Abstract
Cycloxaprid (CYC) is a novel neonicotinoid insecticide with high activity against resistant pests but is safe for mammals. The toxic effects of CYC on earthworms (Eisenia fetida) were studied in this paper. The 14-day exposure results showed that CYC is potentially toxic to earthworms, with a 14d-LC50 of 10.21 mg/kg dry soil, and that it induced tissue damage to the epidermis, gut, and neurochord at sublethal doses. During a 21-day exposure, CYC induced oxidative stress in earthworms, and both enzyme activities of catalase (CAT) and superoxide dismutase (SOD) were impacted. In addition, expression of the genes Cat and Sod were down- and upregulated, respectively. The activity of the enzyme acetylcholinesterase (AChE) was increased at day 7 but decreased at day 21 after CYC exposure, while expression of the signal transduction-related genes was significantly regulated. Our study shows for the first time that negative impacts could be induced by CYC on earthworms under both acute and chronic exposure through oxidative stress and gene regulation. The present study provides a database for assessing the environmental risk to non-target organisms resulting from the use of CYC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neonicotinoid insecticides are the most recently synthesized agrochemicals in the past few decades that have been developed to control an extensive range of crop, vegetable, and fruit pests worldwide by affecting the nicotinic acetylcholine receptors (nAChRs) (Casida 2011, Elbert et al. 2008). Until now, neonicotinoids have drawn great attention because of the speed at which pests develop resistance to them as well as to their high risk to bees (Decourtye and Devillers 2010, Laurino et al. 2011). Studies have shown that even plants can only take up 2–20% of the insecticide from coated seeds, 11 to 24% of pollen, and 17 to 65% of nectar could be contaminated with these neonicotinoids, and at sublethal doses, neonicotinoids still have the potential to induce a variety of behavioral difficulties in honeybees, such as olfactory memory and learning dysfunctions as well as alterations of orientation skills (Sanchez-Bayo 2014, Sanchez-Bayo et al. 2016). Considering these effects, developing new chemicals with a novel structure and mode of action is extremely urgent for comprehensive pest control systems and environmental protection (Su et al. 2012, Xu et al. 2014a).
Cycloxaprid (CYC, Fig. 1) is a novel oxabridged cis-nitromethylene neonicotinoid insecticide developed by East China University of Science and Technology, and it has high activity against imidacloprid-resistant brown plant hopper, aphids, and medfly and lower toxicity to bees (Chang et al. 2015, Cui et al. 2016, Cui et al. 2012, Xu et al. 2014b). Currently, there current knowledge of CYC is lacking. As reported in flooded and anoxic soils, the half-life of CYC is less than 5 days and the chemical changes mainly occur in the chloropyridinyl and seven-member ring (Liu et al. 2015a). In paddy soil with earthworms (Metaphire guillelmi), CYC accumulated in earthworm tissues and the biota-sediment accumulation factors were between 0.59 and 0.82, which resulted in its fast degradation (Liu et al. 2015b). CYC is a good candidate for high-risk neonicotinoid replacement, such as imidacloprid, but currently, there is little relevant knowledge of its toxicity towards non-target organisms. However, this information is critical for the risk evaluation of CYC regarding its environmental impact and for further regulatory decisions.
Neonicotinoids can be used as granules in the soil or as seed-dressings during crop planting. CYC is now registered on rice in China, so the residue in aquatic system and deposition in soil are the main approach for it entering environment and induce potential risks to organisms in these ecosystems. Among many other organisms, earthworms play an important role in maintaining soil fertility and the food chain and have been used as bio-indicators of soil quality and health (Zhang et al. 2014). The earthworm Eisenia fetida is widely available, easily reared, and reproduces rapidly. Therefore, this earthworm has been widely used for toxicological and ecological risk assessments of contamination in soil using lethal endpoints (Feng et al. 2015). To further determine the potential mechanisms of chemicals, inhibition of enzyme activities and gene expression have been used as novel indicators in recent years to characterize toxicity of chemicals to earthworms (Feng et al. 2015, Shi et al. 2017).
Neonicotinoids are toxic to earthworms. For instance, Wang et al. compared the acute toxicity of 24 pesticides towards Eisenia fetida and found that neonicotinoids were more toxic than others in both filter paper toxicity assays and soil toxicity bioassays (Wang et al. 2012). Under sub-lethal concentrations, imidacloprid and thiacloprid can induce significant decreases in earthworm reproduction and weight loss as well as disturb its physiological activity (Capowiez et al. 2006, Capowiez et al. 2005, Dittbrenner et al. 2011). In our earlier studies, imidacloprid, acetamiprid, nitenpyran, clothianidin, and thiacloprid showed high toxicity towards earthworms and seriously affected their reproduction as well as induce tissue damage at sublethal concentrations (Wang et al. 2015b). The toxicity of CYC towards earthworms is unknown. Studies have shown that another novel developed neonicotinoid, Chinese insecticide guadipyr, has low toxicity towards Eisenia fetida but induced significant tissue damage and activity changes of catalase (CAT), superoxide dismutase (SOD) and acetylcholinesterase (AChE) (Wang et al. 2015a). We speculated that CYC might have similar toxicity characteristics towards earthworms. Thus, the goal of this study was to assess the toxic effects of CYC on the earthworm Eisenia fetida using artificial soil and the following outcomes:
-
1.
Fourteen-day acute toxicity of CYC towards earthworms
-
2.
The tissue damage induced by CYC after 14-day exposure at sub-lethal doses
-
3.
The effects of CYC on CAT, SOD, and AChE activity changes
-
4.
The effects of CYC on the expression of the antioxidant genes Sod and Cat in earthworms
-
5.
The effects of CYC on the expression of signal transduction-related genes
Hopefully, this information will help with the registration and application of CYC in the future.
Materials and methods
Chemicals and earthworms
Cycloxaprid (CYC, 99% purity) was obtained from Shanghai Key Laboratory of Chemical Biology of East China University of Science and Technology (Shanghai, China).
Eisenia fetida was purchased from Beijing Dahuan Earthworm Factory (Beijing, China) with the original culture soil and maintained in a box in the laboratory at 20 ± 1 °C. Earthworms were used for experiments after 7 days of acclimation (mortality < 10%) in artificial soil under an artificial climate (20 ± 1 °C, 8 h dark/16 h light photoperiod, illumination of 600 lx, and humidity of 80–85%).
Test soil preparation
Artificial soil (10% sphagnum peat moss, 20% kaolin clay, 68% sand, and 2% CaCO3 to adjust the pH value to 6.0 ± 0.5) was used thorough the study according to the OECD guidelines 207 and 222 (OECD 1984 and 2004) with some modifications. Generally, the desired amount of active ingredient of CYC in 2 mL of acetone was added to 10 g of quartz sand and then mixed thoroughly with 490 g of dry artificial soil after evaporation overnight in fume cupboard. Deionized water was added to give an overall moisture content of 35% of the dry weight. Then, 500 g of artificial soil with insecticide containing 10 earthworms was transferred into a 1-L glass container after 4 h of food deprivation. The container was covered with 1 mm of cotton gauze to ensure sufficient ventilation and kept under the artificial climate described above. Every 7 days at each observation time, 150 mL water was resupplied to retain moisture of test soils. Distilled water was used for the blank control and acetone used for solvent control.
Stability of CYC in artificial soil
The stability of CYC in artificial soil was examined on days 0, 3, 7, 14, and 21 at 0.1, 1.0, and 2.0 mg/kg dry soil. Ten grams of each soil sample was collected into a 50-mL tube within 10 mL of distilled water and 10 mL of acetonitrile. The mixture was homogenized for 3 min and allowed to stand in an ultrasonic water bath for 10 min. Next, 2.5 g of MgSO4 and 5 g of a NaCl mixture was added and shaken for an additional 3 min. After extraction, the sample was centrifuged at 8000 rpm for 5 min and the supernatant was filtered through a 0.2-μM nylon filter into injection bottles. One microliter of the filtrate was directly injected into an Agilent 1200 HPLC-DAD-Q-TOF-MS device (Beijing, China) equipped with an Agilent Proshell 120 SB-C18 analysis column (2.1 × 100 mM, 2.7 μM, Agilent, USA). The column temperature was set at 30 °C, and the flow rate of the mobile phase (acetonitrile: formic acid = 30:70, v/v) was set at 0.2 mL/min.
Acute toxicity test
The acute toxicity of CYC towards earthworms was evaluated referring to the OECD guidelines 207 and 222 (OECD 1984 and 2004). Based on preliminary tests and the test soil preparation, three replicates of 10 earthworms were placed into each container for each treatment with the desired amount of CYC. The containers were kept in the testing chamber, and observations were made at 7 and 14 days to examine the death of earthworms and calculate the median lethal concentrations (LC50s) using a probit regression.
Histology examination
Based on the acute toxicity results, another set of experiments was performed at 0.30, 1.0, and 2.0 mg/kg dry soil of CYC (1/30, 1/10, and 1/5 of the 14d-LC50) plus one blank control. After 14 days of exposure, the effects of CYC on earthworms’ intestines and epidermis were examined according to our earlier studies (Wang et al. 2015b). In brief, three earthworms were collected and kept in beaker covered with two layers of filter paper at the bottom for 6 h at 21 °C to depurate their gut contents fixation with 10% formalin(v/v) for 24 h; the earthworms were then embedded in paraffin. Sections with a thickness of 4 μm at the anterior region were produced by a freezing microtome (LeLCA RM 2016, Germany) and then stained with hematoxylin-eosin (HE) combined with Periodic Acid-Schiff-Alcianblue. A light microscope (OLYMPUS CX31) was used to examine the sections. All of the histology descriptions of the midgut, epidermis, and neurochord were performed qualitatively.
Enzyme activity and gene expression determinations
Five replicates × 10 earthworms were exposure to CYC at 0.10 (1/100 of the 14d-LC50), 1.0 (1/10 of the 14d-LC50), and 2.0 mg/kg dry soil (1/5 of the 14d-LC50) for 21 days to determine the enzyme activity and gene expression changes. At the 7-, 14-, and 21-day from exposure start, two earthworms were randomly collected from each beaker (a total of 10 earthworms were used for each treatment) and deprived of food for 4 h before splitting them into two groups. One group of three earthworms was washed with RNase-free water and then stored at − 80 °C for subsequent total RNA extraction (DP431, RNAprep Pure Kit, Tiangen Biotech, China), cDNA synthesis (G492, 5X All-In-One RT MasterMix Kit, Applied Biological Materials Inc., Canada), and qRT-PCR (RR420A, SYBR Premix Ex Taq Kit, TaKaRa Bio Inc., Japan) (Mu et al. 2015). The relative levels of gene expression were measured in triplicate for each treatment, and the relative increases were calculated using the 2−ΔΔCT method. The evaluated genes were acyl carrier protein (ID: EW1-F1P04-C04, C04), hexosaminidase (ID: EW1-F2P14-D06, D06), neurological dysfunction gene (ID: EW1-F1P10-E08, E08), and signal transduction gene (ID: EW1-F1P07-H02, H02). The primers used in the present study are shown in Table S1 (Supporting information), and β-actin was used as an internal control. The thermal cycle was denaturation for 15 min at 95 °C, followed by 40 cycles at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 32 s.
The second group of three earthworms was individually washed and homogenized on ice within pre-cooled phosphate buffer (50 mmol/L, pH 7.8) in each tube and centrifuged at 5000 rpm, 5 min. The supernatant was transferred to new tubes and centrifuged for another 10 min at 10000 rpm. After 20-fold dilution, protein content of the final supernatant was determined by the Bradford method using Bio-Rad dye reagent and BSA as a standard. Protocols are provided by the Bradford Protein Assay Kit (P0006, Beijing) of Beyotime Company. Then, these diluted supernatants were used for SOD, CAT, and AChE enzyme activity measurements according to the assay kits A001-3, A007-1, and A024, respectively (Nanjing Jiancheng Bioengineering Institute, China) (Wang et al. 2015a). All of the enzyme activities are expressed in enzyme units (EUs) per protein content for comparison, and one EU was defined as the amount of enzyme that degraded one unit of the substrate supplied with the kits.
Statistical analysis
All data are presented as the mean ± standard deviation (SD). During the tests, no significant poison and death was found in acetone control, so only earthworms from blank control were used for the following indicator collection and discussion.
The LC50 was calculated by probit regression using SPSS 17.0 software. For the enzyme activities and relative levels of gene expression, the mean ± standard of each group was calculated in Excel 2017 and then put into software GraphPad Prism 6.0 to tell the differences between each treatment and control using one-way ANOVA analysis method, followed by a Dunnett’s multiple comparison test. The family-wise significance and confidence level was set at 0.05 (95% confidence interval). One asterisk was labeled up the plotted bar in figures when *p < 0.05 and two asterisks when **p < 0.01.
Results
Analysis of CYC in test soil
During the 21-day exposure, the actual doses of CYC in artificial soil are shown in Table 1. The results showed that the deviation of original dose of CYC was less than 20% at the beginning of the test. As the exposure time increased, the concentration of CYC in the soil decreased in a time-dependent pattern. At the end of test, only 2.2–12.6% of the initial dosage was detected. In the present study, test soil was not renewed during exposure. Thus, to avoid ambiguity, a nominal dosage was used for the result expression and discussion.
Acute toxicity and tissue damage of CYC to E. fetida
After exposure to CYC, dead earthworms were counted and the LC50 at 7 and 14 days was calculated to be 13.69 mg/kgdry soil (11.52–16.82) and 10.21 mg/kgdry soil (8.84–11.81), respectively.
At sub-lethal concentrations of 0.30, 1.0, and 2.0 mg/kgdry soil, CYC induced obvious tissue damage to earthworms (Fig. 2). The wall of the earthworm intestine consists of an inner epithelial layer and a circular and longitude muscular layer (1, 2, and 3 in CK in Fig. 2). After 14 days of exposure to CYC, the cuticle had broken holes and underlying circular fibrosis, and longitudinal muscle loss/hyperplasia was also observed. In the gut, the epithelium showed hyperplasia and necrosis, accompanied by a decrease in fold number. In the neurochord, epithelial cells showed necrosis and the inner cells showed fibrosis, with an unclear web structure and some tumor-shaped tissue.
Transverse sections of segments from the anterior region of E. fetida after 14-day exposure to CYC. The first column shows the epidermis and muscles: CK: 1—normal epidermis surface; 2—normal cellular compartmentation; 3—normal longitude muscular cells and 0.3–2.0 mg/kgdry soil groups: 1—irregular epidermis surface; 2—irregular cellular compartmentation; 3—irregular longitude muscular cells; 4—irregular circular muscular cells. The second column shows the control (CK) and damaged guts: 1—irregular cell shape; 2—cytoplasm of sparse density; 3—irregular shape and altered size of nucleus; 4—cells disinterred. The third column shows the control (CK) and treated neurochord: 1—sparse cells; 2—thinner cell walls; 3—swollen nodes; 4—cells disinterred
Effects of CYC on enzyme activities
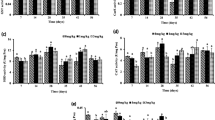
The effects of CYC on the specific activity changes of SOD, CAT, and AChE are shown in Fig. 3. At day 7, the SOD activity was significantly decreased to 41.3 and 69.9% of that of control (2.0 ± 0.1 U/mg pro.) level at 1.0 and 2.0 mg/kgdry soil. At day 14, the specific activity of SOD in the control group was 1.4 ± 0.1 U/mg pro and doubled to 2.6 ± 0.3 and 2.9 ± 0.3 U/mg pro in the presence of CYC at 1.0 and 2.0 mg/kgdry soil, respectively. At day 21, a significant decrease was only found in the 1.0 mg/kgdry soil group, but not after the other treatments.
CAT was less sensitive to CYC exposure. Compared with the control group, no significant changes were found at day 7 and 14 in all groups, while only a significant 32.5% decrease of the control (51.0 ± 10.3 μmol/mg min) was found in the 2.0 mg/kgdry soil treatment group (34.4 ± 5.9 μmol/mg min) at day 21. For AChE, the specific activity was significantly increased by 52.1 and 51.6% of the control level (94.0 ± 6.3 nmol/mg.min) at 1.0 mg/kgdry soil (142.9 ± 19.1 nmol/mg min) and 2.0 mg/kgdry soil (142.5 ± 7.7 nmol/mg min) at day 7, respectively, which was then obviously decreased to 82.8 ± 9.3 nmol/mg min at 1.0 mg/kgdry soil and 66.4 ± 4.5 nmol/mg min at 2.0 mg/kgdry soil at day 21, representing 23 and 38.3% decreases of 107.5 ± 15.2 nmol/mg min in the control group. At day 14, no significant change was observed.
Effects of CYC on gene expression
The effects of CYC on the expression of gene Cat and Sod were shown in Fig. 4. After 7 days of exposure to CYC, expression of Cat was significantly upregulated to 2–3-fold of the control value at 1.0 and 2.0 mg/kgdry soil and 3-fold higher than that of the control at 0.10 and 1.0 mg/kgdry soil on day 14. However, as the exposure time increased to 21 days, expression of Cat gene was significantly downregulated to only 54.2, 45.4, and 21.5% of the control in the 0.10, 1.0, and 2.0 mg/kgdry soil treatments, respectively. Expression of Sod was significantly increased to 3- and 5-fold of that of the control at 1.0 and 2.0 mg/kgdry soil on day 7, 4-fold of that of the control at 2.0 mg/kgdry soil on day 14 and 3-fold of that of the control at 1.0 and 2.0 mg/kgdry soil treatments on day 21.
The genes C04, E08, and D06 are related to nerve dysfunction in earthworms, their relative expression level in earthworms after exposed to CYC were shown in Fig. 5. Throughout the period of exposure to CYC, there was no significant change in expression of C04. Expression of E08 and D06 was significantly increased with the increase of exposure time and concentration. On day 21, the relative expression level of E08 was 2-, 5-, and 5-fold of the control value, respectively, in the 0.10, 1.0, and 2.0 mg/kgdry soil groups and 2-, 3-, and 4-fold for D06 under the same conditions. Expression of H02 was significantly reduced to 20.2 and 16.1% of the control level on day 7 in the 1.0 and 2.0 mg/kgdry soil groups, respectively. When the exposure time increased to 14 days, this downward trend returned to the control level. On day 21, 22.4 and 43.4% decreases were observed at two higher doses, which were also significantly different (Fig. 5).
Discussion
Acute toxicity and tissue damage of neonicotinoids
According to the toxicity classification of chemicals on earthworms in the Chinese guideline (CNSMC, T 31270.15-2014"/T 31270.15-2014), CYC has low toxicity (14d LC50 > 10 mg/kgdry soil) towards earthworms. Compared to the published data, the acute toxicity of CYC to E. fetida was similar to that of thiacloprid but was less toxic than nitenpyram, imidacloprid, acetamiprid, and clothianidin (Supplemental Table S2). Two other new neonicotinoid insecticides, paichongding and guadipyr, have quite low toxicity towards earthworms, and their 14d LC50s were 541.07 and > 100 mg/kgdry soil, respectively (Wang et al. 2015a, Wang et al. 2015b, Wang et al. 2012, Zhang et al. 2017). These differences are mainly due to their various structural activities (Xu et al., 2014a, b).
Histopathology is now a very popular and widely used tool for environmental stress assessment studies at the cellular level (Muangphra et al. 2016). In the present study, after 14 days of exposure under sublethal concentrations, CYC injured the epidermal, gut, and neurochord of earthworms and some cells disintegrated. Similar histopathological examinations have been performed for imidacloprid and other neonicotinoids (Wang et al. 2015b). Skin is the first barrier of earthworms against external stresses, and contaminants in soil are mainly absorbed by the gut in soil; thus, the damage to these tissues would further affect survival or reproduction. Considering the acute toxicity described in this study, CYC has a potential risk to earthworms, and further studies should focus on its chronic toxicity, such as to cell apoptosis, reproduction, and biomass growth.
Neonicotinoids induce antioxidant defense and neurotoxicity in earthworms
Exposure of earthworms to sublethal CYC concentrations induced significant changes in all of the biomarkers studied. Reactive oxygen species (ROS) often increase dramatically in response to damage to cell function and organisms’ homeostasis when environmental stress occurs (Sanchez-Hernandez et al. 2014). During elimination of ROS, SOD and CAT are two important antioxidant enzymes and are a first-line defense against environmental stress. SOD alternately catalyzes the disruption of O2−into O2 or H2O2, which in turn is detoxified by CAT into H2O and O2 (Sanchez-Hernandez et al. 2014, Zhang et al. 2015). SOD and CAT are common used as biomarkers to study the toxicity of contaminants in mammals, fish, insects, water fleas, plants, and so on (Kapoor et al. 2011, Qi et al. 2013, Song et al. 2009). Our results showed that the SOD activity was initially decreased on day 7 and increased on day 14 and then returned to the control level. We assumed that SOD was inactivated by CYC on day 7 because it could not eliminate the vast accumulation of ROS induced by CYC before its fast degradation in soil. Because the doses of CYC significantly decrease after 7 days, the production of ROS also decreased; therefore, SOD was activated to remove the redundant ROS to maintain the balance in earthworms (Zhang et al., 2014). However, as another important antioxidative enzyme, CAT is less sensitive but more effective than SOD at eliminating the effects induced by CYC in earthworms, as CAT activity had no significant change on days 7 and 14 but was slightly inhibited at day 21. The formation of ROS stimulated biosynthesis of SOD and CAT, that is, upregulated expression of Sod and Cat to protect cells against oxidative damage (Zhang et al., 2014, 2017). Inhibition of SOD and CAT corresponded to gene expression increases, which might be due to the excessive generation of ROS along with the increase of the exposure time and concentrations and might require additional enzymes to resolve the excess ROS (Liu et al. 2012). These results showed that CYC was able to induce antioxidant defenses in earthworms and that SOD and CAT as well as Sod and Cat are all good biomarkers for indicating CYC oxidative damage at acute or longer exposures in earthworm.
Life activities are complex and organisms developed a series of mechanisms to defend against diseases and stresses in the long evolution history. Oxidative stress defense is one of the most important self-protection mechanisms and is closely related to function of the nervous system (Federico et al., 2012). The nicotinic nervous system has two principal sites of insecticide action: the nAChR activated by neonicotinoids and AChE inhibited by organophosphorus and methylcarbamate compounds (Shao et al., 2013). AChE is a commonly used biomarker for neonicotinoids as it terminates signal transmission by hydrolyzing acetylcholine (ACh), which binds to ACh receptors on the post-synaptic membrane (Qi et al. 2018). In earthworms after exposed to CYC, AChE activity increased on day 7 but decreased on day 14 at the highest concentrations of 1.0 and 2.0 mg/kgdry soil, which is different from guadipyr (Wang et al. 2015a). We deduce that during CYC binding with nAChRs, it caused ACh elevation in post-synaptic cells which activated AChE to maintain the homeostatic level in cells. As exposure time increase, the content of ACh is too high to eliminate in time and leading to the inhibition on AChE. However, this hypothesis needs more evidence in future. Signals transmitted between nerves contain many pathways, which combined together to maintain cell’s function. C04 is acyl carrier protein controlling gene and is involved in modulation of GABAergic transmission; hexosaminidase plays important roles in the degradation of ganglioside GM2 in neurons (Zhang et al. 2014). Considering all findings in the present study, CYC disturbed the gene expression and the pathways related to antioxidant enzymes, stress responses, neurological dysfunctions, calcium binding and signal transduction (Fig. 6). We predict that CYC cause neurotoxicity in earthworms (Shao et al. 2013; Zhang et al. 2014).
Discovery of neonicotinoid insecticides is a milestone in pesticide research and the fasted-growing leading to their very huge market share and broad application (Han et al. 2018). Chinese researchers also play significant force in the development of neonicotinoids with novel mode of actions. CYC is a brand new compound, and the acute toxicity to earthworm is important endpoint data for its further registration and environmental risk assessment. Even though CYC induced toxicity effects in earthworms, the lower toxicity than imidacloprid and other commercial insecticides to non-target organisms still promise it a good future.
Conclusion
This study is the first to demonstrate the toxic effects of a new neoniotinoid insecticide, CYC, on a soil animal, earthworms. Based on the results, the acute toxicity of CYC to earthworms is low, but CYC induced significant tissue damage and oxidative stress at sublethal dosages in longer exposures. In addition, CYC induced AChE activity changes and affected the transcription of neuronal damage-related genes, indicating possible neurotoxicity of CYC. CYC is a promising insecticide in China, and work is proceeding on evaluating its environmental behavior and characterization (Cui et al. 2016, Cui et al. 2012, Liu et al. 2015a, Liu et al. 2015b), but knowledge of its toxicological effects on non-target organisms is still lacking. This report should be useful for the risk assessment of CYC in soil ecosystems and may provide insight into toxicological research in the future.
References
Azevedo-Pereira HM, Lemos MF, Soares AM (2011) Effects of imidacloprid exposure on Chironomus riparius Meigen larvae: linking acetylcholinesterase activity to behaviour. Ecotox Environ SA 74:1210–1215
Capowiez Y, Rault M, Costagliola G, Mazzia C (2005) Lethal and sublethal effects of imidacloprid on two earthworm species (Aporrectodea nocturna and Allolobophora icterica). Biol Fertil Soils 41:135–143
Capowiez Y, Bastardie F, Costagliola G (2006) Sublethal effects of imidacloprid on the burrowing behaviour of two earthworm species: modifications of the 3D burrow systems in artificial cores and consequences on gas diffusion in soil. Soil Biol Biochem 38:285–293
Casida JE (2011) Neonicotinoid metabolism: compounds, substituents, pathways, enzymes, organisms, and relevance. J Agric Food Chem 59:2923–2931
Chang X, Yuan Y, Zhang T, Wang D, Du X, Wu X, Chen H, Chen Y, Jiao Y, Teng H (2015) The toxicity and detoxifying mechanism of cycloxaprid and buprofezin in controlling Sogatella furcifera (Homoptera: Delphacidae). J Insect Sci 15(1):98
China National Standardization Management Committee (2014) Earthworm acute toxicity test (T 31270.15-2014"/T 31270.15-2014). The guidelines on environmental safety assessment for chemical pesticies. Part 15. CNAM, Beijing, China
Cui L, Sun L, Yang D, Yan X, Yuan H (2012) Effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the feeding behaviour of Sitobion avenae. Pest Manag Sci 68:1484–1491
Cui L, Qi HL, Yang DB, Yuan HZ, Rui CH (2016) Cycloxaprid: a novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic Biochem Physiol 132:96–101
Decourtye A, Devillers J (2010): Insect Ninotinic acetylcholine receptors. In: Thany SH (Editor), Advances in experimental medicine and biology, France, 85–95
Dittbrenner N, Schmitt H, Capowiez Y, Triebskorn R (2011) Sensitivity of Eisenia fetida in comparison to Aporrectodea caliginosa and Lumbricus terrestris after imidacloprid exposure. Body mass change and histopathology. J Soils Sediments 11:1000–1010
Dondero F, Negri A, Boatti L, Marsano F, Mignone F, Viarengo A (2010) Transcriptomic and proteomic effects of a neonicotinoid insecticide mixture in the marine mussel (Mytilus galloprovincialis, Lam.) Sci Total Environ 408:3775–3786
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008) Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64:1099–1105
Englert D, Bundschuh M, Schulz R (2012) Thiacloprid affects trophic interaction between gammarids and mayflies. Environ Pollut 167:41–46
Federico A, Cardaioli E, Pozzo P, Formichi P, Gallus G, Radi E (2012) Mitochondria, oxidative stress and neurodegenetation. J Neurol Sci 322:254–262
Feng L, Zhang L, Zhang Y, Zhang P, Jiang H (2015) Inhibition and recovery of biomarkers of earthworm Eisenia fetida after exposure to thiacloprid. Environ Sci Pollut Res 22:9475–9482
Han W, Tian Y, Shen X (2018) Human exposure to neoicotinoid insecticides and the evaluation of their potential toxicity: an overview. Chemosphere 192:59–65
Kapoor U, Srivastava MK, Srivastava LP (2011) Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol : Int J Published Br Ind Biol Res Assoc 49:3086–3089
Laurino D, Porporato M, Patetta A, Manino A (2011) Toxicity of neonicotinoid insecticides to honey bees: laboratoty tests. B Insectol 64:107–113
Liu S, Zhou Q, Chen C (2012) Antioxidant enzyme activities and lipid peroxidation in earthworm Eisenia fetida exposed to 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-gamma-2-benzopyran. Environ Toxicol 27:472–479
Liu X, Xu X, Li C, Zhang H, Fu Q, Shao X, Ye Q, Li Z (2015a) Degradation of chiral neonicotinoid insecticide cycloxaprid in flooded and anoxic soil. Chemosphere 119:334–341
Liu X, Xu X, Zhang H, Li C, Shao X, Ye Q, Li Z (2015b) Bioavailability and release of nonextractable (bound) residues of chiral cycloxaprid using geophagous earthworm Metaphire guillelmi in rice paddy soil. Sci Total Environ 526:243–250
Mu XY, Wang K, Chai TT, Zhu LZ, Yang Y, Zhang J, Pang S, Wang CJ, Li XF (2015) Sex specific response in cholesterol level in zebrafish (Danio rerio) after long-term exposure of difenoconazole. Environ Pollut 197:278–286
Muangphra P, Tharapoom K, Euawong N, Namchote S, Gooneratne R (2016) Chronic toxicity of commercial chlorpyrifos to earthworm Pheretima peguana. Environ Toxicol 31:1450–1459
OECD (1984) Earthworm, acute toxicity tests. Guideling for testing chemicals. No.207. OECD, Paris, France
OECD (2004) Earthworm reproduction tests. Guideling for testing chemicals. No.222. OECD, Paris, France
Qi SZ, Wand D, Zhu L, Teng M, Wang C, Xue X, Wu L (2018) Neonicotinoid insecticde imidacloprid, guadipyr, and cycoxaprid induce acute oxidative stress in Daphnia magna. Ecotox Environ SA 148:352–358
Qi SZ, Wang C, Chen XF, Qin ZH, Li XF, Wang CJ (2013) Toxicity assessments with Daphnia magna of guadipyr, a new neonicotinoid insecticide and studies of its effect on acetylcholinesterase (AChE), glutathione S-transferase (GST), catalase (CAT) and chitobiase activities. Ecotox Environ SA 98:339–344
Sanchez-Bayo F (2014) The trouble with neonicotinoids: chronic exposure to widely used insecticides kills bees and many other invertebrates. Science 346:806–807
Sanchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N (2016) Are bee diseases linked to pesticides?—a brief review. Environ Int 89-90:7–11
Sanchez-Hernandez JC, Narvaez C, Sabat P, Martinez Mocillo S (2014) Integrated biomarker analysis of chlorpyrifos metabolism and toxicity in the earthworm Aporrectodea caliginosa. Sci Total Environ 490:445–455
Sawasdee B, Kohler HR (2009) Embryo toxicity of pesticides and heavy metals to the ramshorn snail, Marisa cornuarietis (Prosobranchia). Chemosphere 75:1539–1547
Shao X, Xia S, Durkin KA, Casida JE (2013) Insect nicotinic receptor interactions in vivo with neonicotinoid, organophosphorus, and methylcarbamate insecticides and a synergist. P Natl Acad Sci USA 110(43):17273–17277
Shi Z, Tang Z, Wang C (2017) A brief review and evaluation of earthworm biomarkes in soil pollution assessment. Environ Sci Pollut Res 24:13284–13294
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41:905–909
Su WC, Zhou YH, Ma YQ, Wang L, Zhang Z, Rui CH, Duan HX, Qin ZH (2012) N’-nitro-2-hydrocarbylidenehydrazinecarboximidamides: design, synthesis, crystal structure, insecticidal activity, and structure-activity relationships. J Agric Food Chem 60:5028–5034
Wang K, Mu XY, Qi SZ, Chai TT, Pang S, Yang Y, Wang CJ, Jiang JZ (2015a) Toxicity of a neonicotinoid insecticide, guadipyr, in earthworm (Eisenia fetida). Ecotoxicol Environ SA 114:17–22
Wang K, Pang S, Mu XY, Qi SZ, Li DZ, Cui F, Wang CJ (2015b) Biological response of earthworm, Eisenia fetida, to five neonicotinoid insecticides. Chemosphere 132:120–126
Wang Y, Cang T, Zhao X, Yu R, Chen L, Wu C, Wang Q (2012) Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fetida. Ecotoxicol Environ SA 79:122–128
Xu R, Luo M, Xia R, Meng X, Xu X, Xu Z, Cheng J, Shao X, Li H, Li Z (2014a) Seven-membered azabridged neonicotinoids: synthesis, crystal structure, insecticidal assay, and molecular docking studies. J Agric Food Chem 62:11070–11079
Xu R, Xia R, Luo M, Xu X, Cheng J, Shao X, Li Z (2014b) Design, synthesis, crystal structures, and insecticidal activities of eight-membered azabridge neonicotinoid analogues. J Agric Food Chem 62:381–390
Zhang J, Xiong K, Chen A, Li F (2017) Toxicity of a novel neonicotinoid insecticide paichongding to earthworm Eisenia fetida. Soil Sediment Contam 26(3):235–246
Zhang L, Ji F, Li M, Cui Y, Wu B (2014) Short-term effects of Dechlorane plus on the earthworm Eisenia fetida determined by a systems biology approach. J Hazard Mater 273:239–246
Zhang Q, Zhang G, Yin P, Lv Y, Yuan S, Chen J, Wei B, Wang C (2015) Toxicological effects of soil contaminated with spirotetramat to the earthworm Eisenia fetida. Chemosphere 139:138–145
Acknowledgements
This work was mainly funded by the China Post-doctoral Science Foundation (No.2015M570178) and kindly supported by Dr. Wenting Zhao by the Young Teacher Science Fund of Da Bei Nong Group (15ZK002) and Young Teacher Science Fund of Beijng University of Agriculture (2017516006). We thank Dr. Shao Xusheng in East China University of Science and Technology for kindly providing CYC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 106 kb)
Rights and permissions
About this article
Cite this article
Qi, S., Wang, D., Zhu, L. et al. Effects of a novel neonicotinoid insecticide cycloxaprid on earthworm, Eisenia fetida. Environ Sci Pollut Res 25, 14138–14147 (2018). https://doi.org/10.1007/s11356-018-1624-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1624-z