Abstract
Diuron is a widely used pesticide for weed control in Brazil, Australia, the USA, and EU; therefore, environmental consequences are of concern. The aim of the present study was to determine the potential toxic effects of diuron (as formulation Diuron Nortox® 500 SC) on tadpoles of the bullfrog Lithobates catesbeianus, considering three endpoints: lethality, swimming behavior, and avoidance (ability to escape from contaminant). Secondly, it was intended to compare the sensitivity of tadpoles with to fish and other amphibian species by applying species sensitive distribution (SSD). Five diuron concentrations (20, 30, 35, 40, and 45 mg a.i./L) were tested in triplicate to evaluate mortality, swimming speed, and distance traveled after 96 h of exposure to the herbicide. Two avoidance tests were conducted using a multicompartmented system (n = 6) containing three tadpoles per compartment. Dilution gradients were established at 0; 5; 25; 50; 75; and 100% for both systems, corresponding to 0; 0.5; 2.5; 5; 7.5; and 10 mg a.i./L in an experiment, taking into account the results of the acute toxicity test and 0; 5; 25; 50; 100; and 150 μg a.i./L for another experiment, considering reported environmentally relevant concentrations in Brazilian water bodies. The acute toxicity tests (n = 3) with diuron revealed an 96 h-LC50 of 31 ± 3.7 mg a.i./L. The decreased maximum speed was only observed in tadpoles exposed to 30 mg a.i./L (0.15 ± 0.04 m/s) when compared to the control (0.28 ± 0.06 m/s). In the avoidance tests, concentrations of 2.5 and 5 mg a.i./L caused the escape behavior of L. catesbeianus tadpoles. However, tadpoles were also able to avoid diuron contamination at environmental relevance, especially in concentrations of 25 and 50 μg a.i./L. As expected, avoidance and effects on swimming behavior ocurred at lower diuron concentrations than mortality. Sublethal diuron concentrations may thus lead to the displacement of organisms to more favorable areas when they are available and the ability to swim is not affected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Large-scale conversion of native habitats to agriculture represents one of the main threats to biodiversity in continental waters around the world (Lacher and Goldstein 1997; Vörösmarty et al. 2010). This is of particular concern when the expansion or intensification of agriculture takes place in biodiversity-rich tropical countries (Sanchez-Bayo and Hyne 2011). Brazil is a global leader in the production of sugarcane ethanol since the 1970s, but the worldwide interest in biofuels fostered the expansion of sugarcane in the country especially since the early 2000s. Sugarcane is now the third largest crop in the country with more than 10 million hectares planted (UNICA 2015). Pesticide application has been both an important tool in pest and weed control for agricultural commodities in general and an important contributor to the degradation of freshwaters in rural landscapes (Lewis et al. 2016). In 2008, Brazil became the largest world consumer of pesticides (Rigotto et al. 2014), and pesticide consumption remained on the rise since then (Gomes and Barizon 2014; Albuquerque et al. 2016).
There are 441 commercial formulations containing 85 active ingredients registered for pest and weed control in sugarcane plantations in the country (AGROFIT 2017), many of which are toxic to aquatic organisms and capable of eliciting effects of priority concern including carcinogenicity, neurotoxicity, and endocrine disruption among others (Schiesari and Grillitsch 2011). Because pesticide residues were detected in the atmosphere (Moreira et al. 2012), dry and moist precipitation (Nogueira et al. 2012), and surface and groundwater (Dores et al. 2009), there is the potential of non-target organisms to be exposed. The active ingredients most frequently used in the state of São Paulo—the most important sugarcane producing region in the country—are the herbicides 2,4-D, diuron, clomazone, ametryn, hexazinone, MSMA, ethephon, and the insecticide/formicide fipronil (2009–2015; IEA 2016).
Diuron (3-(3.4-dichlorophenyl)-1.1-dimethylurea) is a pre- and post-emergence phenylurea herbicide inhibiting photosynthesis by blocking the electron transport chain at photosystem II (Giacomazzi and Cochet 2004). It is slightly toxic to mammals and birds as well as moderately toxic to aquatic invertebrates (Giacomazzi and Cochet 2004). The compound is bioaccumulative (Mhadhbi and Beiras 2012) and persistent in the environment (half-life > 30 days—aqueous photolysis at pH 7) (PPDB, Pesticide Properties Database 2018). Several studies report contamination of surface and groundwater by diuron in Brazil, highlighting the need for ecotoxicological studies with this herbicide. In Ribeirão Preto (SP), the traditionally most important sugarcane-producing region of Brazil, diuron concentrations of 0.26 to 7.12 μg/L (Dantas et al. 2011) were detected in public supply wells located near sugarcane plantations, reaching the value of 408 μg/L (Paschoalato et al. 2008). At the headwaters of the Poxim River watershed in the Brazilian state of Sergipe, Britto et al. (2012) found diuron at concentrations of 0.90 μg/L, mainly during the rainy season. In a recent review assessing the occurrence of pesticides in surface water in Brazil, the herbicides 2.4-D, bentazone, imazethapyr, and diuron were found to be commonly recorded in the state of Rio Grande do Sul, probably associated with rice plantation (Albuquerque et al. 2016).
In recent decades, amphibian population declines have been interpreted as a warning sign of global environmental degradation (Wake and Vredenburg 2008; Hayes et al. 2010; Blaustein et al. 2011). Because several instances of amphibian population declines and extinctions occurred in spatially isolated preserves, environmental pollution was postulated as one of the contributing factors. Even though many if not most of the declining amphibian species have not been assessed yet for their sensitivity to any contaminants (Schiesari et al. 2007; Awkerman and Raimondo 2018), pesticides are known to impair amphibian growth, development, reproduction, and survivorship (McCoy et al. 2008; Blaustein et al. 2011; Freitas et al. 2016), and there are some compelling cases linking pesticide contamination with amphibian population declines (Davidson et al. 2002; Hayes et al. 2010; Fryday and Thompson 2012). However, even though amphibians in all continents are likely to be exposed to a variety of pesticides given the ubiquity of agriculture (~ 40% of global land surface is covered by agriculture and cattle ranching; Foley et al. 2005), the effects of many compounds, such as diuron, have not been extensively studied. This herbicide is used for weed control in important food and feed producers including Brazil, the USA, Australia, and the European Union (PPDB 2018).
The aim of the present study was to determine the potential toxic effects of diuron (as formulation Diuron Nortox® 500 SC) on tadpoles of the bullfrog Lithobates catesbeianus, considering three endpoints: lethality, swimming behavior (speed and distance moved), and avoidance (ability to escape from contaminant). Secondly, it was intended to compare the sensitivity of tadpoles with to fish and other amphibian species by applying species sensitive distribution (SSD). L. catesbeianus (Anura, Ranidae) is native to North America that was introduced in South America (Giovanelli et al. 2009) and is commonly used as an experimental model in biomedical and ecotoxicological research. Generated results of the acute bioassays were compared with those of other published studies with aquatic and semi-aquatic vertebrates to determine the relative sensitivity of L. catesbeianus and to determine whether the employment of fish as the sole standard aquatic vertebrate test organism in risk assessments sufficiently protects larval amphibians from diuron exposure.
2 Materials and Methods
2.1 Test Organism
All experiments were performed in the laboratory of the Water Resource and Environmental Research Center (CRHEA), located in Itirapina, São Paulo State, Brazil. The authorization for animal experimentation is presented in Supplementary Material 1. Tadpoles of L. catesbeianus were obtained from a commercial frog farm (Ranamat Raniculture, Matão, Brazil) at Gosner’s stage 25 (Gosner 1960) with mean (± standard deviation) weight of 0.262 ± 0.097 g (n = 10). Tadpoles were kept in 500-L polypropylene water tanks containing natural well water under constant aeration (density, 1 animal/L). Two-thirds of the water was renewed every 3 days. Animals were fed daily with commercial fish food (Total®, protein content 32%) ad libitum up to 24 h before the beginning of test. Water tanks were kept at 24 ± 2 °C following a 12:12-h light: dark photoperiod with ± 1000 lx light intensity.
2.2 Acute Toxicity Tests
Acute toxicity tests were conducted exposing L. catesbeianus larvae to diuron for 96 h. From a 5 g a.i./L stock solution and based on a bibliographic review (see section 2.5 below), five test concentrations of diuron were prepared (20, 30, 35, 40, and 45 mg a.i./L; applied as Diuron Nortox® 500 SC) in natural well water control. The natural well water was also used as dilution water. Each experimental unit consisted of a plastic bucket containing 5 L test solution and five test organisms (density, 1 animal/L), with four replicates per treatment. The water was not renewed during the experiment, the feces were removed daily, and the buckets were aerated for 1 min every 24 h. Mortality was determined daily for the exposure period of 96 h. Acute tests were carried out under the same conditions of the organisms culture (24 ± 2 °C and 12:12-h light: dark photoperiod). Basic water physicochemical parameters including pH (micronal B374), conductivity (ORION 145 plus), temperature, and dissolved oxygen (YSI 55–25 ft) were measured at the beginning and at the end of the test. Three independent tests with three replicates each were performed, to determine the mean value of 96 h-LC50 (mean ± standard deviation).
2.3 Swimming Behavior Analysis
At the end of the acute toxicity test, video analyses were performed to record the swimming behavior of three randomly selected surviving individuals from each tested concentration. One tadpole at the time was placed in a transparent plastic tray (147 × 100 × 40.5 mm) and filmed under high definition for 1 min, being the observations made under light. The tadpoles were acclimated for 10 min before the videos were started. During the filming time, the organism was stimulated 3 times with a slight touch with a Pasteur pipette. Videos were analyzed using the software Kinovea (2018) v. 0.8.26 (https://www.kinovea.org/). This software was calibrated, using the measurements of the tray, to measure maximum speed (m/s) and distance traveled (cm) as swimming behavior endpoints.
2.4 Avoidance Tests
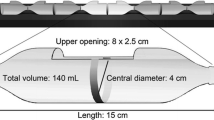
Tests assessing the avoidance behavior of L. catesbeianus tadpoles to diuron were conducted using the methodology developed by Moreira-Santos et al. (2008). In summary, a multicompartmented system was constructed by glueing six 300-mL vials in a row (Fig. 1). Vials were open at both ends so that tadpoles could move freely between them. Initially, a calibration procedure was performed in duplicate with sodium chloride (NaCl) to verify the stability of the concentration gradient over time. Five NaCl concentrations (17, 33, 50, 66, and 83 mg/L) were prepared using a stock solution of 100 mg/L (considered 100%) plus a control (0%) of natural well water used in the dilutions. The initial and final concentrations of NaCl and conductivity were measured. Subsequently, a distribution control experiment was performed in triplicate to validate the assumption that in the absence of a chemical stressor tadpoles had no preferences and distributed randomly among the six compartments. Three tadpoles were transferred to each of the compartments containing water only, and their distribution was recorded after 12 h.
The avoidance of tadpoles of L. catesbeianus to diuron was tested in two separate experiments. In the first experiment, performed in triplicate, a concentration gradient was established by diluting a stock solution (1 g a.i. /L) to yield concentrations of 0; 0.5; 2.5; 5; 7.5; and 10 mg a.i./L in each compartment. The highest tested concentration (10 mg a.i./L) corresponded to half of the lowest concentration in the acute toxicity test not leading to any toxic effect. A second experiment, performed in duplicate, was carried out with environmentally relevant diuron concentrations, comprising the range of concentrations most detected in the environment, as shown in the introduction: 0; 5; 25; 50; 100; and 150 μg a.i./L, obtained by diluting a stock solution of 1 g a.i./L. Tests were performed at 24 ± 2 °C in the dark to avoid any possible influence of light on swimming behavior and orientation. As before, three tadpoles were placed in each compartment. Initially, the concentration gradient was established by filling each individual compartment with the corresponding test solution at each dilution percentage.
To avoid mixture of test solutions between adjacent compartments, compartments were isolated from each other with non-toxic modeling clay. Subsequently, the tadpoles were introduced and finally the modeling clay divisions were removed. The distribution of individuals among experimental compartments was recorded after 12 h.
2.5 Species Sensitivity Distribution
To compare the sensitivity of L. catesbeianus to diuron with that of other freshwater vertebrates, a species sensitivity distribution (SSD) was constructed using mean 96 h-LC50 derived for L. catesbeianus and for other amphibian and fish species reported in the US-EPA database (US-EPA 2019), see references used in Supplementary Material—Table S1. Geometric means were calculated when more than one toxicity value was reported for a given species. The log-normal distributions of the values were constructed using the ETX program, version 2.0 (Van Vlaardingen et al. 2004). Since the model assumes a lognormal distribution of the data, log-normality was tested with the Anderson-Darling test included in the ETX software, which was evaluated at the 5% significance level.
2.6 Chemical Analysis
The 5 g/L and 1 g/L stock solutions used in acute toxicity test and avoidance experiment, respectively, were filtered using a syringe filter (nylon 0.22 μm), diluted 50,000 and 10,000 times, respectively, in water/MeOH 50:50 v/v mixture after and transferred to a vial of 2.0 mL of lid capacity with septum (Agilent). Diuron quantification was performed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). An Agilent 1200 model chromatograph, equipped with a binary pump, automatic injector, and thermostatized column compartment, was used in this analysis. The chromatographic separation was performed with a Zorbax SB-C18 column (2.1 × 30 mm, particle size of 3.5 μm) at 30 °C, injection volume of 10 μL, mobile phase flow of 0.3 mL/min and running time of 2 min. It was used as mobile phase ultrapure water containing 0.1% (v/v) of formic acid (additive that favors the formation of ions that ionize in the positive mode) and methanol (isocratic elution 40:60 v/v). The solvents were previously filtered in membranes with 0.2 μm of porosity. The identification and quantification of the compound were performed by mass spectrometry in an Agilent equipment with triple quadrupole (model 6410b). The analytical curve was constructed according to the area obtained for each compound in function of its concentration in the column.
Regarding the validation of the analytical method, the quantification limit (LOQ) was obtained by the signal-to-noise method comparing the analytical signal of samples at low diuron concentrations with baseline noise. It was considered the concentration where the signal-to-noise ratio was observed in the proportion 10:1. It was determined that the LOQ of the method is 0.5 μg/L. Accuracy and precision were evaluated using recovery and repeatability assays, respectively.
2.7 Data Analysis
Values of 96 h-LC50 were calculated by nonlinear regression, using the logistic curve of three parameters in the software Statistica 7.0 (StatSoft 2004).
For the swimming behavior data (maximum speed and distance moved) were carried out through the analysis of variance (one-way ANOVA) using the SigmaPlot v11.0 software (Systat Software Inc. 2008). The normality of data was tested using the Shapiro-Wilk test. A Dunnett test was carried out when differences were obtained in data that followed a normal distribution. For data that did not meet these requirements, the nonparametric Kruskall-Wallis test, followed by Dunn’s post hoc test, was used. All statistical tests were considered significantly different when p < 0.05 (Systat 2008).
For the avoidance experiments, the number of avoiders was determined for each compartment as the difference between the expected (NE) and the observed numbers (NO): Avoiders = NE–NO. NE was determined as described by Moreira-Santos et al. (2008): for the compartment with the highest diuron concentration, NE was equal to the number of tadpoles introduced into the compartment at the beginning of the test. For the remaining compartments, NE included the organisms initially introduced into the compartment, plus the organisms introduced from the adjacent compartment(s) with higher concentration(s). The percentage of avoidance in each compartment was calculated as follows: (NA/NE) × 100.
3 Results and Discussion
3.1 Test Performance
Water physicochemical conditions remained stable over the course of the experiments for all treatments, with temperatures ranging from 23.8 to 24.3 °C; pH from 6.9 to 7.6; electrical conductivity from 26 to 82 μS/cm, and dissolved oxygen from 6.4 to 7.0 mg/L.
The NaCl calibration procedure in the avoidance test indicated that the gradient of concentrations in the six compartments was closely maintained over the course of the experiment, since conductivity values 12 h after the start of the experiment were 82–102% (minimum-maximum) of those measured at the beginning of the test (Table 1). These results indicate that the redistribution of contaminants between adjacent compartments by swimming tadpoles was minimal. No mortality or unusual behavior was observed in the control treatments of any of the experiments.
Regarding the chemical analysis, concentrations measured in the stock solutions (0.9 and 5.6 g a.i./L) differed by less than 12% (11 ± 1.41) (mean ± standard deviation) from the nominal concentrations (1 and 5 g a.i./L), respectively. For the avoidance tests, concentrations measured in each compartment at the end of the experiments differed by less than 31% from the nominal concentrations (30.46 ± 18.91 mg a.i./L) (mean ± standard deviation). For the avoidance experiment based on environmental concentrations, the measured concentrations were 5; 25; 50; 100; and 150 μg a.i/L and nominal concentrations were 4.8; 24.1; 41.7; 80.5; and 162.5 μg a.i/L, respectively. For other avoidance experiment based on the result of acute toxicity tests, the measured concentrations were 0.8; 2; 3.4; 6.8; and 6.9 mg a.i./L and nominal concentrations were 0.5; 2.5; 5; 7.5; and 10 mg a.i./L, respectively. Therefore, the toxicity values were calculated based on nominal concentrations.
3.2 Acute Diuron Toxicity to L. catesbeianus and Its Sensitivity Compared with Other Amphibians and Fish
The acute toxicity tests of L. catesbeianus exposed to the pesticide Diuron Nortox® 500 SC resulted in a 96 h-LC50 (mean ± SD) of 31 ± 3.7 mg a.i./L (95% CI, 23–39 mg a.i./L) (n = 3). The percentages of mortality for the concentrations tested of 20, 30, and 35 mg a.i./L were respectively 8.87 ± 0.07%; 46.65 ± 0.40%, and 73.30 ± 0.2% with 100% mortality in the concentrations of 40 and 45 mg a.i./L. This 96 h-LC50 value is considerably higher than those reported for 18 fish species, including the rainbow trout Oncorrhynchus mykiss (7.0 mg a.i./L), commonly employed as a model freshwater vertebrate species for determination of environmental quality standards (e.g., EFSA 2013; Fig. 2). These results indicate that a toxicity assessment for diuron based on the toxicity value of rainbow trout is also protective for L. catesbeianus. Whether environmental quality standards for diuron based on toxicity data of rainbow trout are protective for a larger range of amphibian taxa remains uncertain, considering that only two other amphibian species—Xenopus laevis (Schuytema and Nebeker 1998) and Agalychnis callidryas (Ghose et al. 2014)—have been tested so far. This observation echoes previous quantitative studies documenting that amphibian ecotoxicological knowledge is almost exclusively based on a handful of common, widely distributed generalist species, usually from the northern hemisphere (Schiesari et al. 2007; Ortiz-Santaliestra et al. 2018). However, there are analyses that systematically compared the relative sensitivity of amphibians and fish and have suggested that additional amphibian tests are not necessary during chemical risk assessment (Weltje et al. 2013).
Species sensitivity distribution (SSD) constructed based on 96 h-LC50 values for diuron obtained in the present study for Lithobates catesbeianus (in bold), supplemented with data for other amphibian and fish species (geometric means) from the US-EPA database (US-EPA 2019)
Although diuron has a specific mode of action for primary producers, e.g., Mansano et al. (2017) verified that diuron significantly inhibited R. subcapitata growth and caused physiological alteration (chlorophyll a content) and morphological changes in cells (complexity and cell size), some studies have shown that this compound can cause deleterious effects in non-target heterotrophic organisms. For example, a low concentration of diuron (1 μg/L) may affect the immune parameters in the oyster Crassostrea gigas by inhibiting the activity of antioxidant enzymes (Luna-Acosta et al. 2012). The toxicity of pure diuron and its commercial formulation was similar for the protozoan Paramecium caudatum, with 6 h-LC50 of 64.6 ± 3.3 mg/L and 62.4 ± 2.5 mg/L, respectively. Chronic tests showed that the diuron (1.75 mg/L) cause significant decrease on population growth, generation number, and biomass of P. caudatum (Mansano et al. 2016a). Another study by Mansano et al. (2016b) evaluated the toxicity of diuron and the insecticide carbofuran (active ingredient and commercial formulation) to the Neotropical cladoceran Ceriodaphnia silvestrii. The 8-day lowest observed effect concentration (LOEC) for diuron was 8000 and > 800 μg/L for active ingredient and commercial formulation, respectively. The authors indicated that this cladoceran is the most sensitive species to both compounds when compared to other invertebrates.
Freitas et al. (2016) reported the thyroidogenic effects of diuron in tadpoles of L. catesbeianus, especially at high test temperatures. Moreover, degradation of diuron to metabolites, such as the 3.4-DCA, does not minimize the deleterious effects of this compound to the tadpoles, especially when associated with temperature changes (Freitas et al. 2016). Orton et al. (2009) verified the inhibition of testosterone and ovulation levels in the amphibian Xenopus laevis after exposure to diuron. The lowest values NOAELs (NOEC) calculated for different species of tadpoles according Schuytema and Nebeker (1998) were Pseudacris regilla, 14.5 mg/L (14 days); Rana catesbeiana, 7.6 mg/L (21 days); Rana aurora, 7.6 mg/L (14 days); and Xenopus laevis, 29.1 mg/L (14 days). P. regilla and X. laevis embryos had reduced growth and developed increased deformities in diuron concentrations over 20 mg/L. Hindlimb bud and forelimb development were retarded in R. aurora after 14 days exposure to diuron concentrations of 7.6 mg/L (Schuytema and Nebeker 1998).
For fish, diuron was reported to cause teratogenic effects in embryo-larva stages of Psetta maxima. Laboratory studies with P. maxima showed a significant decrease in hatching success, malformations (embryos), pericardium edema, and deformation of the skeleton (larvae) (Lazhar et al. 2012). For zebrafish (Danio rerio) embryos and larvae diuron presented 96 h-LC50 of 6.5 mg/L and values of 96 h-EC50 of approximately 4 mg/L (Velki et al. 2017a). It was observed a decrease in spontaneous embryo coiling movements and reduction of thigmotaxis in larvae when exposed to diuron. Abnormalities were observed at 48 h, showing lack of pigmentation (4 mg/L) and occurrence of edema (8 mg/L). The enzymatic activity and alterations of gene expression in zebrafish embryos and larvae exposed to diuron were evaluated by Velki et al. (2017b), where biochemical and molecular changes were detected. For example, prolonged exposure to diuron (2 hpf–98 hpf) lead to increased carboxylesterase activity (CES) at concentrations of 0.086 μM and 2.15 μM and expression of the corresponding ces2 gene at the concentrations of 0.086, 2.15, and 8.6 μM. This was not observed after the early exposure to diuron (2 hpf–50 hpf) for CES; however, in the treatment of 8.6 μM, ces2 was induced. A possible explanation, according to the authors, is that exposure to diuron induces detoxification and stimulates the expression of ces2 which leads to an increase in CES activity.
3.3 Swimming Behavior of L. catesbeianus Exposed to Diuron
Tadpoles of L. catesbeianus exposed to the concentrations used in the acute toxicity tests presented 100% mortality at concentrations of 40 and 45 mg a.i./L. Subsequently, swimming velocity and distance could only be determined in the treatment of 35 mg a.i./L and lower. Although mean maximum speed and distance traveled were lower in all treatments relative to the control, only tadpoles exposed to 30 mg a.i./L showed a significant decrease in their speed (0.15 ± 0.04 m/s) when compared with the control (0.28 ± 0.06 m/s; Fig. 3; one-way ANOVA; F3,9 = 4.89; p < 0.05; Dunnett’s test, p < 0.05).
Avoidance of tadpoles Lithobates catesbeianus to a concentration gradient of diuron after 12 h of exposure. The reference line indicates the number of tadpoles in each compartment at the beginning of the experiment. Bars represent the mean + SD of avoidance rates recorded in three replicates of each treatment
The tadpoles exposed to 30 and 35 mg a.i./L showed low swimming activity and weaker reaction to stimuli. This response is possibly related to the neurotoxic effects of diuron, as Bretaud et al. (2000) observed inhibition of acetylcholinesterase activity in juveniles of the fish Carassius auratus exposed to diuron at concentrations 600 times lower (500 μg/L). Exposure to diuron may have caused alterations in neuro-muscular function and consequently affected their ability to swimming (Chen et al. 2007). When exposed to concentrations in which the orientation response is impaired, they cannot escape from the toxic habitat, thus, the organisms would be more susceptible to suffer the effects of the contaminant, and feeding efficiency would be reduced, and hence, the energy available for general metabolism, growth, and reproduction as demonstrated by Denoël et al. (2012) when exposing the species Rana temporaria to endosulfan. The ecological relevant consequence of this is that the contaminated animals would be more vulnerable to some predators; they could explore smaller portions of their habitat and suffer reductions in the foraging and fitness.
3.4 Avoidance Behavior of L. catesbeianus Exposed to Diuron
As can be deduced from Fig. 4, the 2.5 and 5 mg a.i./L diuron concentrations caused the greatest avoidance behavior in tadpoles. At these test concentrations, they presented the lowest numbers of tadpoles per compartments (on average < 1). Interestingly, little avoidance behavior was observed for the higher test concentrations (7.5 and 10 mg a.i./L). It is probable that the consequent exhaustion of available energy and the increased physiological damage caused a reduction ability to escape. Similar observations of a lack of behavioral avoidance to high contaminant concentrations has been similarly recorded in cladocerans and copepods exposed to metals and the insecticide endosulfan (Gutierrez et al. 2012). Several other studies evaluating other contaminants have shown that the ability of organisms to escape is often diminished when the swimming capacity of tadpoles is affected (Wojtaszek et al. 2004; Chen et al. 2007; Shinn et al. 2008; Denoël et al. 2013).
Avoidance of tadpoles Lithobates catesbeianus to an environmentally relevant concentration gradient of diuron after 12 h of exposure. The reference line indicates the number of tadpoles in each compartment at the beginning of the experiment. Bars represent the mean + SD of avoidance rates recorded in two replicates of each treatment
The results of the present study also showed that diuron at environmentally relevant concentrations may trigger an avoidance response in L. catesbeianus tadpoles (Fig. 5). At the end of the experiment, the lowest number of tadpoles was observed in the compartments with the concentrations of 25 and 50 μg a.i./L. Avoidance behavior can potentially lead to the displacement of organisms towards most favorable areas, when they occurred. Avoidance has been shown to be a sensitive sublethal response, which may have important repercussions as such concentrations of effect can be considered as habitat deregulators, by making the latter, at least partially, unsuited to accommodate aquatic life (Vasconcelos et al. 2016).
The ability to detect and avoid contaminants has been observed for different fish species exposed to metals (Svecevičius 1999; Hartwell et al. 1989), domestic and industrial effluents (Smith and Bailey 1990), ammonia and low dissolved oxygen (Richardson et al. 2001), and different herbicides, such as 2,4-D, glyphosate, diquat, and Aquathol® K (Folmar 1976). Recently, Araújo et al. (2018) also demonstrated avoidance behavior of the guppy Poecilia reticulata when exposed to atrazine at environmental concentrations. Likewise, the ability to detect and avoid contaminants has been observed for different species of larval amphibians exposed to insecticide abamectin (Vasconcelos et al. 2016) for copper and the fungicide pyrimethyl (Araújo et al. 2014a, 2014b).
4 Concluding Remarks and Indications for Future Research
The sensitivity comparison between amphibians and fishes for the herbicide diuron in the present study indicated that standard acute tests using the rainbow trout and other fishes as model organism adequately cover the sensitivity of larval amphibians such as L. catesbeianus. However, it is important to emphasize that only a limited number of amphibian species and compounds have been tested so far, and there is an urgent need to increase the toxicity dataset for neotropical and endemic amphibians (Schiesari et al. 2007).
As pointed out by other researchers (Vasconcelos et al. 2016), our results highlight that acute toxicity tests that solely evaluate mortality may underestimate adverse effects on amphibians, as sublethal effects can seriously affect individual performance under real field conditions. The sublethal effects observed in this study at low and environmentally relevant concentrations, for example, are tightly linked to possible effects of foraging rate and predator avoidance, seriously affecting growth, development, and survival in natural environments.
Tadpoles of L. catesbeianus were able to detect and avoid diuron at environmentally relevant concentrations. Therefore, it is possible to conclude that the non-forced exposure system can help to understand how contaminants can affect the spatial distribution of organisms. Further research is needed to evaluate the real implications of avoidance behavior on tadpoles in the environment, such as vulnerability to some predators, reductions in the foraging, and fitness. As previously recommended by Araújo et al. (2016), avoidance tests can be an important tool evaluating toxicity of environmental contaminants to amphibians, and then its inclusion as laboratory test could be considered in the risk assessments of aquatic contaminants in the future.
References
AGROFIT - Phytosanitary Agrochemicals System (2017). Freely accessible via: <http:// agricultura.gov.br/agrofit> (Accessed in 02 July 2017).
Albuquerque, A. F., Ribeiro, J. S., Kummrow, F., Nogueira, A. J. A., Montagner, C. C., & Umbuzeiro, G. A. (2016). Pesticides in Brazilian freshwaters: A critical review. Environmental Science: Processes & Impacts, 18, 779–787.
Araújo, C. V. M., Shinn, C., Vasconcelos, A. M., Ribeiro, R., & Espíndola, E. L. G. (2014a). Preference and avoidance responses by tadpoles: The fungicide pyrimethanil as a habitat disturber. Ecotoxicology, 23, 851–860.
Araújo, C. V. M., Shinn, C., Moreira-Santos, M., Lopes, I., Espíndola, E. L. G., & Ribeiro, R. (2014b). Copper-driven avoidance and mortality in temperate and tropical tadpoles. Aquatic Toxicology, 146, 70–75.
Araújo, C. V. M., Moreira-Santos, M., & Ribeiro, R. (2016). Active and passive spatial avoidance by aquatic organisms from environmental stressors: A complementary perspective and a critical review. Environment International, 92-93, 405–415.
Araújo, C. V. M., Silva, D. C. V. R., Gomes, L. E. T., Acayaba, R. D., Montagner, C. C., Moreira-Santos, M., Ribeiro, R., & Pompêo, M. L. M. (2018). Habitat fragmentation caused by contaminants: Atrazine as a chemical barrier isolating fish population. Chemosphere, 193, 24–31.
Awkerman, J. A., & Raimondo, S. (2018). Simulated developmental and reproductive impacts on amphibian populations and implications for assessing long-term effects. Ecotoxicology and Environmental Safety, 149, 233–240.
Blaustein, A. R., Han, B. A., Relyea, R. A., Johnson, P. T. J., Julia, C., Buck, J. C., Gervasi, S. S., & Kats, L. B. (2011). The complexity of amphibian population declines: Understanding the role of cofactors in driving amphibian losses. Ecology and Conservation Biology, 1223, 108–119.
Bretaud, S., Toutant, J. P., & Saglio, P. (2000). Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicology and Environmental Safety, 47, 117–124.
Britto, F. B., Vasco, A. N. D., Pereira, A. P. S., Júnior, M., Vieira, A., & Nogueira, L. C. (2012). Herbicides in the upper Poxim River, Sergipe, and the risk of contamination of water resources. Revista Ciência Agronômica, 43, 390–398.
Chen, T.-H., Gross, J. A., & Karasov, W. H. (2007). Adverse effects of chronic copper expo-sure in larval northern leopard frogs (Rana pipiens). Environmental Toxicology and Chemistry, 26, 1470–1475.
Dantas, A. D. B., Paschoalato, C. F., Martinez, M. S., Ballejo, R. R., & Di Bernardo, L. (2011). Removal of diuron and hexazinone from Guarany aquifer groundwater. Brazilian Journal of Chemical Engineering, 28, 415–424.
Davidson, C., Mahony, N., Struger, J., Ng, P., & Pettit, K. (2002). Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate change hypothesis for California amphibian declines. Conservation Biology, 16, 1588–1601.
Denoël, M., D’Hooghe, B., Ficetola, G. F., Brasseur, C., De Pauw, E., Thomé, J. P., & Kestemont, P. (2012). Using sets of behavioral biomarkers to assess short-term effects of pesticide: A study case with endosulfan on frog tadpoles. Ecotoxicology, 21, 1240–1250.
Denoël, M., Libon, S., Kestemont, P., Brasseur, C., Focant, J.-F., & De Pauw, E. (2013). Effects of a sublethal pesticide exposure on locomotor behavior: A video-trackinganalysis in larval amphibians. Chemosphere, 90, 945–951.
Dores, E. F. G. C., Spadotto, C. A., Weber, O. L., Carbo, L., Vecchiato, A. B., & Pinto, A. A. (2009). Environmental behaviour of metolachlor and diuron in a tropical soil in the central region of Brazil. Water Air Soil Pollution, 197, 175–183.
EFSA - European Food Safety Authority. (2013). Guidance on tiered risk assessment for plant protection products for aquatic organisms in the edge-of-field surface waters. EFSA Journal, 11, 3290.
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, I. C., Ramankutty, N., & Snyder, P. K. (2005). Global consequences of land use. Science, 309, 570–574.
Folmar, L. C. (1976). Overt avoidance reaction of rainbow trout fry to nine herbicides. Bulletin of Environmental Contamination and Toxicology, 15, 509–514.
Freitas, J. S., Kupsco, A., Diamante, G., Felicio, A. A., Almeida, E. A., & Schlenk, D. (2016). Influence of temperature on the thyroidogenic effects of diuron and its metabolite 3,4-DCA in tadpoles of the american bullfrog (Lithobates catesbeianus). Environmental Science & Technology, 50, 13095–13104.
Fryday, S., & Thompson, H. (2012). Toxicity of pesticides to aquatic and terrestrial life stages of amphibians and occurrence, habitat use and exposure of amphibian species in agricultural environments. EFSA (European Food Safety Authority) supporting publications 2012: EN-343.
Ghose, S. L., Donnelly, M. A., Kerby, J., & Whitfield, S. M. (2014). Acute toxicity tests and meta-analysis identify gaps in tropical ecotoxicology for amphibians. Environmental Toxicology and Chemistry, 33(9), 2114–2119.
Giacomazzi, S., & Cochet, N. (2004). Environmental impact of diuron transformation: A review. Chemosphere, 56, 1021–1032.
Giovanelli, J. G. R., Haddad, C. F. B., & Alexandrino, J. (2009). Predicting the potential distribution of the alien invasive American bullfrog (Lithobates catesbeianus) in Brazil. Biological Invasion, 10, 585–590.
Gomes, M. A. F., & Barizon, R. R. M. (2014). Panorama da contaminação Ambiental por Agrotóxicos e nitrato de origem agrícola no Brasil: Cenário 1992/2011 (p. 36). Jaguariúna: Embrapa Meio Ambiente.
Gosner, K. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16, 183–190.
Gutierrez, M. F., Paggi, J. C., & Gagneten, A. M. (2012). Microcrustaceans escape behavioras an early bioindicator of copper, chromium and endosulfan toxicity. Ecotoxicology, 21, 428–438.
Hartwell, S. I., Jin, J. H., Cherry, D. S., & Cairns, J., Jr. (1989). Toxicity 574 versus avoidance response of golden shiner, Notemigonus crysoleucas, to five metals. Journal Fish of Biology, 35, 447–456.
Hayes, T. B., Falso, P., Gallipeau, S., & Stice, M. (2010). The cause of global amphibian declines: A developmental endocrinologist’s perspective. Journal of Experimental Biology, 213, 921–933.
IEA - Instituto De Economia Agrícola. (2016). Banco de dados, São Paulo. Freely accessible via: <http://www.iea.sp.gov.br/>. (Accessed in 20 May 2016).
Kinovea. (2018). Software v.0.8.26. Freely accessible via <https://www.kinovea.org/>. (Accessed in 16 May 2018).
Lacher, T. E., & Goldstein, M. I. (1997). Tropical ecotoxicology: Status and needs. Environmental Toxicology and Chemistry, 16, 100–111.
Lazhar, M., Hela, T., Moncef, B., & Néji, A. A. (2012). Toxicity of three selected pesticides (alachlor, atrazine and diuron) to the marine fish (turbot Psetta maxima). African Journal of Biotechnology, 11, 11321–11328.
Lewis, S. E., Silburn, D. M., Kookana, R. S., & Shaw, M. (2016). Pesticide behavior, fate, and effects in the tropics: An overview of the current state of knowledge. Journal of Agricultural and Food Chemistry, 64, 3917–3924.
Luna-Acosta, A., Renault, T., Thomas-Guyon, H., Faury, N., Saulnier, D., Budzinski, H., Menach, K. L., Pardon, P., Fruitier-Arnaudin, I., & Bustamante, P. (2012). Detection of early effects of a single herbicide (diuron) and a mix of herbicides and pharmaceuticals (diuron, isoproturon, ibuprofen) on immunological parameters of Pacific oyster (Crassostrea gigas) spat. Chemosphere, 87, 1335–1340.
Mansano, A. S., Moreira, R. A., Pierozzi, M., Oliveira, T. M., Vieira, E. M., Rocha, O., & Regali-Seleghim, M. H. (2016a). Effects of diuron and carbofuran pesticides in their pure and commercial forms on Paramecium caudatum: The use of protozoan in ecotoxicology. Environmental Pollution, 213, 160–172.
Mansano, A. S., Moreira, R. A., Dornfeld, H. C., Diniz, L. G. R., Vieira, E. M., Daam, M. A., Rocha, O., & Seleghim, M. H. R. (2016b). Acute and chronic toxicity of diuron and carbofuran to the neotropical cladoceran Ceriodaphnia silvestrii. Environmental Science and Pollution Research, 25, 13335–13346 1–12.
Mansano, A. S., Moreira, R. A., Dornfeld, H. C., Freitas, E. C., Vieira, E. M., Sarmento, H., Rocha, O., & Seleghim, M. H. R. (2017). Effects of diuron and carbofuran and their mixtures on the microalgae Raphidocelis subcapitata. Ecotoxicology and Environmental Safety, 142, 312–321.
McCoy, K. A., Bortnick, L. J., Campbell, C. M., Hamlin, H. J., Guillette, J. R., & Mary, C. (2008). Agriculture alters gonadal form and function in the toad Bufo marinus. Environmental Health Perspectives, 116, 1526–1532.
Mhadhbi, L., & Beiras, R. (2012). Acute toxicity of seven selected pesticides (alachlor, atrazine, dieldrin, diuron, pirimiphos-methyl, chlorpyrifos, diazinon) to the marine fish (turbot, Psetta maxima). Water, Air, Soil Pollution, 223, 5917–5930.
Moreira, J. C., Peres, F., Simões, A. N., Pignati, W. A., Dores, E. C., Vieira, S. N., Strüssemann, C., & Mott, T. (2012). Contaminação de águas superficiais e de chuva por agrotóxicos em uma região do estado do Mato Grosso. Ciência e Saúde coletiva, 18, 1557–1568.
Moreira-Santos, M., Donato, C., Lopes, I., & Ribeiro, R. (2008). Avoidance tests with small fish: Determination of the median avoidance concentration and of the lowest observed-effect gradient. Environmental Toxicology and Chemistry, 27, 1576–1582.
Nogueira, E. N., Dores, E. F. G. C., Pinto, A. A., Amorim, R. S. S., Ribeiro, M. R., & Lourencetti, C. (2012). Currently used pesticides in water matrices in Central-Western Brazil. Journal of the Brazilian Chemical Society, 23, 1476–1487.
Ortiz-Santaliestra, M. E., Maia, J. P., Egea-Serrano, A., & Lopes, I. (2018). Validity of fish, birds and mammals as surrogates for amphibians and reptiles in pesticide toxicity assessment. Ecotoxicology, 27(7), 819–833.
Orton, F., Lutz, I., Kloas, W., & Routledge, E. J. (2009). Endocrine disrupting effects of herbicides and pentachlorophenol: In vitro and in vivo evidence. Environmental Science & Technology, 43, 2144–2150.
Paschoalato, C., Dantas, A., Rosa, I., Faleiros, R., & Bernardo, L. D. (2008). Use of activated carbon to remove the herbicides diuron and hexazinone from water. Revista DAE, 179, 34–41.
PPDB - Pesticide Properties Database. (2018). Freely accessible via: < https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/4.htm>. (Accessed in 20 October 2018).
Richardson, J., Williams, E. K., & Hickey, C. W. (2001). Avoidance behaviour of freshwater fish and shrimp exposed to ammonia and dissolved oxygen separately and in combination. New Zealand Journal of Marine and Freshwater Research, 35, 625–633.
Rigotto, R. M., Vasconcelos, D. P., & Rocha, M. M. (2014). Pesticide use in Brazil and problems for public health. Cadernos de Saúde Pública, 30, 1360–1362.
Sanchez-Bayo, F., & Hyne, R. V. (2011). Comparison of environmental risks of pesticides between tropical and nontropical regions. Integrated Environmental Assessment and Management, 7(4), 577–586.
Schiesari, L., & Grillitsch, B. (2011). Pesticides meet megadiversity in the expansion of biofuel crops. Frontiers in Ecology and the Environment, 4, 215–221.
Schiesari, L., Grillitsch, B., & Grillitsch, H. (2007). Biogeographic biases in research and their consequences for linking amphibian declines to pollution. Conservation Biology, 21, 465–471.
Schuytema, G. S., & Nebeker, A. V. (1998). Comparative toxicity of diuron on survival and growth of Pacific treefrog, bullfrog, red-legged frog, and African clawed frog embryos and tadpoles. Archives of Environmental Contamination and Toxicology, 34, 370–376.
Shinn, C., Marco, A., & Serrano, L. (2008). Inter- and intra-specific variation on sensitivity of larval amphibians to nitrite. Chemosphere, 71, 507–514.
Smith, E. H., & Bailey, H. C. (1990). Preference/avoidance testing of waste discharges on anadromous fish. Environmental Toxicology and Chemistry, 9, 77–86.
Statsoft. (2004). Statistica version 07. Freely accessible via <www.statsoft.com> (Accessed in 16 May 2018).
Svecevičius, G. (1999). Fish avoidance response to heavy metal and their mixtures. Acta Zoologica Lithuania, 9, 103–113.
Systat. (2008). Systat software, Incorporation SigmaPlot for Windows version 11.0.
UNICA - União da Indústria de cana de açúcar. (2015). Freely accessible via: <http://www.unica.com.br/noticia/25950095920326811142/safra-2015-por-cento2F2016-no-centro-sul-deve-atingir-590-milhoes-de-toneladas-de-cana-processadas-por-cento2C-com-prioridade-para-a-producao-de-etanol>. (Accessed in 17 August 2018).
US-EPA - US Environmental Protection Agency. (2019). ECOTOX knowledgebase. Freely accessible via: <https://cfpub.epa.gov/ecotox/> (Accessed in 6 Mar 2019).
Van Vlaardingen, P., Traas, T. P., Wintersen, A. M., & Aldenberg, T. (2004). ETX 2.0. A program to calculate hazardous concentrations and fraction affected, based on normally distributed toxicity data. Bilthoven, the Netherlands: RIVM Report No. 601501028/2004. National Institute of Public Health and the Environment (RIVM).
Vasconcelos, A. M., Daam, M. A., Santos, L. R. A., Sanches, A. L. M., Araújo, C. V. M., & Espíndola, E. L. G. (2016). Acute and chronic sensitivity, avoidance behavior and sensitive life stages of bullfrog tadpoles exposed to the biopesticide abamectin. Ecotoxicology, 25, 500–509.
Velki, M., Di Paolo, C., Nelles, J., Seiler, T.-B., & Hollert, H. (2017a). Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere, 180, 65–76.
Velki, M., Meyer-Alerta, H., Seilera, T.-B., & Hollert, H. (2017b). Enzymatic activity and gene expression changes in zebrafish embryos and larvae exposed to pesticides diazinon and diuron. Aquatic Toxicology, 193, 187–200.
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S., Sullivan, C. A., Liermann, C. R., & Davies, P. M. (2010). Global threats to human water security and river biodiversity. Nature, 467, 555–561.
Wake, D. B., & Vredenburg, V. T. (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. PNAS, 105, 11467.
Weltje, L., Simpson, P., Gross, M., Crane, M., & Wheeler, J. R. (2013). Comparative acute and chronic sensitivity of fish and amphibians: A critical review of data. Environmental Toxicology and Chemistry, 32(5), 984–994.
Wojtaszek, B. F., Staznik, B., Chartrand, D. T., Stephenson, G. R., & Thompson, D. G. (2004). Effects of vision® herbicide on mortality, avoidance response, and growth ofamphibian larvae in two forest wetlands. Environmental Toxicology and Chemistry, 23, 832–842.
Funding
We thank the support of the National Council for Scientific and Technological Development through a postdoc grant to the first author (Process number: CNPq 171233/2017-4). Financial support was also provided by FAPESP (2015/187903) and the Portuguese government (FCT) through a postdoc grant to M. Daam (SFRH/BPD/109199/2015) and the research center CENSE (UID/AMB/04085/2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Moreira, R.A., Freitas, J.S., da Silva Pinto, T.J. et al. Mortality, Spatial Avoidance and Swimming Behavior of Bullfrog Tadpoles (Lithobates catesbeianus) Exposed to the Herbicide Diuron. Water Air Soil Pollut 230, 125 (2019). https://doi.org/10.1007/s11270-019-4168-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4168-z