Abstract
Purpose
Polypharmacy is an important strategy for managing high blood pressure (BP). Combination treatment with a Ca2+ channel blocker (CCB) and an angiotensin II type 1 receptor blocker (ARB) can be used for this purpose. If combination therapy had additional beneficial effects such as protection against cardiovascular injury and regulation of blood glucose, it would be an effective tool for managing metabolic syndrome. Thus, this study investigated the effects of combination treatment with the CCB cilnidipine and the ARB telmisartan on hypertension, cardiovascular injury, and hyperglycemia.
Methods
A telemetry system was used to measure BP and heart rate (HR) in spontaneous hypertensive rats (SHRs). The effectiveness of combination therapy in protecting against cardiovascular injury was examined using a myocardial ischemia/reperfusion (MI/R) system in SHRs and a cuff-placement-induced neointima hyperplasia model in C57BL/6 mice. An oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were also performed in obese diabetic mice.
Results
Combination treatment with a fixed (human equivalent) dose of cilnidipine and telmisartan can effectively lower BP without reflex tachycardia. This combination therapy may also induce cardioprotective effects by increasing the expression of endothelial nitric oxide synthase (eNOS) and vasoprotective effects by inhibiting DNA synthesis in cuff-induced vascular injury. In addition, it may also attenuate high blood glucose levels.
Conclusion
The results of this study suggest that combination treatment with cilnidipine and telmisartan can be used as an effective strategy for the treatment of hypertension and related complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is defined as a high arterial blood pressure (BP) with a measured systolic/diastolic BP above 140/90 mmHg (Perumareddi 2019). Hypertension can be divided into primary hypertension and secondary hypertension. Primary hypertension refers to high BP without a specific cause, and secondary hypertension develops as a symptom of a specific cause (Charles et al. 2017). More than 90% of hypertensive patients have primary hypertension, most having multifactorial causes, with genetic factors reported as being particularly important (Oliveras and Sierra De La 2014; Singh et al. 2016). Hypertension is closely related to aging as blood vessels lose elasticity and BP increases, which puts a strain on the heart and can lead to heart failure, chronic hypertension, and cardiovascular diseases such as stroke, heart attack, and myocardial infraction (Suzuki et al. 2005; Buford 2016). Therefore, as society ages rapidly, the prevalence of hypertension and related complications will increase, which could become a worldwide problem (Taddei et al. 2006; Mills et al. 2016).
The treatment of hypertension focuses on BP control using drug therapy and non-drug therapy (Lee et al. 2019). Non-drug therapy involves a low-salt and low-cholesterol diet, as well as addressing lifestyle factors such as smoking cessation, stress reduction, and exercise (Ozemek et al. 2018). Current antihypertensive agents mainly act on the pathophysiological mechanisms that cause hypertension. The main types of antihypertensive agents used today include angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and Ca2+ channel blockers (CCBs) (Nguyen et al. 2010). Each can be used as monotherapy or in combination (Azizi et al. 2019).
ARBs, which regulate BP by blocking the activity of the angiotensin II receptor type 1 (AT1 receptor), are mainly used for patients who experience adverse effects from ACEIs, such as dry cough (Helmer et al. 2018). CCBs control BP by blocking Ca2+ channels in vascular smooth muscle cells (VSMCs) and cardiomyocytes, resulting in vasodilation and negative inotropic activity in the heart (Tamargo and Ruilope 2016). This study used telmisartan, which has a higher selectivity for the AT1 receptor than other ARBs and is absorbed rapidly after oral administration (Galzerano et al. 2010; Akhrass and Mcfarlane 2011). The dihydropyridine CCB cilnidipine, which reduces BP by blocking N-type and L-type Ca2+ channels in both arterioles and veins (Nezu et al. 2018), was also tested. Theoretically, combination treatment with telmisartan and cilnidipine could synergistically lower BP, which could be used to better manage hypertension. Combination therapy may also be beneficial to control cardiovascular disease and hyperglycemic conditions. Therefore, this study was designed to examine, using animal disease models, whether cilnidipine and telmisartan combination therapy could synergistically regulate high BP and effectively manage myocardial ischemia/reperfusion injury, neointima hyperplasia, and high blood glucose.
Materials and methods
Materials
Telmisartan and cilnidipine were purchased from Masung Co. (Seongnam, Korea). Phosphate-buffered saline (PBS) was purchased from Gibco (Grand Island, NY, USA), polyethylene tubing-50 (PE-50) from Becton, Dickinson & Co. (Franklin Lakes, NJ, USA) and mouse anti-bromodeoxyuridine (BrdU) monoclonal antibody from Chemicon International, Inc. (Seongnam, Korea). Normal saline was purchased from Daihan Co. (Seoul, Korea), formaldehyde from Samchun Co. (Yeosoo, Korea) and anti-mouse IgG from Vector (Burlingame, CA, USA). Pentobarbital sodium was purchased from Hanlim (Seoul, Korea), the endothelial nitric oxide synthase (eNOS) ELISA kit was purchased from R&D systems (Minneapolis, MN, USA), and the Accu-Chek active strip was purchased from Roche Diagnostics (GmbH, Mannheim, Germany).
Animals
Spontaneous hypertensive rats (SHRs) aged 7–8 weeks (body weight 200–220 g) and 5-week-old C57BL/6 mice (body weight 25–28 g) were purchased from Orient Bio, Inc. (Seongnam, Korea). The animals were placed in an animal room under a 12 h/12 h dark/light cycle at constant temperature (22 ± 2 °C) and humidity (50 ± 5%). Prior to the experiment, the animals were allowed to acclimate for 1 week. All experimental procedures using animals were performed according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were approved by the Chungnam National University Animal Care and Use Committee (2009-2-19).
Drug dosing and experimental animal protocols
The doses for animals were calculated using the animal equivalent dose calculation (Nair and Jacob 2016; Park et al. 2020) and are shown in Table 1. The experimental rats were randomly divided into eight protocols to evaluate the effects of reducing BP (Table 2). BP and heart rate (HR) were monitored after oral administration of drug or vehicles using a cross-over study type as previously described (Park et al. 2020). This cross-over dosing protocol provided data (n = 9) for each group. At least 3 days of washout were given between different drug doses in each animal. The dosing protocol for the vascular protection study is shown in Table 3. The drugs were orally administered to cuff-placed mice for 2 weeks. Normal saline was administered to both normal control (sham-operated) and cuff-placed control groups. To study the effects of the drug on blood glucose regulation in type 2 diabetes, obese diabetic mice were induced as previously described (Nan et al. 2010; Choi et al. 2011; Jung et al. 2019).
Measurement of blood pressure (BP) and heart rate (HR)
The BP (mmHg) and HR (beat/min) of SHRs were measured using a telemetry system (Data Sciences International, St. Paul, MN, USA) as previously described (Shin et al. 2009; Lee et al. 2010). The implantable transmitter (radio frequency transducer model TA11PA C40) was inserted into the abdominal artery of SHRs. Each rat was allowed 2 weeks of recovery after the surgical procedure. The systolic blood pressure (SBP), mean arterial pressure (MAP), and HR were measured for 3 days prior to drug administration, and the average of these values was used as a baseline. The area over the curve (AOC) values were calculated as a change from the BP baseline. Telemetered hemodynamic data were collected at 10-s intervals every 5 min. Each SHR received the next dose after the SBP reached approximately 200 mmHg.
Measurement of myocardial ischemia/reperfusion (MI/R) injury
To evaluate the cardioprotective effects of monotherapy and combination treatments using telmisartan and cilnidipine, a myocardial ischemia/reperfusion (MI/R) injury model was used as previously described (Park et al. 2014, 2020). After anesthetizing SHRs with pentobarbital (60 mg/kg, IP), the left anterior descending coronary artery (LAD) was ligated for coronary occlusion and reperfusion. Vehicle or drug was injected through the femoral vein, and ischemia was induced for 30 min by complete tightening of the ligature around the LAD. Reperfusion was performed for 2.5 h by untying the ligature. After reoccluding the LAD, 1% Evans blue was added to analyze the area at risk of infarction in the left ventricle (AAR/LV) and the infarct zone (IZ). The AAR/LV and IZ/AAR were calculated after the analysis of the heart slices (2-mm-thick slices of the left ventricle immediately below the ligature).
Measurement of endothelial nitric oxide synthase (eNOS)
Since eNOS has a cardioprotective effect through vasodilation and regulation of endothelial cell function (Kulandavelu et al. 2015; Siragusa and Fleming 2016), the expression of eNOS protein was measured in coronary artery tissues, including the occluded area, in MI/R injured SHRs. An ELISA kit (R&D Systems, MN, USA) was used to measure eNOS expression as previously described (Lee et al. 2017; Park et al. 2020). A standard curve was constructed using the eNOS standard material included in the ELISA kit, and eNOS protein was quantified as a percentage.
Cuff placement for the induction of vascular remodeling and histological analysis
A cuff was used to narrow the inner diameter of blood vessels by inducing blood vessel damage (Kokubo et al. 2008). Cuff placement was surgically performed as previously described (Yi et al. 2010). After placing a polyethylene tube (2-mm long PE-50, 0.58 mm inner diameter) around the left femoral artery, study drugs were administered to C57BL/6 mice orally for 2 weeks. Computerized morphometric analysis (ImageJ software, NIH, MD, USA) was used to measure the intimal area between the lumen and the internal elastic lamina, and the medial area between the internal and external elastic lamina at the cuffed artery. The average of 12 5-mm thick serial sections was used as the value for each animal.
Measurement of DNA synthesis
Since BrdU specifically incorporates into newly synthesized DNA strands in the S phase of the cell cycle (Mead and Lefebvre 2014), the proportion of BrdU-positive cells in femoral artery cross sections was determined using anti-BrdU antibodies (BrdU-Cell Proliferation Kit, Amersham) as previously described (Yi et al. 2010; Lee et al. 2017; Park et al. 2020). To calculate the extent of proliferation activity in both the intima and media of each cross section, the BrdU index was generated as the ratio of BrdU-positive nuclei to total nuclei.
Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT)
After 2 h of drug or vehicle treatment, blood samples were collected and denoted as a sample at time 0. A high amount of glucose (2 g/kg, PO) was administered to obese diabetic mice after 12 h of fasting as previously described (Choi et al. 2011; Jung et al. 2019). Blood was collected from the tail vein of each animal at 30, 60, and 120 min after glucose administration to measure the blood glucose level using a blood glucose meter (Accu-Check; Roche Diagnostics Korea Co., Ltd., Seoul, Korea). OGTT data were analyzed by comparing the area under the curve (AUC) of each line graph. For ITT, insulin (1 U/kg, SC) instead of glucose was administered under the same conditions as the OGTT. Blood was collected at 30, 60, 120, and 240 min after insulin treatment, and blood glucose levels were measured. Similarly, the AUC was used to compare the significant difference between groups.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Student’s t test or one-way analysis of variance (ANOVA) with Dunnett’s analysis was performed to determine statistical significance using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). A p value < 0.05 was considered a statistically significant difference.
Results
Effect of monotherapy or combination treatment with cilnidipine and telmisartan on BP and HR
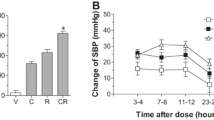
SBP, MAP and HR were measured in SHR via a telemetry system to determine the effect of combination treatment of telmisartan and cilnidipine on hypertension. Regardless of the dose, telmisartan or cilnidipine monotherapy lowered the SBP (Fig. 1a) and MAP (Fig. 1c), with similar patterns. As expected, high (human equivalent, see Tables 1–2) doses of both drugs showed higher efficacy than low (1/4 of high) doses. Thus, the combination group using a fixed-dose of both drugs (TC-H) resulted in higher AOC values of the SBP and MAP than those of the low-dose combination (TC-L). Interestingly, the efficacy of telmisartan was higher than that of cilnidipine, suggesting that combination treatment with cilnidipine and telmisartan is more effective in reducing SBP and MAP than cilnidipine monotherapy. The change in SBP over time was the highest after high-dose telmisartan and fixed-dose combination therapy (Fig. 1b). Although cilnidipine monotherapy increased the HR, the combined treatment of cilnidipine and telmisartan reduced the HR (Fig. 1d). These results suggest that combination treatment with fixed-dose cilnidipine and telmisartan can effectively manage BP without reflex tachycardia.
Effect of monotherapy or combined treatment of cilnidipine with telmisartan on blood pressure (BP) and heart rate (HR) in spontaneous hypertensive rats (SHRs). a Effect of telmisartan and cilnidipine on systolic blood pressure (SBP). b Changes in SPB after dosing for 24 h. c Effect of telmisartan and cilnidipine on mean arterial pressure (MAP). d Effect of telmisartan and cilnidipine on HR. The average of SBP, MAP and HR values measured for 3 days prior to drug administration was used as the baseline and the changes from baseline were considered as the activity of drugs. BP and HR were recorded continuously in telemetered SHRs and data were collected during drug administration period. The area over the curve (AOC) and the area under the curve (AUC) were calculated for the quantification of BP and HR, respectively. Data are expressed as mean ± SEM (n = 9). **p < 0.01, significant difference. V1 (0.9% NaCl), V2 (0.15 N NaOH), T-H (high-dose telmisartan-treated), C-H (high-dose cilnidipine-treated), TC-H (high-dose combined treatment of cilnidipine with telmisartan), T-L (low-dose telmisartan–treated), C-L (low-dose cilnidipine-treated), TC-L (low-dose combined treatment of cilnidipine with telmisartan)
Effect of monotherapy or combination treatment with cilnidipine and telmisartan on protection against MI/R injury
To confirm the cardioprotective effect of combination therapy with cilnidipine and telmisartan, MI/R injured SHRs were conducted and the AAR/LV and IZ/AAR were calculated. Although both monotherapy and combination treatment reduced the IZ/AAR in both groups compared with both vehicle groups, the combination treatment with cilnidipine and telmisartan showed no significant difference as compared to the monotherapy groups (Fig. 2a). However, the expression of eNOS protein in the combination therapy group was significantly higher than in the monotherapy groups (Fig. 2b). These results suggest that the fixed-dose combined treatment of telmisartan with cilnidipine may induce cardioprotective effects by increasing eNOS expression.
Effect of monotherapy or combined treatment of cilnidipine with telmisartan on myocardial ischemia/reperfusion (MI/R) injury in spontaneous hypertensive rats (SHRs). a Effect of combination therapy on the area at risk (AAR) and infarct zone (IZ) in left ventricle (LV). Myocardial ischemia (MI) was induced by the occlusion of the left anterior descending coronary artery (LAD) for 30 min in anesthetized SHRs and reperfusion injury was produced by releasing the ligature for 150 min. Drugs or vehicles were intravenously injected once 15-min prior to the induction of MI. Data were expressed as the percentage of the AAR/LV (risk mass/left ventricle mass) and IZ/AAR (infarct mass/risk mass). b Effect of combination therapy on endothelial nitric oxide synthase (eNOS) level in coronary arterial tissues. Data are expressed as mean ± SEM (n = 8). **p < 0.01, significant difference. V1 (0.9% NaCl), V2 (0.15 N NaOH), T (high-dose telmisartan-treated), C (high-dose cilnidipine-treated), TC (high-dose combined treatment of cilnidipine with telmisartan)
Effect of monotherapy or combination treatment with cilnidipine and telmisartan on protection against vascular remodeling in cuff-placed mouse
To examine the protective effect of monotherapy or fixed-dose combination treatment with cilnidipine and telmisartan on cuff-induced neointima formation, study drugs were orally administered for 2 weeks to cuff-placed mice. Although cilnidipine monotherapy did not significantly reduce neointima formation, the fixed-dose combination treatment with cilnidipine and telmisartan resulted in a significant decrease in neointima hyperplasia (Fig. 3a). No significant change in thickness in the media layer was observed in any of the experimental groups (Fig. 3b). In both the intima and media layers, the ratios of BrdU-positive cells were significantly decreased in all experimental groups. The fixed-dose combination treatment with cilnidipine and telmisartan potentiated the reduction of the ratio of BrdU-positive cells, in contrast to monotherapies with either drug (Fig. 3c and d). These results suggest that the fixed-dose combination treatment with cilnidipine and telmisartan protects against vascular injury induced by cuff placement through the inhibition of DNA synthesis.
Effect of monotherapy or combined treatment of cilnidipine with telmisartan on neointima formation and DNA synthesis in cuff-placed mouse. Single or combined treatment of cilnidipine with telmisartan were orally administered to cuff-placed mice for 2 weeks, and the thickness of the intima (a) and media (b) layers was measured. DNA synthesis in the intima (c) and media (d) layers of cross sections of femoral artery was measured using bromodeoxyuridine (BrdU)-incorporation assay. Data are expressed as mean ± SEM (n = 6). *p < 0.05; **p < 0.01, significant difference
Effects of monotherapy or combination treatment with cilnidipine and telmisartan on blood glucose level
To determine the effect of cilnidipine and telmisartan combination therapy on glucose tolerance, an OGTT and ITT was conducted in obese diabetic mice. Cilnidipine monotherapy significantly decreased the AUC (Fig. 4a), indicating that cilnidipine efficiently removed glucose from the blood. The fixed-dose cilnidipine combination treatment with telmisartan also significantly reduced the AUC. However, there was no difference in the ITT results among the experimental groups (Fig. 4b). These results suggest that the fixed-dose cilnidipine and telmisartan combination treatment can regulate high blood glucose levels.
Effect of monotherapy or combined treatment of cilnidipine with telmisartan on the regulation of blood glucose level in obese diabetic mice. C57BL/6 mice fed on high-fat diet (60% kcal fat) were intraperitoneally (IP) administered by nicotinamide (240 mg/kg) 15 min before injecting streptozotocin (100 mg/kg, IP) to induce obese diabetic animal model as described under “Materials and method” section. a Oral glucose tolerance test (OGTT). Drugs or vehicles were administered 2 h prior to the oral administration of glucose (2 g/kg), and blood samples were collected at 0, 30, 60, and 120 min after glucose administration and blood glucose levels were measured. b Insulin tolerance test (ITT). At 2 h after drugs or vehicle administration, insulin (1 U/kg) was subcutaneously injected and bloods were collected at 0, 30, 60, 120, and 240 min after insulin treatment and blood glucose levels were measured. Date were analyzed by quantifying the changes produced by area under the curve (AUC) of each group. Data are expressed as mean ± SEM (n = 6). *p < 0.05 and **p < 0.01, significant difference. Con (control), T (high-dose telmisartan-treated), C (high-dose cilnidipinetreated), TC (high-dose combined treatment of cilnidipine with telmisartan)
Discussion
Since hypertension is a chronic disease, long-term drug treatment is required depending on the patient’s condition (Cuspidi et al. 2018). Polypharmacy is usually required for adequate BP control with agents that act on different pharmacological mechanisms (Musini et al. 2015; Guerrero-Garcia and Rubio-Guerra 2018). The major therapeutic mechanisms used for lowering high BP include AT1 receptor blockade and the regulation of intracellular Ca2+ concentration. AT1 receptor blockade is associated with the proliferation and migration of VSMCs, one of the main causes of atherosclerosis, and the intracellular Ca2+ concentration is important for vasodilation (Kramer et al. 2019). Thus, the combination of a CCB with an ARB has been a rational strategy for the management of BP. There are several examples of CCB/ARB combinations, such as amlodipine/olmesartan (Punzi et al. 2010) and amlodipine/telmisartan (Moen 2010).
In this study, the effect on BP of combination therapy with the CCB cilnidipine and the ARB telmisartan was evaluated. The effectiveness of cilnidipine and telmisartan in protecting against vascular injury and myocardial ischemia and promoting blood glucose regulation was also evaluated. Cilnidipine alone was less effective than telmisartan in lowering BP, as measured by SBP and MAP. Moreover, combination therapy with telmisartan had no additive or synergistic activity (Fig. 1), as the low dose of either drug alone or in combination had results similar to those of the high doses. These results indicate that cilnidipine and telmisartan combination therapy at lower doses results in meaningful BP reduction effects. However, this effect was lower than with fixed-dose combination therapy. Thus, the fixed-dose combination treatment is more desirable, as it maximizes the BP-lowering activity without reflex tachycardia.
Neither cilnidipine nor telmisartan showed protective effects against MI/R injury. However, the fixed-dose combination treatment significantly increased the expression of eNOS in coronary artery tissue (Fig. 2). These results suggest that combination therapy can induce coronary vasodilation and enhance the myocardial O2 supply, which may protect the heart and vessels from cardiovascular injuries. Moreover, combination therapy significantly reduced neointima hyperplasia induced by cuff placement around the femoral arteries of mice (Fig. 3). Long-term hypertension causes damage to blood vessels and excessive proliferation of VSMCs, which leads to cardiovascular diseases, including atherosclerosis (Barton 2013). Thus, the results suggest that combination therapy can protect from abnormal excessive proliferation of VSMCs by inhibiting DNA synthesis in both the intima and media layers of blood vessels. Protection against vascular damage is an important goal of treating hypertension.
Diabetes is a risk factor for cardiovascular disease, and cardiovascular complications are a major cause of death in diabetic patients (Nasrallah et al. 2016). Cilnidipine monotherapy managed high blood glucose levels (Fig. 4), unlike telmisartan alone. Although cilnidipine and telmisartan combination treatment resulted in a significant tolerance after a high glucose load in 2 h, the combination therapy was no more effective than the single dose of cilnidipine. Thus, combination therapy is no more effective than monotherapy for controlling hyperglycemia.
Conclusion
Combination therapy with fixed-dose cilnidipine and telmisartan can effectively manage BP without reflex tachycardia and may induce cardioprotective activity by increasing eNOS expression. Moreover, cilnidipine and telmisartan combination therapy may protect against cuff-induced neointima formation by inhibiting DNA synthesis and manage high blood glucose levels. Taken together, these results suggest that combination treatment with cilnidipine and telmisartan may be an effective tool for treating metabolic syndrome consisting of hypertension, atherosclerosis, and diabetes.
References
Akhrass PR, Mcfarlane SI (2011) Telmisartan and cardioprotection. Vasc Health Risk Manag 7:677–683
Azizi M, Rossignol P, Hulot JS (2019) Emerging drug classes and their potential use in hypertension. Hypertension 74:1075–1083
Barton M (2013) Mechanisms and therapy of atherosclerosis and its clinical complications. Curr Opin Pharmacol 13:149–153
Buford TW (2016) Hypertension and aging. Ageing Res Rev 26:96–111
Charles L, Triscott J, Dobbs B (2017) Secondary hypertension: discovering the underlying cause. Am Fam Physician 96:453–461
Choi WS, Lee JJ, Kim Y, Kim IS, Zhang WY, Myung CS (2011) Synergistic improvement in insulin resistance with a combination fenofibrate and rosiglitazone in obese type 2 diabetic mice. Arch Pharm Res 34:615–624
Cuspidi C, Tadic M, Grassi G, Mancia G (2018) Treatment of hypertension: the ESH/ESC guidelines recommendations. Pharmacol Res 128:315–321
Galzerano D, Capogrosso C, Di Michele S, Galzerano A, Paparello P, Lama D, Gaudio C (2010) New standards in hypertension and cardiovascular risk management: focus on telmisartan. Vasc Health Risk Manag 6:113–133
Guerrero-Garcia C, Rubio-Guerra AF (2018) Combination therapy in the treatment of hypertension. Drugs Context 7:
Helmer A, Slater N, Smithgall S (2018) A review of ACE inhibitors and ARBs in black patients with hypertension. Ann Pharmacother 52:1143–1151
Jung SH, Han JH, Park HS, Lee DH, Kim SJ, Cho HS, Kang JS, Myung CS (2019) Effects of unaltered and bioconverted mulberry leaf extracts on cellular glucose uptake and antidiabetic action in animals. BMC Complement Altern Med 19:55
Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, Okayama A, Kawano Y (2008) Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension 52:652–659
Kramer HJ, Townsend RR, Griffin K, Flynn JT, Weiner DE, Rocco MV, Choi MJ, Weir MR, Chang TI, Agarwal R, Beddhu S (2019) KDOQI US Commentary on the 2017 ACC/AHA Hypertension Guideline. Am J Kidney Dis 73:437–458
Kulandavelu S, Balkan W, Hare JM (2015) Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci USA 112:6254–6255
Lee JJ, Shin CY, Park HJ, Zhang WY, Kim Y, Kim IS, Lee KH, Myung CS (2010) Drug synergism of antihypertensive action in combination of telmisartan with lercanidipine in spontaneous hypertensive rats. Arch Pharm Res 33:1411–1418
Lee DH, Jo EJ, Ga EJ, Han JH, Jung SH, Park HS, Heo KS, Myung CS (2017) Effects of combination therapy with candesartan and ramipril on hypertension and related complications. J Pharm Investig 47:365–371
Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, Kim KI, Kim JH, Lee JH, Park JM, Pyun WB, Chae SC (2019) 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens 25:20
Mead TJ, Lefebvre V (2014) Proliferation assays (BrdU and EdU) on skeletal tissue sections. Methods Mol Biol 1130:233–243
Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J (2016) Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134:441–450
Moen MD (2010) Telmisartan/amlodipine: single-pill combiation in hypertension. Am J Cardiovasc Drugs 10:401–412
Musini VM, Rezapour P, Wright JM, Bassett K, Jauca CD (2015) Blood pressure-lowering efficacy of loop diuretics for primary hypertension. Cochrane Database Syst Rev:CD003825
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Nan MH, Park JS, Myung CS (2010) Construction of adiponectin-encoding plasmid DNA and gene therapy of non-obese type 2 diabetes mellitus. J Drug Target 18:67–77
Nasrallah R, Hassouneh R, Hebert RL (2016) PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol 27:666–676
Nezu T, Hosomi N, Aoki S, Suzuki N, Teshima T, Sugii H, Nagahama S, Kurose Y, Maruyama H, Matsumoto M (2018) Effects of cilnidipine, an L/N-type calcium channel blocker, on carotid atherosclerosis in Japanese post-stroke hypertensive patients: results from the CA-ATTEND Study. J Atheroscler Thromb 25:490–504
Nguyen Q, Dominguez J, Nguyen L, Gullapalli N (2010) Hypertension management: an update. Am Health Drug Benefits 3:47–56
Oliveras A, De La Sierra A (2014) Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens 28:213–217
Ozemek C, Laddu DR, Arena R, Lavie CJ (2018) The role of diet for prevention and management of hypertension. Curr Opin Cardiol 33:388–393
Park ES, Kang DH, Yang MK, Kang JC, Jang YC, Park JS, Kim SK, Shin HS (2014) Cordycepin, 3’-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovasc Toxicol 14:1–9
Park HS, Lee DH, Han JH, Jung SH, Lee M, Jang KW, Myung CS (2020) The effects of combined treatment of losartan and ramipril on hypertension and related complications. J Pharm Investig 50:573–581
Perumareddi P (2019) Prevention of hypertension related to cardiovascular disease. Prim Care 46:27–39
Punzi H, Neutel JM, Kereiakes DJ, Shojaee A, Waverczak WF, Dubiel R, Maa JF (2010) Efficacy of amlodipine and olmesartan medoxomil in patients with hypertension: the AZOR Trial Evaluating Blood Pressure Reductions and Control (AZTEC) study. Ther Adv Cardiovasc Dis 4:209–221
Shin CY, Choi WS, Yi I, Nan MH, Myung CS (2009) Synergistic decrease in blood pressure by captopril combined with losartan in spontaneous hypertensive rats. Arch Pharm Res 32:955–962
Singh M, Singh AK, Pandey P, Chandra S, Singh KA, Gambhir IS (2016) Molecular genetics of essential hypertension. Clin Exp Hypertens 38:268–277
Siragusa M, Fleming I (2016) The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch 468:1125–1137
Suzuki H, Kanno Y, Efficacy of Candesartan on Outcome in Saitama Trial G (2005) Effects of candesartan on cardiovascular outcomes in Japanese hypertensive patients. Hypertens Res 28:307–314
Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A (2006) Endothelium, aging, and hypertension. Curr Hypertens Rep 8:84–89
Tamargo J, Ruilope LM (2016) Investigational calcium channel blockers for the treatment of hypertension. Expert Opin Investig Drugs 25:1295–1309
Yi I, Lee JJ, Park JS, Zhang WY, Kim IS, Kim Y, Shin CY, Kim HS, Myung CS (2010) Enhanced effect of losartan and rosuvastatin on neointima hyperplasia. Arch Pharm Res 33:593–600
Acknowledgements
This study was financially supported by the research fund of Chungnam National University (2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors (J.-H. Jo, D.-H. Lee, J.-H. Han, M. Lee, K.-W. Jang, and C.-S. Myung) declare that they have no conflict of interest.
Human and animal rights
This work complies with all ethical standards. Animal experiments were approved by the Chungnam National University Animal Care and Use Committee (2009-2-19).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, JH., Lee, DH., Han, JH. et al. Effects of combination treatment with cilnidipine and telmisartan on hypertension, cardiovascular injury, and high blood glucose. J. Pharm. Investig. 51, 337–346 (2021). https://doi.org/10.1007/s40005-021-00522-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-021-00522-2