Abstract
Diuron is a widely used herbicide worldwide, and its toxic effects on aquatic and amphibious organisms have received extensive concerns. However, little information is available regarding the impacts of diuron on non-target soil organism earthworm. Thus, this work investigated the toxic effects of environmentally relevant concentrations (i.e., 0.05, 0.5, and 5.0 mg/kg soil) of diuron on earthworm Eisenia fetida according to multiple biomarkers including oxidative stress, DNA damage, and histopathology. Results showed that diuron significantly induced the production of reactive oxygen species (ROS) and presented a dose–effect relationship (R2 > 0.6) during the first 21 days of exposure. On the seventh day, the activity of superoxide dismutase (SOD) in E. fetida exposed to 0.05, 0.5, and 5.0 mg/kg of diuron decreased by 3.3%, 17.8%, and 37.4% with respect to the control, respectively, while the activities of catalase (CAT), peroxidase (POD), and glutathione S-transferase (GST), as well as the content of malondialdehyde (MDA) increased to varying degrees. Diuron resulted in low damage of coelomocyte DNA in E. fetida, while no tissue damage was observed on days 7 and 14. At the end of exposure period (28 d), except for ROS, all other biomarkers in the diuron-treated groups were restored to the control level. Integrated biological response (IBR) showed that ROS and GST are sensitive biomarkers to monitor the potential toxicity effect of diuron on E. fetida. The results of this study provide valuable information for risk assessment of diuron on soil ecosystem health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, herbicides are playing an increasingly important role in weeds control and crop productivity improvement. Nevertheless, with the ubiquitous use of herbicides, a myriad of negative effects on environmental safety and human health are inevitably expected (Fugère et al. 2020; Van Bruggen et al. 2018). Among herbicides, phenyl urea herbicide diuron (N-(3,4-dichlorophenyl)-N, N-dimethylurea) is widely used during pre-emergence and post-emergence to control annual and perennial monocotyledonous and dicotyledonous weeds in agricultural soils. It is also often used in non-agricultural soils, such as industrial and domestic sites, for weed and vegetation control (Liu et al. 2010). The water solubility at 25 °C and octanol/water coefficient (log Kow) of diuron is 42.0 mg/L and 2.68, respectively, which means diuron has moderate mobility in soils and can enter the aquatic ecosystems from terrestrial environments by leaching and runoff (Dores et al. 2009). At present, the environmental consequences of diuron on aquatic ecosystems are of great concern. Previous studies showed that diuron could cause various levels of toxic effects on fish and amphibian such as zebrafish (Velki et al. 2017), goldfish (Bretaud et al. 2000), flounder (Moon et al. 2019), medaka (Kamarudin et al. 2020), and tadpole (Moreira et al. 2019). In the soil environment, diuron is considered high persistence, with several studies which have reported the half-lives of diuron in soil ranging from one month to one year (Madhum and Freed 1987; Rocha et al. 2013; Rouchaud et al. 2000). The quantity of diuron in soil could be reached about 0.5 mg/kg when it was used as recommended dose (Giacomazzi and Cochet, 2004; Gooddy et al. 2002). Up to date, most of studies analyzed the adsorption and desorption behavior of diuron in various types of soils (El-Nahhal et al. 2013; Kasozi et al. 2010; Liu et al. 2010; Muendo et al. 2021). However, little information about the toxicity of this herbicide on important soil organisms such as earthworms is reported.
Earthworms are the largest group of soil organisms, which provide vital ecosystem services, and are often referred to as ‘soil ecosystem engineers’ (Jouquet et al. 2006). They are susceptible to exogenous stress and often used as an important model organism to monitor the toxicity effects of xenobiotics on soil ecosystems (Button et al. 2010; Calisi et al. 2009; Li et al. 2020; Zhang et al. 2015). In this regard, many biomarkers such as growth, reproduction, avoidance behavior, oxidative stress, DNA damage, changes in subcellular morphology, and histology are often used alone or together to evaluate the responses of earthworms to different pesticides (Pelosi et al. 2014). Among which, anti-oxidative defense system at the physiological–biochemical level and DNA damage at the molecular level are very roust to capture earthworms’ responses to herbicides at very low concentrations (Shi et al. 2017). A previous study reported that earthworm Eisenia andrei showed an obvious avoidance behavior in the soil spiked with 5–100 mg/kg diuron (Lackmann et al. 2018). However, whether diuron can affect earthworms at physiological–biochemical and molecular levels at environmentally relevant concentrations still need to be clarified.
Based on standard of the Organization for Economic Cooperation and Development (OECD 1984), in this study, the earthworm E. fetida was selected as model organism to evaluate the toxicity effect of diuron. The changes of oxidative stress, DNA damage, and histopathology were tested. Moreover, the Integrated Biological Response version 2 (IBRv2) was employed to analyze the integrated responses in E. fetida toward diuron. The objective of this work was to further figure out the sublethal toxic effects of environmentally relevant concentrations of diuron on the earthworms. The findings from this study will provide valuable data regarding the ecological risk assessment of diuron in the soil ecosystems.
Materials and methods
Chemicals, soil, and earthworms
Diuron (95% purity) was obtained from Hailir Pesticides and Chemicals Group located in Qingdao city, China. Other reagent-grade chemicals were purchased from commercial corporations. The soil used in exposure experiment was taken from surface soil (0–20 cm) located in garden of Qingdao Agricultural University, China (120°23′12.22384″E, 120°23′12.22384″E) with no pesticide application history in the past five years. The soil sample belongs to typical silty clay loam in China, which consists of 31.2% sand, 68.4% silt, 0.4% clay and pH value is 6.5 (1:2.5 w/v in water). The air-dried soil samples were ground to pass through a 2-mm sieve and then defaunated in an incubator at 60 °C for 48 h prior to exposure experiment. The adult earthworms (E. fetida) were purchased from the Wangjun Earthworm Breeding Farm (Jurong, Jiangsu, China) and acclimatized at 20 ± 1 °C in the laboratory for two weeks prior to exposure experiment.
Exposure experiment
According to published studies, the initial residue concentrations of diuron in soils were around 0.5 mg/kg at the recommended application usage (Baer and Calvet 1999; Gooddy et al. 2002). Therefore, in this study, the environmentally relevant concentrations (ERC) of diuron were set as 0.05 (0.1 × ERC), 0.5 (1 × ERC), 5.0 mg/kg (10 × ERC) dry soil. Firstly, 62.5 mL of 0.4, 4, and 40 mg/L diuron aqueous solution were thoroughly mixed with the 500 g soil to obtain the nominal concentration. The soil spiked with 62.5-mL distilled water was set as control. Each treatment was repeated four times. Each spiked soil sample was placed into a 1-L glass beaker, and the soil moisture was set to 35% by adding distilled water (Zhang et al. 2015). Secondly, 300–600 mg of healthy E. fetida with well-developed clitella were transferred to moist filter papers for 24 h at 20 ± 1 °C to empty their gut contents. The gut-cleaned E. fetida were randomly put into each beaker. Each beaker contains twenty E. fetida. Finally, the beaker was covered with perforated sealing film and maintained in an incubator with the 12 h/12 h light–dark cycle at 20 ± 1 °C for 28 days (Li et al. 2022). On days 7, 14, 21, and 28, five E. fetida were randomly sampled from each beaker (total of twenty E. fetida for each treatment). Among them, five E. fetida were used for determining reactive oxygen species (ROS), five for determining the activity of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione S-transferase (GST), and content of malondialdehyde (MDA), five for determining DNA damage, and five for histopathological observation. The selected E. fetida were rinsed with 0.9% saline and maintained on clean moist filter paper at 20 ± 1 °C in the dark for 24 h to purge their gut contents before tests. No dead earthworms were observed during the experimental period.
Determination of enzymatic activity, MDA content, and ROS
The gut-cleaned E. fetida were weighed and placed into a glass mortar and ground in liquid nitrogen. The ground E. fetida powders were transferred into a tube containing ice-cold phosphate buffer solution (50 mM, pH 7.5, 1:9 w/v). The mixtures were centrifuged at 10,000 rpm for 20 min at 4 °C. The yielded supernatants were used for determination of protein content, enzymatic activity, and MDA content. The protein concentration was measured by dye binding method described by Bradford (1976) with bovine serum albumin as the standard. The absorbance was determined at 595 nm, and the protein content was calculated based on the constructed standard curve. The SOD activity was determined by nitro blue tetrazolium method (Durak et al. 1993). The CAT activity was determined by H2O2 consumption method reported by Xu et al. (1997). The POD activity was assayed by guaiacol method (Kochba et al. 1977). The GST activity was assayed by 1-chloro-2,4-dinitrobenzene colorimetry (Habig et al. 1974). The MDA content was measured by thiobarbituric acid method (Xiang and Wang 1990). All the detailed operating procedures of above biomarkers are given in the Supplementary Information.
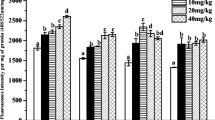
The ROS level was determined according to the method of 2,7-dichlorofluorescin diacetate (DCFH-DA) fluorescence (Lawler et al. 2003). Briefly, the ground earthworms were mixed with ice-cold potassium phosphate buffer (100 mM, pH 7.5, 1:9 w/v). The mixtures were firstly centrifuged at 3000 rpm at 4 °C for 10 min and the supernatants were again centrifuged at 20,000 rpm at 4 °C for 20 min. The pellet was re-suspended with potassium phosphate buffer (pH 7.5) to obtain a mitochondrial suspension. Next, the DCFH-DA solution was added into mitochondrial suspension (2 μM) and incubated at 37 °C for 30 min. The reaction was terminated by adding 200 μL1 mol/L of HCl, and the fluorescence intensity was assayed 488 nm excitation and 522 nm emission by a fluorescence spectrophotometer (F7000, Hitachi, Japan). The result was defined as the fluorescence intensity per mg protein.
DNA damage and histology examination
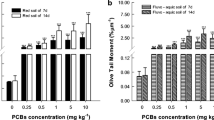
In this study, comet assay was employed to examine the DNA damage in E. fetida caused by diuron. Firstly, the gut-cleaned E. fetida were placed in 1 mL of extrusion medium (5% ethanol, 95% saline, 2.5 mg/mL EDTA, and 10 mg/mL guaiacol glyceryl ether) for 3 min to induce excretion of coelomocytes (Eyambe et al. 1991). The coelomocytes suspensions were centrifuged at 3000 rpm at 4 °C for 10 min and the supernatant was discarded. The coelomocytes sediment was suspended by adding 1 mL of phosphate buffer (pH 7.4) and used for comet assay. The comet assay was conducted by a method of Singh et al. (1988), and the detailed protocols are provided in the Supplementary Information. Finally, the prepared samples were stained with 0.03 mg/mL of ethidium bromide for 10 min and viewed by a fluorescence microscope (Olympus BX53, Japan). At least 100 cells were captured from each treatment and analyzed by Comet Assay Software Project (CASP) software (Końca et al. 2003). The olive tail moment (OTM) was used for quantitation of DNA damage extent.
In this study, the hematoxylin–eosin (HE) staining method was used to study the epidermal and intestinal histology of E. fetida after 28-d exposure (Wang et al. 2015). Briefly, the clitellum posterior parts of gut-cleaned earthworms were cut transversely and then fixed for 24 h using 10% formalin. The severed tissues were embedded in paraffin and sliced vertically for 4-µm thick with a freezing microtome. Sections of 4 µm thickness were stained with HE and observed using an optical microscope for histology analysis.
Data treatment and statistical analysis
The IBRv2 values of SOD, CAT, POD, GST, ROS, and MDA were calculated according to the method reported by Sanchez et al. (2013). All data are presented as the mean ± standard deviation (SD) and analyzed by SPSS 19.0 statistical software. The homoscedasticity and normality of variance were checked prior to statistical analysis. If the data meet homoscedasticity and normality, the differences between various treatments were compared using one-way analysis of variance (ANOVA) followed by the Tukey’s test (p < 0.05). If not, a nonparametric Kruskal–Wallis test was employed to compare the statistical significance (p < 0.05). The software OriginPro 2019 (Origin Lab, Northampton, Massachusetts, USA) was used to plot.
Results and discussion
Oxidative stress of diuron on E. fetida
The ROS, by-products of aerobic metabolism, contain superoxide anion (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH⋅), which serve as signaling molecules in regulation of molecular and physiological processes in the cells of living organisms (Finkel 2011). Moreover, ROS are often associated with the oxidative stress, which indicates that ROS can induce pathology through damaging proteins, lipids, and DNA (Schieber and Chandel 2014). Generally, ROS maintains dynamic balance under normal physiological activities. Once this balance is interrupted, the overproduction of ROS will produce oxidative stress in the organisms (Sharma et al. 2012). The result of this study showed that diuron treatments significantly (p < 0.05) induced the production of ROS in E. fetida as compared to the control during the first 21 days of exposure. In addition, the quantity of ROS and diuron concentration showed an obvious dose–effect relationship. The correlation coefficients (R2) are 0.8537, 0.6169, and 0.7373 on days 7, 14, and 21, respectively (Fig. 1). These findings suggested occurrences of oxidative stress and the probable damage of cells in E. fetida exposed to diuron (Hou et al. 2016). Moreover, the results showed that exposure dose (diuron concentration) played a more important role than exposure time during the first 21 days of exposure (Fig. 1). At the last exposure time (28 d), only the highest concentration (5.0 mg/kg, 10 × ERC) of diuron induced significant (p < 0.05) production of the ROS in E. fetida as compared to the control (Fig. 1), thus indicating that oxidative stress caused by diuron was reversible and temporary. This can be attributed to the adaption of the earthworms to the contaminated soil, regulation of their antioxidative systems, and metabolism of diuron (Wang et al. 2016; Zhang et al. 2014).
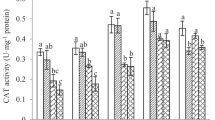
In living organisms, there are enzymatic and non-enzymatic mechanisms involved in the regulation of ROS for protection of cells from the exogenous stress (Lin et al. 2010). Among antioxidant enzymatic systems, SOD, CAT, and POD play crucial roles in scavenging excessive ROS. The SOD can decompose free radical O2– into H2O2, and the H2O2 can be further catalyzed into H2O and O2 by CAT and POD (Lin et al. 2012; Soares et al. 2016). As expected, the result of this study showed that activities of three antioxidant enzymes changed obviously in the earthworm exposed to diuron as compared to the control especially at the early stages of exposure (Fig. 2). The SOD activity was remarkably inhibited by diuron on day 7. Its activity decreased by 3.3%, 17.8%, and 37.4% in 0.05, 0.5, and 5.0 mg/kg diuron treatments as compared with the control, respectively (Fig. 2a). The reduced SOD activity could be attributed to the higher concentrations of diuron-induced excess ROS (Fig. 1) which exceeded the catalytic threshold of SOD, thereby inhibiting the generation of SOD and making them inactive (Li et al. 2020). With the exposure extension, the activities of SOD were significantly stimulated only by the highest concentration of diuron on days 14 and 21, and this stimulation effect restored to the control level at the last exposure time (28 d, Fig. 2a). The increase in SOD activity indicated that SOD in the earthworms recovered its activity and had capacity to eliminate the excessive ROS with time (Zhang et al. 2015). In accordance with our results, similar effects were reported in case of pesticides such as atrazine and thifluzamide which induced an increase in the SOD activity in E. fetida (Song et al. 2009; Yao et al. 2020). As for CAT, its activity has no obvious changes at low (0.05 mg/kg, 10 × ERC) and medium (0.5 mg/kg, 1 × ERC) concentrations of diuron treatments as compared with the control during the whole exposure period, while the highest concentration of diuron (5 mg/kg, 10 × ERC) significantly (p < 0.05) stimulated the activity of CAT on days 7, 14, and 21 (Fig. 2b). This indicated that CAT in E. fetida could effectively scavenge the excessive H2O2 under the stress of 0–5.0 mg/kg of diuron (Li et al. 2020). However, the POD activity exhibited a different response to diuron stress as compared to CAT activity in our study. Under the stress of 5.0 mg/kg diuron, the POD activity showed a changing trend of activation–inhibition–activation-return to the control level (Fig. 2c). This difference between CAT and POD activities may be attributed to both antioxidant enzymes that perform different roles in scavenging the H2O2. The CAT is present in cytosol, peroxisomes, and mitochondria, and it can directly scavenge H2O2 (Gill and Tuteja 2010). However, the POD can utilize co-substrates such as guaiacol and ascorbate to indirectly decompose H2O2 (Soares et al. 2016). In addition, based on our results, both CAT and POD have a synergistic complementary effect against the stress of ROS induced by diuron. Similar to our results, this synergistic effect of antioxidant enzymes in earthworms to resist stress caused by xenobiotics have also been stated by previous studies (Łaszczyca et al. 2004; Wang et al. 2017; Zhang et al. 2013). The GST is an important detoxification enzyme which can participate in eliminating the excessive ROS, contaminants, and metabolites of lipid peroxidation via interacting with glutathione and electrophiles (Oliveira et al. 2009). It has been widely used as a sensitive biomarker to assess the impact of contaminants on earthworms. In this study, the activities of GST in 0.05 and 0.5 mg/kg of diuron treatments have no obvious difference with respect to the control. However, a significant stimulation (p < 0.05) was observed under the highest concentration of diuron (5.0 mg/kg) treatment (Fig. 2d). This indicated that GST effectively participated in the detoxification of diuron in E. fetida. This phenomenon has been confirmed by the study of Aly and Schröder (2008), where herbicide metolachlor was found a substrate for the GST. It is worth mentioning that at the last exposure time (28 d), four enzymatic activities were all returned to the control level. This indicated that the oxidative stress caused by diuron (0–5 mg/kg) in the earthworms will gradually disappear with the extension of exposure time and diuron degradation (Bretaud et al. 2000; Gooddy et al. 2002; Zhang et al. 2014).
Changes of SOD a, CAT b, POD c, and GST d activity in E. fetida exposed to diuron. Each column is the mean of three replicates, the error bar is the deviation (SD). The letters indicated significant differences at p < 0.05 among treatments at the same exposure time. Pr is the abbreviation of protein
The MDA is a product of lipid membrane peroxidation and its content can indicate lipid peroxidation level from the side (Saint-Denis et al. 2001). Therefore, the content of MDA in E. fetida was determined for further clarifying whether environmentally relevant concentrations of diuron could result in severe lipid peroxidation in the earthworms. The results showed that low concentration of diuron (0.05 mg/kg) did not cause increment in MDA contents. However, medium concentration of diuron (0.5 mg/kg) result in the significant (p < 0.05) increment in MDA contents on day 7, while the contents of MDA were significantly increased under high concentration (5.0 mg/kg) of diuron on days 7 and 14 (Fig. 3). Based on the result of ROS and enzymatic activity (Fig. 1 and Fig. 2), this might be attributed to the fact that the excessive ROS induced by high concentrations of diuron could not be completely scavenged by antioxidative enzyme systems at the early stage of exposure in E. fetida, thereby causing peroxidation of the lipid membranes. Likewise, previous studies also reported that the environmentally relevant concentrations of herbicides (e.g., sulfentrazone and fomesafen) only resulted in a short-term lipid peroxidation at the early days of exposure in E. fetida (Li et al. 2020; Zhang et al., 2013). On days 21 and 28, the MDA contents in all diuron treatments have no significant differences with those of the control treatments (Fig. 3), thus indicating that diuron-induced lipid peroxidation in E. fetida was gradually alleviated by the combined action of antioxidant and detoxification enzymes with time (Li et al. 2022). Overall, according to the results of ROS, enzymatic activities, and MDA contents, we conclude that environmentally relevant concentrations of diuron (0–5 mg/kg) only lead to a mild oxidative stress to E. fetida at the early exposure period (< 14 d).
DNA damage and histopathology in E. fetida under the stress of diuron
Oxidative damage is often closely related to genotoxicity in organisms since DNA damage is usually considered as the result of oxidative stress and lipid peroxidation (Dogan et al. 2011). Because ever since Ostling and Johanson (1984) developed comet assay, it has been widely accepted as a rapid, sensitive, and simple technology to detect DNA damage of living organisms (Button et al. 2010; Mitchelmore et al. 1998). Therefore, we used comet assay to determine whether diuron can result in DNA damage of E. fetida. In this study, results showed that OTM values in the diuron treatments have no statistical difference with those of the control treatments on day 7 (Fig. 4), indicating that diuron (0–5 mg/kg) cannot cause DNA damage to E. fetida in a short time. However, with the exposure extension, the significant enhancement (p < 0.05) of OTM values was observed in E. fetida exposed to both 0.5 and 5.0 mg/kg of diuron on day 14 (Fig. 4). Moreover, the OTM values were positively related to the concentrations of diuron. This indicated that medium and high concentrations of diuron can cause a potential DNA damage in the coelomocytes of E. fetida at the early exposure. The reason, according to our results and previous studies, can be attributed to the accumulation of ROS and oxidative stress, which ultimately led to DNA damage (Dogan et al. 2011; Song et al. 2019). Interestingly, after 14 days, the OTM values in diuron treatment gradually declined and restored to the control level on day 28 (Fig. 4), indicating that DNA damage caused by diuron was alleviated over exposure time. In line with our study, many previous studies also stated that DNA damage could be alleviated after longer exposure time in the earthworms exposed to pesticide spirotetramat and sulfentrazone (Li et al. 2020; Zhang et al. 2015). The mitigation of DNA damage could be attributed to the pesticide dissipation, antioxidant enzymes protection, and DNA self-repair (Ching et al. 2001; Li et al. 2018; Wang et al. 2018). According to the DNA damage criterion described by Mitchelmore et al. (1998), the higher concentrations of diuron (0.5 and 5.0 mg/kg) only resulted in low damage to the coelomocytes of E. fetida on days 7 and 14. On days 21 and 28, diuron cannot result in DNA damage to E. fetida, indicating that DNA damage will be self-repaired over time when the earthworms are subjected to lower stress (Li et al. 2020).
Histopathology has become an important and effective tool for investigating the impacts of pollutants on earthworms at the cellular level (Li et al. 2022; Yang et al. 2018). In this study, both epidermis and gut tissues of E. fetida were observed because xenobiotics can be contacted via direct and digestive absorption ways (Qi et al. 2018). The result of this study showed that all parts of epidermis and gut tissues in E. fetida have no obvious differences between control and diuron treatments (0–5.0 mg/kg) after 28 days of exposure (Fig. 5), suggesting that environmentally relevant concentrations of diuron could not result in tissue damage of E. fetida in a longer exposure period. This may be attributed to the fact that earthworms have a great power of regeneration when they were suffered from slight damage by xenobiotic pollutants (Morowati 2000; Rouchaud et al. 2000).
Integrated assessment of diuron exposure
In order to further clarify the comprehensive influence caused by diuron, the IBRv2 index was applied because it can provide an intuitive comparison of evaluating the responses of multiple biomarkers in living organisms under environmental stress (Li et al. 2020; Sanchez et al. 2013). In this study, the integrated responses of ROS, SOD, POD, CAT, GST, and MDA were analyzed and compared. The result showed that the distance from zero in 5.0 mg/kg diuron treatment was obviously far away from 0.05 and 0.5 mg/kg diuron treatments (Fig. 6a), indicating that the highest concentrations of diuron caused the most serious oxidative damage in the E. fetida (Yang et al. 2018). Among the biomarkers, the IBRv2 values of ROS and GST were higher than those of other biomarkers (Fig. 6a), suggesting ROS and GST are sensitive biomarkers in the earthworms toward the stress of diuron. Similar to our study, previous studies also demonstrated that ROS could be firstly induced in the organisms once they were under stress of xenobiotics (e.g., mandipropamid and sulfentrazone) and GST likely played more important role in alleviating the oxidative stress compared to other.
antioxidases (Fang et al. 2021; Li et al. 2022). The IBRv2 values of SOD, CAT, and POD are intertwined with each other in various exposure times, thus further indicating that these three antioxidases in E. fetida work together against the oxidative stress from diuron. On the timeline, the total IBRv2 values of 5.0 mg/kg diuron treatment showed a tendency of first rise and then decline (Fig. 6b), implying that the diuron-induced oxidative stress effect in E. fetida was related with exposure time, and it was reduced over time (Du et al. 2015; Song et al. 2019).
Conclusion
Natural soil test showed that the environmentally relevant concentrations of diuron (0–5.0 mg/kg) induced slight oxidative stress and DNA damage to E. fetida in a short-term exposure (< 14 d). At the end of exposure period (28 d), these negative effects diminished and/or disappeared. The epidermis and gut tissues of E. fetida were not damaged by diuron (< 5.0 mg/kg). IBR showed that ROS and GST can be used as sensitive biomarkers to monitor ecotoxicological toxicity of diuron on the earthworm in the early exposure. Overall, the results of this study indicated that herbicide diuron is relative safety to the earthworms when it is used at the recommended doses in the fields.
References
Aly MAS, Schröder P (2008) Effect of herbicides on glutathione S-transferases in the earthworm, Eisenia fetida. Environ Sci Pollut Res 15:143–149. https://doi.org/10.1065/espr2007.02.385
Baer U, Calvet R (1999) Fate of soil applied herbicides: experimental data and prediction of dissipation kinetics. J Environ Qual 28:1765–1777. https://doi.org/10.2134/jeq1999.00472425002800060012x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bretaud S, Toutant JP, Saglio P (2000) Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotox Environ Safe 47:117–124. https://doi.org/10.1006/eesa.2000.1954
Button M, Jenkin GRT, Bowman KJ et al (2010) DNA damage in earthworms from highly contaminated soils: Assessing resistance to arsenic toxicity by use of the comet assay. Mutat Res-Gen Tox En 696:95–100. https://doi.org/10.1016/j.mrgentox.2009.12.009
Calisi A, Lionetto MG, Schettino T (2009) Pollutant-induced alterations of granulocyte morphology in the earthworm Eisenia foetida. Ecotox Environ Safe 72:1369–1377. https://doi.org/10.1016/j.ecoenv.2009.03.010
Ching EWK, Siu WHL, Lam PKS et al (2001) DNA adduct formation and DNA strand breaks in green-lipped Mussels (Perna viridis) exposed to benzo[a]pyrene: dose-and time-dependent relationships. Mar Pollut Bull 42:603–610. https://doi.org/10.1016/S0025-326X(00)00209-5
Dogan D, Can C, Kocyigit A, Dikilitas M, Taskin A, Bilinc H (2011) Dimethoate-induced oxidative stress and DNA damage in Oncorhynchus mykiss. Chemosphere 84:39–46. https://doi.org/10.1016/j.chemosphere.2011.02.087
Dores EFGC, Spadotto CA, Weber OLS et al (2009) Environmental behaviour of metolachlor and diuron in a tropical soil in the central region of Brazil. Water Air Soil Poll 197:175–183. https://doi.org/10.1007/s11270-008-9801-1
Du L, Li G, Liu M et al (2015) Evaluation of DNA damage and antioxidant system induced by di-n-butyl phthalates exposure in earthworms (Eisenia fetida). Ecotox Environ Safe 115:75–82. https://doi.org/10.1016/j.ecoenv.2015.01.031
Durak I, Yurtarslanl Z, Canbolat O, Akyol Ö (1993) A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta 214:103–104. https://doi.org/10.1016/0009-8981(93)90307-P
El-Nahhal Y, Abadsa M, Affifi S (2013) Adsorption of diuron and linuron in Gaza soils. Amer J Anal Chem 4:94–99. https://doi.org/10.4236/ajac.2013.47A013
Eyambe GS, Goven AJ, Fitzpatrick LC, Venables BJ, Cooper EL (1991) A non-invasive technique for sequential collection of earthworm ( Lumbricus terrestris ) leukocytes during subchronic immunotoxicity studies. Lab Anim 25:61–67. https://doi.org/10.1258/002367791780808095
Fang K, Han L, Liu Y, Fang J, Wang X, Liu T (2021) Enantioselective bioaccumulation and detoxification mechanisms of earthworms (Eisenia fetida) exposed to mandipropamid. Sci Total Environ 796:149051. https://doi.org/10.1016/j.scitotenv.2021.149051
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15. https://doi.org/10.1083/jcb.201102095
Fugère V, Hébert M-P, da Costa NB et al (2020) Community rescue in experimental phytoplankton communities facing severe herbicide pollution. Nat Ecol Evol 4:578–588. https://doi.org/10.1038/s41559-020-1134-5
Giacomazzi S, Cochet N (2004) Environmental impact of diuron transformation: a review. Chemosphere 56(11):1021–1032. https://doi.org/10.1016/j.chemosphere.2004.04.061
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gooddy DC, Chilton PJ, Harrison I (2002) A field study to assess the degradation and transport of diuron and its metabolites in a calcareous soil. Sci Total Environ 297:67–83. https://doi.org/10.1016/S0048-9697(02)00079-7
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hou J, You G, Xu Y et al (2016) Antioxidant enzyme activities as biomarkers of fluvial biofilm to ZnO NPs ecotoxicity and the integrated biomarker responses (IBR) assessment. Ecotox Environ Safe 133:10–17. https://doi.org/10.1016/j.ecoenv.2016.06.014
Jouquet P, Dauber J, Lagerlöf J, Lavelle P, Lepage M (2006) Soil invertebrates as ecosystem engineers: intended and accidental effects on soil and feedback loops. Appl Soil Ecol 32:153–164. https://doi.org/10.1016/j.apsoil.2005.07.004
Kamarudin NA, Zulkifli SZ, Azmai MNA, Abdul AFZ, Ismail A (2020) Herbicide diuron as endocrine disrupting chemicals (EDCs) through histopathalogical analysis in gonads of javanese medaka (Oryzias javanicus, Bleeker 1854). Animals 10:525. https://doi.org/10.3390/ani10030525
Kasozi GN, Nkedi-Kizza P, Agyin-Birikorang S, Zimmerman AR (2010) Characterization of adsorption and degradation of diuron in carbonatic and noncarbonatic soils. J Agric Food Chem 58:1055–1061. https://doi.org/10.1021/jf902792p
Kochba J, Lavee S, Spiegel-Roy P (1977) Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’ orange ovular callus lines1. Plant Cell Phys 18:463–467. https://doi.org/10.1093/oxfordjournals.pcp.a075455
Końca K, Lankoff A, Banasik A et al (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res-Gen Tox En 534:15–20. https://doi.org/10.1016/S1383-5718(02)00251-6
Lackmann C, Velki M, Seiler T-B, Hollert H (2018) Herbicides diuron and fluazifop-p-butyl affect avoidance response and multixenobiotic resistance activity in earthworm Eisenia andrei. Chemosphere 210:110–119. https://doi.org/10.1016/j.chemosphere.2018.07.008
Łaszczyca P, Augustyniak M, Babczyńska A et al (2004) Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland). Environ Int 30:901–910. https://doi.org/10.1016/j.envint.2004.02.006
Lawler JM, Song W, Demaree SR (2003) Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Bio Med 35:9–16. https://doi.org/10.1016/S0891-5849(03)00186-2
Li X, Zhu L, Du Z et al (2018) Mesotrione-induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Ecol Indic 95:436–443. https://doi.org/10.1016/j.ecolind.2018.08.001
Li M, Ma X, Saleem M et al (2020) Biochemical response, histopathological change and DNA damage in earthworm (Eisenia fetida) exposed to sulfentrazone herbicide. Ecol Indic 115:106465. https://doi.org/10.1016/j.ecolind.2020.106465
Li M, Ma X, Wang Y, Saleem M, Yang Y, Zhang Q (2022) Ecotoxicity of herbicide carfentrazone-ethyl towards earthworm Eisenia fetida in soil. Comp Biochem Phys C 253:109250. https://doi.org/10.1016/j.cbpc.2021.109250
Lin D, Zhou Q, Xie X, Liu Y (2010) Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida). Chemosphere 81:1328–1333. https://doi.org/10.1016/j.chemosphere.2010.08.027
Lin D, Xie X, Zhou Q, Liu Y (2012) Biochemical and genotoxic effect of triclosan on earthworms (Eisenia fetida) using contact and soil tests. Environ Toxicol 27:385–392. https://doi.org/10.1002/tox.20651
Liu Y, Xu Z, Wu X, Gui W, Zhu G (2010) Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J Hazard Mater 178:462–468. https://doi.org/10.1016/j.jhazmat.2010.01.105
Madhum YA, Freed VH (1987) Degradation of the herbicides bromacil, diuron and chlortoluron in soil. Chemosphere 16:1003–1011. https://doi.org/10.1016/0045-6535(87)90037-3
Mitchelmore CL, Birmelin C, Livingstone DR, Chipman JK (1998) Detection of DNA strand breaks in isolated mussel (Mytilus edulisL.) digestive gland cells using the “Comet” assay. Ecotox Environ Safe 41:51–58. https://doi.org/10.1006/eesa.1998.1666
Moon Y-S, Kim M, Hong CP, Kang JH, Jung JH (2019) Overlapping and unique toxic effects of three alternative antifouling biocides (Diuron, Irgarol 1051®, Sea-Nine 211®) on non-target marine fish. Ecotox Environ Safe 180:23–32. https://doi.org/10.1016/j.ecoenv.2019.04.070
Moreira RA, Freitas JS, da Silva Pinto TJ et al (2019) Mortality, spatial avoidance and swimming behavior of bullfrog tadpoles (Lithobates catesbeianus) exposed to the herbicide diuron. Water Air Soil Pollut 230:125. https://doi.org/10.1007/s11270-019-4168-z
Morowati M (2000) Histochemical and histopathological study of the intestine of the earthworm (Pheretima elongata) exposed to a field dose of the herbicide glyphosate. Environmentalist 20:105–111. https://doi.org/10.1023/A:1006704009184
Muendo BM, Shikuku VO, Getenga ZM, Lalah JO, Wandiga SO, Rothballer M (2021) Adsorption-desorption and leaching behavior of diuron on selected Kenyan agricultural soils. Heliyon 7:e06073. https://doi.org/10.1016/j.heliyon.2021.e06073
OECD., (1984) Test No 207: Earthworm Acute Toxicity Tests. Organisation for Economic Co-operation and Development, Paris
Oliveira M, Maria VL, Ahmad I et al (2009) Contamination assessment of a coastal lagoon (Ria de Aveiro, Portugal) using defence and damage biochemical indicators in gill of Liza aurata–An integrated biomarker approach. Environ Pollut 157:959–967. https://doi.org/10.1016/j.envpol.2008.10.019
Ostling O, Johanson KJ (1984) Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Bioph Res Co 123:291–298. https://doi.org/10.1016/0006-291X(84)90411-X
Pelosi C, Barot S, Capowiez Y, Hedde M, Vandenbulcke F (2014) Pesticides and earthworms. A Review Agron Sustain Dev 34:199–228. https://doi.org/10.1007/s13593-013-0151-z
Qi S, Wang D, Zhu L et al (2018) Effects of a novel neonicotinoid insecticide cycloxaprid on earthworm, Eisenia fetida. Environ Sci Pollut Res 25:14138–14147. https://doi.org/10.1007/s11356-018-1624-z
Rocha PRR, Faria AT, Silva GSD, Queiroz M, Silva A (2013) Half-life of diuron in soils with different physical and chemical attributes. Ciência Rural 43:1961–1966. https://doi.org/10.1590/S0103-84782013001100007
Rouchaud J, Neus O, Bulcke R, Cools K, Eelen H, Dekkers T (2000) Soil dissipation of diuron, chlorotoluron, simazine, propyzamide, and diflufenican herbicides after repeated applications in fruit tree orchards. Arch Environ Con Tox 39:60–65. https://doi.org/10.1007/s002440010080
Saint-Denis M, Narbonne JF, Arnaud C, Ribera D (2001) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of lead acetate. Soil Biol Biochem 33:395–404. https://doi.org/10.1016/S0038-0717(00)00177-2
Sanchez W, Burgeot T, Porcher J-M (2013) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Shi Z, Tang Z, Wang C (2017) A brief review and evaluation of earthworm biomarkers in soil pollution assessment. Environ Sci Pollut Res 24:13284–13294. https://doi.org/10.1007/s11356-017-8784-0
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Soares C, de Sousa A, Pinto A et al (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bo 122:115–125. https://doi.org/10.1016/j.envexpbot.2015.09.010
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41:905–909. https://doi.org/10.1016/j.soilbio.2008.09.009
Song P, Gao J, Li X et al (2019) Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Environ Int 129:10–17. https://doi.org/10.1016/j.envint.2019.04.074
Van Bruggen AHC, He MM, Shin K et al (2018) Environmental and health effects of the herbicide glyphosate. Sci Total Environ 616–617:255–268. https://doi.org/10.1016/j.scitotenv.2017.10.309
Velki M, Di Paolo C, Nelles J, Seiler TB, Hollert H (2017) Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 180:65–76. https://doi.org/10.1016/j.chemosphere.2017.04.017
Wang K, Pang S, Mu X et al (2015) Biological response of earthworm, Eisenia fetida, to five neonicotinoid insecticides. Chemosphere 132:120–126. https://doi.org/10.1016/j.chemosphere.2015.03.002
Wang X, Martínez MA, Wu Q et al (2016) Fipronil insecticide toxicology: oxidative stress and metabolism. Crit Rev Toxicol 46:876–899. https://doi.org/10.1080/10408444.2016.1223014
Wang C, Zhang Q, Wang F, Liang W (2017) Toxicological effects of dimethomorph on soil enzymatic activity and soil earthworm (Eisenia fetida). Chemosphere 169:316–323. https://doi.org/10.1016/j.chemosphere.2016.11.090
Wang C, Harwood JD, Zhang Q (2018) Oxidative stress and DNA damage in common carp (Cyprinus carpio) exposed to the herbicide mesotrione. Chemosphere 193:1080–1086. https://doi.org/10.1016/j.chemosphere.2017.11.148
Xiang R, Wang DN (1990) The improvement of lipid peroxidation thiobarbituric acid spectrophotometry. Prog Biochem Biophys 17:241–242
Xu J, Yuan X, Lang P (1997) The determination of enzymic activity and its inhibition on catalase by ultraviolet spectrophotometry. Environ Chem 16:73–76
Yang Y, Xiao Y, Chang Y, Cui Y, Klobučar G, Li M (2018) Intestinal damage, neurotoxicity and biochemical responses caused by tris (2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotox Environ Safe 158:78–86. https://doi.org/10.1016/j.ecoenv.2018.04.012
Yao X, Zhang F, Qiao Z et al (2020) Toxicity of thifluzamide in earthworm (Eisenia fetida). Ecotox Environ Safe 188:109880. https://doi.org/10.1016/j.ecoenv.2019.109880
Zhang Q, Zhu L, Wang J et al (2013) Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ Sci Pollut Res 20:201–208. https://doi.org/10.1007/s11356-012-0962-5
Zhang Q, Zhang B, Wang C (2014) Ecotoxicological effects on the earthworm Eisenia fetida following exposure to soil contaminated with imidacloprid. Environ Sci Pollut Res 21:12345–12353. https://doi.org/10.1007/s11356-014-3178-z
Zhang Q, Zhang G, Yin P et al (2015) Toxicological effects of soil contaminated with spirotetramat to the earthworm Eiseniafetida. Chemosphere 139:138–145. https://doi.org/10.1016/j.chemosphere.2015.05.091
Acknowledgements
We appreciate the support of the project from Natural Science Foundation (ZR2016CM11) and Primary Research & Development Plan (2017GSF21112) of Shandong Province, China. We thank three anonymous reviewers for their valuable suggestions on our manuscript. We also thank Ms. Mu Yalin (a member of our laboratory) for her assistance in paper revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Editorial responsibility: Jing Chen.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Wang, Y., Ma, X. et al. Ecotoxicity of herbicide diuron on the earthworm Eisenia fetida: oxidative stress, histopathology, and DNA damage. Int. J. Environ. Sci. Technol. 20, 6175–6184 (2023). https://doi.org/10.1007/s13762-022-04348-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04348-9