)-treated (C)
-
Protocol b: single average-dose losartan-treated (Lo-A)
-
Protocol c: single average-dose ramipril-treated (R-A)
-
Protocol d: average-dose of combined drugs-treated (LoR-A)
-
Protocol e: single low-dose losartan-treated (Lo-L)
-
Protocol f: single low-dose ramipril-treated (R-L)
-
Protocol g: low-dose of combined drugs-treated (LoR-L)
Eight telemetered-SHRs were administered every dosing protocol, from protocol a to g as shown in Table 3. This cross-over type of dosing protocols provided n = 8 of each group. SHRs showed high BP (≈ 200 mmHg) and each animal was daily (09:00AM) received drugs by oral administration. Between dosing in each animal, at least 3 days were given for full wash-out to exclude the effect of BP-lowering drug previously administered and recover high BP for the next experiment. Losartan was prepared in 0.15 N NaOH and ramipril in 0.9% normal saline. In this experiment, control rats were received 0.15 N NaOH (0.5 mL regardless of weight) as a vehicle.
For the study of protective action against vascular injury, the drugs were orally administered daily for 2 weeks to cuff-placed mice. Dosing protocol for this study was summarized in Table 4. The oral injection of 0.15 N NaOH (200 μL) was applied to both normal and cuff-placed mice as controls. The experimental mice were randomly divided into five groups as follows:
-
Group 1: normal control (sham-operated and 0.15 N NaOH-treated),
-
Group 2: control (cuff-placed and 0.15 N NaOH-treated)
-
Group 3: losartan (cuff-placed and single average-dose losartan-treated)
-
Group 4: ramipril (cuff-placed and single average-dose ramipril-treated)
-
Group 5: combination (cuff-placed and single-dose both drugs-treated)
Measurement of blood pressure and heart rate using the telemetry system
The BP (mmHg) and heart rate (HR, beat/min) of SHRs were determined using a telemetry system (Data Sciences International, St. Paul, MN, USA) as described previously (Lee et al. 2010). Briefly, SHRs were anesthetized with sodium pentobarbital (60 mg/kg, IP) and maintained at 37 °C on a heated rodent operating table. The catheter of the implantable transmitter (radio frequency transducer model TA11PA C40) was inserted into the abdominal aorta and the body of transmitter was placed in the peritoneal cavity. After surgical procedure, each rat was allowed to recover for 2 weeks in individual cage placed over the receiver panel that was connected to data acquisition system. Systolic blood pressure (SBP), mean arterial pressure (MAP), and HR were measured for 3 days prior to drug administration, and the average of these responses was used as a baseline measurement (Lee et al. 2010). The area over the curve (AOC) value of BP was determined as the change from baseline. Hemodynamic data were collected for 10 s every 5 min.
Measurement of myocardial ischemia/reperfusion (MI/R) injury
To examine the cardioprotective effect of combined treatment of losartan with ramipril, anesthetized SHRs (60 mg/kg, IP) were subjected to ischemic/reperfusion (I/R) injury as described previously (Lee et al. 1997; Park et al. 2014). Briefly, after being intubated by laryngotracheotomy on the heating pad (37 °C), the trachea was connected to a rodent ventilator (SAR 830/P ventilator; CWE, Inc., Ardmore, PA, USA) for artificial ventilation with room air (mechanical breathing with a volume of 1 mL per breath and 60 breaths per min). After opening the chest by a left thoracotomy in the 5th-6th intercostal space, the heart was exteriorized and a ligature (5–0 silk) was placed around the left anterior descending coronary artery (LAD) for coronary occlusion and reperfusion. After stabilizing for 20 min, SHRs injected through the femoral vein with vehicle (0.15 N NaOH) or study drugs (10.30 mg/kg for losartan or 0.49 mg/kg for ramipril or both, IV bolus). After 15 min, SHRs were subjected to 30 min ischemia by complete tightening of the ligature around LAD, and reperfusion was allowed for 2.5 h by releasing the ligature. After reperfusion, LAD was reoccluded and 2 mL of 1% Evans blue (Sigma-Aldrich Korea, Ltd.) was administered into the catheterized femoral vein. The images of heart slices (2-mm thick of the left ventricle immediately below the ligature) were captured for analysis of area at risk of infarction in left ventricle (AAR/LV). To determine the infarct zone (IZ), the heart slices were incubated with 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich Korea, Ltd.) in phosphate buffer (pH 7.4) at 37 °C for 15 min and then fixed overnight in 10% formalin solution. The AAR/LV and IZ/AAR were calculated.
Measurement of endothelial nitric oxide synthase (eNOS)
The eNOS has a cardio-protective effect through nitric oxide-cyclic guanosine monophosphate-protein kinase G pathway and/or direct protein S-nitrosylation (Correa et al. 2015; Simon et al. 2014). The coronary arterial tissues including area at occlusion in MI/R injury-induced SHRs were extracted and the expression of eNOS protein was quantified by ELISA kit (R&D System, MN, USA) according to manufacturer's recommendation. The quantification of eNOS protein was determined using the eNOS standard material included in the ELISA kit and calculated as a percentage.
Cuff placement and histological analysis of vascular remodeling
The surgical procedure of cuff placement was performed as described previously (Yi et al. 2010). A polyethylene tube (2-mm long PE-50, 0.58-mm inner diameter) was placed loosely around the left femoral artery of the mouse anesthetized with pentobarbital (65 mg/kg, IP) and tied with suture. After placing the cuff, mice orally administered for 2 weeks with study drugs were euthanized by overdose of pentobarbital, and perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde via a catheter inserted into the left ventricle. The cuffed artery was excised and the arterial tissue was fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin. Serial cross sections (5-mm thick) were obtained throughout the entire length of the cuffed femoral artery and paraffin sections of the femoral artery were stained with hematoxylin and eosin (H&E) (Kim et al. 2016). The intimal cross-sectional area between the lumen and the internal elastic lamina and the medial area between the internal and external elastic lamina were measured using computerized morphometric analysis (Image J, National Institute of Health). The average of 12 sections was taken as the value for each animal.
Immunohistochemical analysis for the measurement of DNA synthesis
The measurement of BrdU-positive cells was performed as previously described (Mead et al. 2014). The DNA synthesis in S phase of cell cycle can be reacted with BrdU as a specific marker for labeling newly synthesized DNA (Cappella et al. 2015). For the detection and quantification of cells that had entered into the cell cycle within 24 h of sacrifice, BrdU was injected at 18 h (100 mg/kg, SC + 30 mg/kg, IP) and at 12 h (30 mg/kg, IP) prior to sacrifice. The cross sections of femoral artery performed in the same manner described under “Cuff placement and histological analysis of vascular remodeling” were processed for immunohistochemistry using anti-BrdU antibodies (BrdU-Cell Proliferation Kit, Amersham). The sections were stained with contrast dye, H&E, and the proportion of BrdU-positive cells were determined by cell counts under light microscopy using a computerized morphometric analysis (Image J, National Institute of Health). The BrdU index defined as the ratio of BrdU-positive nuclei vs. total nuclei was calculated for determining the extent of the proliferative activity in both intima and media of each cross section. The average of 12 sections was taken as the value for each animal.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical significance was determined by Student’s t-test or one-way analysis of variance (ANOVA) with Dunnett's analysis using Prism 5.01 software (GraphPad Software, San Diego, CA, USA). The results were considered as significant if the p value is < 0.05.
Results
Effect of single or combined treatment of losartan with ramipril on SBP, MAP and HR
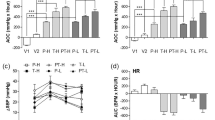
To confirm the effect of combined treatment of losartan with ramipril on high blood pressure, the SBP, MAP and HR in SHRs were measured using a telemetry system. The combination group with fixed average-dose of both drugs (LoR-A; 373.3 ± 31.8 mmHg∙h) significantly increased the AOC values of SBP compared to that with single treatment of each drug (Lo-A; 283.3 ± 24.0, R-A; 240.0 ± 32.1 mmHg∙h) (Fig. 2a). The changes of SBP in the combination group with fixed average-dose of both drugs were significantly larger than the monotherapy groups with average-dose of each drug until 8 h after drug administration, however, there was no significant difference among cases with low dose-treating groups (Fig. 2b). As expected, MAP in combination therapy group with fixed average-dose (LoR-A; 376.7 ± 22.0 mmHg·h) was significantly greater than single treatment groups (Lo-A; 210.0 ± 21.9, R-A; 176.7 ± 18.3 mmHg∙h) (Fig. 2c). In both SBP and MAP, there was no significant difference among cases with low-dose trials (Fig. 2a, c). HR in groups with monotherapy (fixed average- or low-dose) showed no significant difference. However, the groups with combination therapy showed tendency to lower HR as compared with those with monotherapy (Fig. 2d). These results suggest that the combined treatment of fixed average-dose of losartan with ramipril reduce BP without reflex tachycardia.
Effect of single or combined treatment of losartan with ramipril on systolic blood pressure (SBP), mean arterial pressure (MAP) and heart rate (HR) in telemetered SHRs. a Effect of losartan and ramipril in single or combined treatment on SBP. b The SBP-lowering profile after dosing for 24 h. c Effect of losartan and ramipril in single or combined treatment on MAP. The average of SBP and MAP values measured for 3 days prior to drug administration was used as the baseline measurement. According to the dosing protocol shown in Table 2, drugs were orally administered and the area over the curve (AOC) values of SBP and MAP was calculated as described under “Materials and methods” section. d Effect of losartan and ramipril in single or combined treatment on HR. For the comparison of HR among groups, AUC was calculated. Values represent 24 h-averages for each treatment group (n = 8) and are expressed as the mean ± SEM. Significantly different between indicated groups at *p < 0.05. C, vehicle (0.15 N NaOH)-treated control; Lo-A, single average-dose losartan-treated; R-A, single average-dose ramipril-treated; LoR-A, average-dose combination treatment of losartan and ramipril; Lo-L, single low-dose losartan-treated; R-L, single low-dose ramipril-treated; LoR-L, low-dose combination treatment of losartan and ramipril
Effect of single or combined treatment of losartan with ramipril on protection against MI/R injury
We examined the preventive role of combined treatment of losartan with ramipril in MI/R injury in anesthetized SHRs. The heart sections stained with blue by Evans blue were considered as the normal myocardium, and unstained area as AAR (Park et al. 2014). TTC stained the area of viable myocardium with brick red and unstained the area of necrotic myocardium (IZ) (Park et al. 2014). The AAR/LV (risk mass/left ventricular mass) and IZ/AAR (infarct mass/risk mass) were calculated and compared. As shown in Fig. 3a, AAR/LV was similar in the control (vehicle-treated) and study drug-treated groups. Single treatment of losartan or ramipril did not significantly reduce the myocardial infarct size (IZ/AAR). However, note that the combination therapy, given 15 min before the onset of ischemia, tended to reduce IZ/AAR, but the results were not statistically significant. The expression of eNOS in group with combined treatment was significantly increased as compared with control group (Control; 46.7 ± 4.4%, LoR-A; 100.0 ± 14.3%) (Fig. 3b). These results suggest that the combination therapy with fixed average-dose of losartan and ramipril may induce cardio-protective action through the increase of eNOS levels.
Effect of single or combined treatment of losartan with ramipril on MI/R injury in SHRs (a) and eNOS level (b) in coronary arterial tissues. Anesthetized SHRs were subjected to 30 min occlusion of the left anterior coronary artery (LAD) followed by 150-min reperfusion, and LAD was re-occluded after reperfusion as described under “Materials and methods” section. After staining with Evans blue and TTC, the area at risk (AAR) and infarct zone (IZ) in left ventricle (LV) were calculated as the percentage of the AAR/LV (risk mass/left ventricle mass) and IZ/AAR (infarct mass/risk mass). Drugs or vehicle (0.15 N NaOH) were intravenously injected once 15-min before induction of MI by complete tightening of the ligature around LAD. Significantly different from control at **p < 0.01. C, vehicle-treated control; Lo-A, single average-dose losartan-treated; R-A, single average-dose ramipril-treated; LoR-A, average-dose combined treatment of losartan with ramipril. Values are mean ± SEM (n = 7)
Effect of single or combined treatment of losartan with ramipril on protection against vascular injury
To investigate the effect of single or combined treatment of fixed average-dose of losartan with ramipril on cuff-induced neointima hyperplasia, a fixed-average dose of each drug or combined was orally administered for 14 days after cuff placement, and the extents of cuff-induced vascular neointima formation and proliferative activity were measured. As shown in Fig. 4a, the neointima formation was significantly induced by cuff placement (1060.45 ± 136.4 to 6007.22 ± 467.4 μm2). Losartan at 20.60 mg/kg/day decreased neointima formation without statistical significant (Fig. 4a). The combined treatment of losartan with ramipril did not show any protective effect against cuff-induced neointima formation. However, the combined treatment of both drugs was significantly decreased thickness of media layer (16,174 ± 1302 to 10,333 ± 1202 μm2) (Fig. 4b). The ratios of BrdU-positive cells in the intima layer were significantly reduced the single (Lo-A; 18.3 ± 0.8%, R-A; 19.1 ± 1.6%) and combined (LoR-A; 10.3 ± 0.7%) treatment as compared with control (cuff-induced & no treatment, 22.67 ± 1.4%) (Fig. 4c). Moreover, the combined treatment of both drugs induced the significant decrease in ratio of BrdU-positive cells in the media layer as compared with control (11.3 ± 0.8% to 9.0 ± 1.1%) (Fig. 4d). These results suggest that the combined treatment of fixed average-dose of losartan with ramipril may protect vascular injury induced by cuff placement via inhibition of DNA synthesis.
Effect of single or combined treatment of losartan with ramipril on cuff-induced neointima formation and DNA synthesis. According to the dosing protocol shown in Table 4, drugs were orally administered for 14 days after cuff placement, and the extents of thickness in both intima (a) and media (b) layer were measured. The extents of DNA synthesis were measured using BrdU-incorporation assay within intima (c) and media (d) layer. Data are expressed as mean ± SEM. The number of animals tested in this experiment were indicated in Table 4. Significantly different from cuff-control (cuff-placed, but no treatment) at *p < 0.05 and **p < 0.01
Discussion
Our current study show three findings: combined treatment of fixed average dose of losartan with ramipril (1) lowers SBP and MAP without reflex tachycardia in SHRs, (2) increases the expression of eNOS in coronary arterial tissues including area at occlusion in MI/R injury-induced SHRs, and (3) decreases DNA synthesis in intima and thickness of media in cuff-placed vessels. For the management of hypertension, a therapeutic strategy is polypharmacy by fixed-dose combination with drugs having different mode of action such as the renin–angiotensin–aldosterone system (RAAS) inhibitor with diuretic, RAAS inhibitor with CCB, and β-blockers with diuretics (Gradman et al. 2011). Therefore, the combined treatment of losartan with ramipril was expected to synergistically decrease BP through regulation of angiotensin production and its receptor binding. As expected, the combination therapy with fixed-average dose of losartan with ramipril lowered SBP and MAP, and this action was greater than monotherapy of each drug. Note that this combination did not show any significant change in HR as compared with no treatment group (Fig. 2). Thus, these results indicate that the combined treatment of losartan with ramipril has an excellent BP-lowering effect without reflex tachycardia.
Hypertensive patients are at high risk for complications such as arteriosclerosis (Alexander 1995), diabetes (Arauz-Pacheco et al. 2003), heart failure (Messerli et al. 2017), and stroke (Bath et al. 2018). The increased expression of eNOS in cardiac cells indicates cardioprotective effect (Simon et al. 2014). Although the combination therapy of losartan with ramipril did not show any significant improvement of AAR/LV and IZ/AAR in MI/R-injured SHRs, the expression of eNOS in coronary arterial tissues including area at occlusion was increased (Fig. 3). These results suggest that the combination therapy of losartan with ramipril may have cardio-protective effect from MI/R-associated heart diseases such as myocardial infarction.
The installation of the cuff around blood vessels induces vascular stress, resulting in thickening of intima and media layer, which narrows the internal diameter of the blood vessels (Kockx et al. 1993; Kokubo et al. 2018). Angiotensin II is known to induce vasoconstriction, vascular cell proliferation and differentiation (Wynne et al. 2009; Daemen et al. 1991). The combined treatment of losartan with ramipril decreased the thickness of media layer in cuff-placed vessels. Furthermore, this combination significantly decreased DNA synthesis in both intima and media of cuff-placed blood vessels (Fig. 4). These results suggest that the combination therapy of losartan with ramipril may exert vascular-protective effect from vascular narrowing-associated diseases such as atherosclerosis. However, the current study did not show any beneficial effect of combined treatment of losartan with ramipril on lowering blood glucose level and improving glucose tolerance (Data did not shown).
Recently, large-scale clinical studies for the combination therapy of ARBs with ACEIs have performed from multiple angles. The combination therapy of telmisartan with ramipril in patients with vascular disease or high-risk diabetes showed more adverse events without an increase in benefit (ONTARGET Investigators et al. 2008). Furthermore, the combined ramipril and telmisartan contributed to a higher rate of adverse renal outcomes than monotherapy (Chaudhary et al. 2009). However, several reports have represented that a combination of ramipril and telmisartan does not increase strokes or alter other major cardiovascular or renal events in patients with diabetes (Mann et al. 2013). Moreover, the combination therapy reduces proteinuria and prevents structural lesions more effectively than either monotherapy (Ruggenenti et al. 2008; Gentile et al. 2015). Although much better controlled experiments need to be performed in the future, the combination therapy might demonstrate beneficial effects in certain clinical conditions.
Conclusion
The combination therapy of fixed average-dose of losartan with ramipril shows the greater BP-lowering effect than monotherapy in hypertension, and may provide cardio- and vascular-protective effects against myocardial infarction and atherosclerosis. As indicated in mechanism of action, the combination of these two drugs can inhibit vasoconstriction and angiotensin II-induced cardiac and vascular remodeling, which could improve blood circulation. Therefore, our current finding suggests that this combination therapy may be a useful strategy in the treatment of hypertension and cardiovascular complications.
References
Alexander RW (1995) Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 25:155–161
Arauz-Pacheco C, Parrott MA, Raskin P, American Diabetes A (2003) Treatment of hypertension in adults with diabetes. Diabetes Care 26(Suppl 1):S80–82
Avdeev SN, TsarEva NA, Gaisin IR (2018) Combination therapy is a new standard for treatment of pulmonary arterial hypertension. Ter Arkh 90:72–80
Azar RR, Sarkis A (2019) Global impact of the new European and American hypertension guidelines: a perspective from Lebanon. J Clin Hypertens (Greenwich) 21:684–686
Bath PM, Appleton JP, Krishnan K, Sprigg N (2018) Blood pressure in acute stroke: to treat or not to treat: that is still the question. Stroke 49:1784–1790
Becker RA, Scholkens B (1987) Ramipril: review of pharmacology. Am J Cardiol 59:3D–11D
Cappella P, Gasparri F, Pulici M, Moll J (2015) Cell proliferation method: click chemistry based on BrdU coupling for multiplex antibody staining. Curr Protoc Cytom 72:1–17
Chaudhary K, Nistala R, Whaley-Connell A (2009) Dual renin-angiotensin system blockade in the ONTARGET study: clinically relevant risk for the kidney? Curr Hypertens Rep 11(5):375–381
Chen YJ, Li LJ, Tang WL, Song JY, Qiu R, Li Q, Xue H, Wright JM (2018) First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev 11:CD008170
Correa F, Buelna-Chontal M, Chagoya V, Garcia-Rivas G, Vigueras RM, Pedraza-Chaverri J, Garcia-Nino WR, Hernandez-Pando R, Leon-Contreras JC, Zazueta C (2015) Inhibition of the nitric oxide/cyclic guanosine monophosphate pathway limited the cardioprotective effect of post-conditioning in hearts with apical myocardial infarction. Eur J Pharmacol 765:472–481
Crozier I, Ikram H, Awan N, Cleland J, Stephen N, Dickstein K, Frey M, Young J, Klinger G, Makris L et al (1995) Losartan in heart failure. Hemodynamic effects and tolerability. Losartan Hemodynamic Study Group. Circulation 91:691–697
Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM (1991) Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 68:450–456
de Leeuw PW, Birkenhager WH (1987) Short- and long-term effects of ramipril in hypertension. Am J Cardiol 59:79D–82D
Gentile G, Remuzzi G, Ruggenenti P (2015) Dual renin-angiotensin system blockade for nephroprotection: still under scrutiny. Nephron 129(1):39–41
Gradman AH, Basile JN, Carter BL, Bakris GL, American Society of Hypertension Writing G (2011) Combination therapy in hypertension. J Clin Hypertens (Greenwich) 13:146–154
Guerrero-Garcia C, Rubio-Guerra AF (2018) Combination therapy in the treatment of hypertension. Drugs Context 7:212531
Heeneman S, Sluimer JC, Daemen MJ (2007) Angiotensin-converting enzyme and vascular remodeling. Circ Res 101:441–454
Hill RD, Vaidya PN (2019) Angiotensin II receptor blockers (ARB, ARb). StatPearls, Treasure Island (FL)
Kim HJ, Yoo HY (2016) Hypoxic pulmonary vasoconstriction and vascular contractility in monocrotaline-induced pulmonary arterial hypertensive rats. Korean J Physiol Pharmacol 20:641–647
Kockx MM, De Meyer GR, Andries LJ, Bult H, Jacob WA, Herman AG (1993) The endothelium during cuff-induced neointima formation in the rabbit carotid artery. Arterioscler Thromb 13:1874–1884
Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Nakamura F, Miyamoto Y (2018) Impact of intima-media thickness progression in the common carotid arteries on the risk of incident cardiovascular disease in the Suita study. J Am Heart Assoc 7(11):e007720
Lee JJ, Shin CY, Park HJ, Zhang WY, Kim Y, Kim IS, Lee KH, Myung CS (2010) Drug synergism of antihypertensive action in combination of telmisartan with lercanidipine in spontaneous hypertensive rats. Arch Pharm Res 33:1411–1418
Lee YM, Peng YY, Ding YA, Yen MH (1997) Losartan attenuates myocardial ischemia-induced ventricular arrythmias and reperfusion injury in spontaneously hypertensive rats. Am J Hypertens 10:852–858
Lin Y, Ma L (2018) Blood pressure lowering effect of calcium channel blockers on perioperative hypertension: a systematic review and meta-analysis. Medicine (Baltimore) 97:e13152
Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Rydén L, Sleight P, Teo KK, Yusuf S, ONTARGET Investigators (2013) Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 31(2):414–421
Mann SJ (2017) Redefining β-blocker use in hypertension: selecting the right β-blocker and the right patient. J Am Soc Hypertens 11:54–65
McComb MN, Chao JY, Ng TM (2016) Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther 21:3–19
Mead TJ, Lefebvre V (2014) Proliferation assays (BrdU and EdU) on skeletal tissue sections. Methods Mol Biol 1130:233–243
Messerli FH, Rimoldi SF, Bangalore S (2017) The transition from hypertension to heart failure: contemporary update. JACC Heart Fail 5:543–551
Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM (2014) Angiotensin II and vascular injury. Curr Hypertens Rep 16:431
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31
ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358(15):1547–1559
Overlack A (1996) ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf 15:72–78
Parish RC, Miller LJ (1992) Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf 7:14–31
Park ES, Kang DH, Yang MK, Kang JC, Jang YC, Park JS, Kim SK, Shin HS (2014) Cordycepin, 3'-deoxyadenosine, prevents rat hearts from ischemic/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovasc Toxicol 14:1–9
Pongsuthana S, Chutpongtada K (2016) A comparison of the efficacy and renal side effects of antihypertensive drugs “angiotensin receptor blockers” (ARBs) in Rajavithi hospital. J Med Assoc Thai 99(Suppl 2):S56–62
Roush GC, Sica DA (2016) Diuretics for hypertension: A review and update. Am J Hypertens 29:1130–1137
Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, Perna A, Remuzzi G (2008) Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 19:1213–1224
Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS, Council on the Kidney in Cardiovascular D, the Council for High Blood Pressure Research of the American Heart A (2001) Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation 104:1985–1991
Simon JN, Duglan D, Casadei B, Carnicer R (2014) Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol 73:80–91
Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH (2015) Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 116:960–975
Tedesco MA, Ratti G, Aquino D, Limongelli G, di Salvo G, Mennella S, Galzerano D, Iarussi D, Iacono A (1998) Effects of losartan on hypertension and left ventricular mass: a long-term study. J Hum Hypertens 12:505–510
Wermelt JA, Schunkert H (2017) Management of arterial hypertension. Herz 42:515–526
Wynne BM, Chiao CW, Webb RC (2009) Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. J Am Soc Hypertens 3:84–95
Yi I, Lee JJ, Park JS, Zhang WY, Kim IS, Kim Y, Shin CY, Kim HS, Myung CS (2010) Enhanced effect of losartan and rosuvastatin on neointima hyperplasia. Arch Pharm Res 33(4):593–600
Acknowledgements
This study was financially supported by the research fund of Chungnam National University (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This work complies with all ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, HS., Lee, DH., Han, JH. et al. The effects of combined treatment of losartan and ramipril on hypertension and related complications. J. Pharm. Investig. 50, 573–581 (2020). https://doi.org/10.1007/s40005-020-00478-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-020-00478-9