Abstract
Imidacloprid, a neonicotinoid insecticide, has been used widely in agriculture worldwide. The adverse effects of imidacloprid on exposed biota have brought it increasing attention. However, knowledge about the effects of imidacloprid on antioxidant defense systems and digestive systems in the earthworm is vague and not comprehensive. In the present study, the changes in the activity of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), cellulase, reactive oxygen species (ROS), and malondialdehyde (MDA) in the earthworm Eisenia fetida exposed to artificial soil treated with imidacloprid were examined systematically. The results showed that the activity of these biomarkers was closely related to the dose and duration of the exposure to imidacloprid. The activity of SOD was stimulated significantly at doses of 0.66 and 2 mg kg−1 imidacloprid but markedly inhibited at a dose of 4 mg kg−1 imidacloprid with prolonged exposure. The activities of CAT and POD increased irregularly at 0.2–4 mg kg−1 imidacloprid over different exposure times. The level of ROS at a dose of 2 or 4 mg kg−1 imidacloprid was significantly increased over the entire exposure period. When the concentration of imidacloprid was above 0.66 mg kg−1, the balance of the activity of the antioxidant enzymes and ROS level was interrupted. The activity of cellulase decreased significantly with prolonged exposure. At the stress of 4 mg kg−1 imidacloprid, the content of MDA was significantly increased with increasing exposure time. The results of the present study suggest that imidacloprid has a potentially harmful effect on E. fetida and may be helpful for assessment of the risk of imidacloprid to the soil ecosystem environment. However, to obtain more comprehensive toxicity data, it is necessary to investigate the effects of imidacloprid on earthworm using native soils in the future work.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imidacloprid [1-(6-chloro-3-pyridylmethyl)-N-nitro-imidazolidin-2-ylideneamine], a neonicotinoid insecticide, has been used widely to control sucking insects, soil insects, termites, and some chewing insects in agriculture worldwide (Sur and Stork 2003; Laycock et al. 2012). Imidacloprid works by disrupting the insect nervous system, causing irreversible blockage of nicotinic acetylcholine receptors and thus producing lethal effects (Matsuda et al. 2001). In addition to locating in target organisms, imidacloprid may disperse in surface water, ground water, soil, plants, and other nontarget organisms after different types of application (Felsot et al. 1998; Phillips and Bode 2002; Laurent and Rathahao 2003; Juraske et al. 2009). Due to the concern over the widespread use of this insecticide, the effects of imidacloprid on nontarget species and the environment has been extensively investigated in previous studies. Imidacloprid is considered to be highly toxic to bees. Suchail et al. (2001) performed a 10-day chronic exposure test on honeybees (Apis mellifera) and found that mortality increased over the control at doses as low as 0.1 μg L−1. Laycock et al. (2012) found that the fecundity of bumble bees (Bombus terrestris) declined by one third when the bees were exposed to environmentally realistic concentrations of imidacloprid near 1 μg L−1. Kobori and Amano (2004) reported that imidacloprid applied at field rates could result in 71 and 67 % mortality to the female adults and pupae of the aphid parasitoid Aphidius gifuensis. Jemec et al. (2007) showed that the measured environmental levels of imidacloprid pose little potential chronic risk to the water flea Daphnia magna, and the toxicity of imidacloprid is highly species specific. Sardo and Soares (2010) found that growth, behavior, and avoidance were modified in freshwater oligochaete Lumbriculus variegatus exposed to 0–5 mg kg−1 imidacloprid-contaminated sediments. The toxicity of imidacloprid to the earthworm had also been reported in several earlier studies. Luo et al. (1999) and Zang et al. (2000) found that imidacloprid at a concentration of 0.5 mg kg−1 could cause sperm deformities in Eisenia fetida. Under field conditions, a decrease in the production of earthworm casts was observed by Lal et al. (2001) during a period of 120 days. Capowiez et al. (2003, 2006) and Dittbrenner et al. (2011) observed that the behavior of two earthworm species (Aporrectodea caliginosa and Lumbricus terrestris) was significantly altered, including decreases in burrow length, the rate of burrowing, the distance covered, and the number of surface casts, at concentrations of imidacloprid below 4 mg kg−1 in dry soil. However, information regarding biochemical responses (e.g., oxidative stress and lipid peroxidation) of imidacloprid in earthworms is scarce or limited.
Biochemical responses of living organisms have been reported to be changed under the stress of exposure to pollutants at earlier stages and lower concentrations. These changes are sensitive, informative, and reproducible and can indicate the potential toxicity of contaminants preceding tissue damage or disease initiation. Thus, biomarkers at the biochemical level have been regarded as an effective tool to investigate and assess the influence of xenobiotics on the environment (Lukkari et al. 2004; Lin et al. 2010). Although the formation of reactive oxygen species (ROS) is a normal metabolic process, ROS can also be generated in living organisms under the stress of environmental contaminants. The overproduction of ROS can result in oxidative stress, disorders of digestion, lipid peroxidation, and cell death (Xiao et al. 2006). Malondialdehyde (MDA), the end product of lipid peroxidation, has been used as a biomarker for membrane damage. Moreover, the MDA level may also indicate the levels of ROS in living organisms (Jain et al. 2001). In the digestive system of the earthworm, cellulase plays a beneficial role in promoting the decomposition of organic matter in soil (Tejada et al. 2010). Cellulase has been used extensively as an important biomarker to assess the toxicity of pesticides to earthworms (Luo et al. 1999; Xiao et al. 2006; Shi et al. 2007). To counter the toxic effect of pollutants, some endogenous antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (POD), as well as nonenzymatic mechanisms scavenge the excess ROS and alleviate their deleterious effects in living organisms (Maity et al. 2008). Thus, changes in ROS, MDA, and enzymatic activity should indirectly indicate the toxic effects of exposure to pollutants on living organisms.
Among the various earthworm species, E. fetida is the most abundant in compost and the upper straw layer of soil. Use of E fetida is especially appropriate for the toxicity tests due to easy breeding and rapid propagation (Yasmin and D’Souza 2007). Sensitivity of E. fetida to xenobiotics is comparatively less than the sensitivity of other species of earthworm (Ma and Bodt 1993; Fitzgerald et al. 1996). In the guidelines of the Organization for Economic Co-operation and Development (OECD), E. fetida is also used as a standard model organism for assessing the toxicity of xenobiotics in soil (OECD 2004). In the present study, we used E. fetida to investigate the effects of imidacloprid on antioxidative, digestive, and biochemical activities, including SOD, CAT, POD, and cellulase activities, as well as the ROS level and MDA content. The aim of this study was to provide fundamental data and comprehensive understanding from a biochemical level in earthworms exposed to imidacloprid under standard laboratory conditions.
Materials and methods
Chemicals and reagents
Imidacloprid (CAS No. 138261-41-3, purity 98.5 %) was purchased from Dr. Ehrenstorfer GmbH (Augsburg) in Germany. Thiobarbituric acid (TBA), guaiacol, nitro blue tetrazolium (NBT), dichloro-dihydro-fluorescein diacetate (DCFH-DA), and l-methionine were purchased from the Sigma-Aldrich Shanghai Trading Co Ltd, China. All other chemicals used in the following tests were of analytical grade.
Earthworms and soil
Earthworms (E. fetida) were purchased from an earthworm-culturing farm in Qingdao, China. The earthworms were acclimatized in the laboratory for at least 2 weeks prior to use. The earthworms were placed in culture pots containing soil and cattle manure at 20 ± 1 °C, and the moisture content was adjusted to 50 %. Healthy earthworms with adult clitella weighing approximately 300–600 mg (fresh weight) were selected for the toxicity test. Before the exposure experiment, the earthworms were carefully removed from the culture, rinsed with distilled water to remove litter particles, and then placed on damp filter paper in the dark at 20 ± 1 °C for 24 h to empty the contents of their guts.
OECD artificial soil was used in this toxicity test. The soil was prepared using 20 % kaolin clay with 10 % sphagnum peat moss, and the pH was adjusted to 6.0 ± 0.5 by adding calcium carbonate (OECD 2004).
Experimental design
Imidacloprid exposure concentrations were modeled after the design scheme of Dittbrenner et al. (2011), who placed earthworms into soil mixed with 0 (solvent control), 0.2, 0.66, 2, and 4 mg kg−1 of imidacloprid and investigated changes in the burrowing behavior of the earthworms. Soil spiking was performed according to the protocol of Lin et al. (2010). Appropriate amounts of imidacloprid dissolved in 5 mL acetone were added to 50 g of artificial soil that was first placed in a clean glass jar (1,000 mL, diameter 105 mm), and then, the resulting mixture was blended for 1 min in the fume hood to mix well and allow the acetone to evaporate completely. Subsequently, the remaining 450 g of artificial soil was divided into three equal portions, and the contaminated soil was gradually added, blending for 3–5 min after each addition. Then, 175 mL of distilled water was added to the test soil, reaching a soil moisture content of 35 %. All soil samples were stabilized overnight prior to the earthworm toxicity test. Ten E. fetida were placed in each soil sample, and each treatment was replicated three times. All exposures were maintained in an incubator at 20 ± 1 °C under a 12/12-h light –dark cycle for 14 days. Five grams of wetted cattle manure was added to each test soil to maintain the earthworms during the entire exposure period. Three E. fetida were collected randomly from each replicate on the 1st, 3rd, and 14th day after the application of imidacloprid for analysis in MDA and ROS assays. No mortality was observed during the exposure experiments.
Enzyme extraction and determination of protein concentration
The extraction of enzymes was carried out on ice throughout the entire process. Gut-cleaned E. fetida were placed into a prechilled mortar and homogenized in 50 mM Tris–sucrose buffer (1:9, w/v, pH 7.5) for 3 min. The homogenate was centrifuged at 10,414 g at 4 °C for 20 min. The resulting supernatant was stored at −20 °C prior to the assays of enzyme activities, MDA content, and protein concentrations.
Protein concentration was determined using the Bradford (1976) method, and bovine serum albumin was used as the standard. The absorbance of the sample was detected at 595 nm.
Enzyme activity assay
SOD activity was assayed according the method of Durak et al. (1993). The activity was measured based on the inhibition of photochemical reduction of NBT. One unit (U) of SOD activity was defined as 50 % inhibition of the NBT photoreduction rate, and the result was expressed as U per milligram protein. CAT activity was measured following the method described by Xu et al. (1997). One unit of enzyme activity was equal to the amount of enzyme consumed after decreasing half of H2O2 over 100 s at 25 °C. POD activity was determined according to the process described by Song et al. (2009). After supernatant containing the enzyme was added to 3 mL of the reaction mixture (100 mM potassium phosphate buffer, pH 6.0, 50 mL; 30 % H2O2, 19 μL; and guaiacol, 28 μL), the variation of absorbance at 470 nm was recorded to calculate POD activity. One activity unit of POD was defined as the amount of enzyme that caused an increase of 0.01 absorbance units per minute, and the result was expressed as U per milligram protein. Cellulase activity was measured according to the method of Ghose (1987). The absorbance of the reaction solution was measured at 550 nm. The concentration of reducing sugar was estimated utilizing 3,5-dinitrosalicylic acid reagent, and glucose was used as the standard for reducing sugars. The result was expressed as milligrams glucose per milligram protein per hour.
ROS measurement
ROS production was determined according to the method of Lawler et al. (2003), with a slight modification. Gut-cleaned E. fetida were placed into a prechilled mortar containing 100 mM ice-cold potassium phosphate buffer (pH 7.4) and homogenized for 3 min. The resulting homogenate was centrifuged at 1,157 g at 4 °C for 10 min. The supernatant was then centrifuged at 38,012 g at 4 °C for 20 min, and the mitochondrial protein was obtained through resuspension. DCFH-DA was added into the mitochondrial suspension to obtain a 2-μM solution and was incubated for 30 min at 37 °C in a water bath. Fluorescence was monitored using a fluorescence spectrophotometer (Cary Eclipse Varian, Palo Alto, USA), with an excitation wavelength of 488 nm and an emission wavelength of 522 nm. The result was expressed as fluorescence intensity per milligram protein.
MDA detection
Determination of MDA content was based on the TBA assay described by Xiang and Wang (1990), with slight modifications. The 200 μL enzymatic supernatant was mixed with the reaction solution (0.2 mL of 8.1 % dodecylsulfate (SDS), 1.5 mL of 20 % acetic acid, pH 3.5, 1 % TBA, and 1 mL of water), and the resulting mixture was incubated at 90 °C for 60 min in a water bath. The mixture was centrifuged at 1,157 g for 15 min. The absorbance of the supernatant was measured at 532 nm, and MDA content was defined as nanomole TBA-reactive substance per milligram protein.
Statistical analysis
Each treatment was replicated three times, and all statistics were conducted in the SPSS software (SPSS 16.0). All of the values are presented as the mean ± standard deviation (SD). Parametric tests were preceded by tests to evaluate homogeneity of variances. One-way analysis of variance (ANOVA) followed by post hoc comparisons (LSD tests) were carried out to test for significant differences between the exposed and control groups. A significant difference from the control is indicated as p < 0.05 or p < 0.01.
Results
Effects of imidacloprid on the activity of SOD, CAT, POD, and cellulase in E. fetida
Compared with the control, no significant (p > 0.05) changes were observed in SOD activity at a dose of 0.2 mg kg−1 imidacloprid during the 14 days of exposure (Fig. 1a, Table 1). However, at doses of 0.66 and 2 mg kg−1 imidacloprid, the activity of SOD was significantly (p < 0.05 and p < 0.01) increased with increasing exposure time, reaching approximately 149 % of the value in the controls. After exposure to 4 mg kg−1 imidacloprid, the activity of SOD was significantly (p < 0.05) increased after 1 day of exposure but was significantly (p < 0.05) inhibited at 7 and 14 days of exposure. Univariate analyses (ANOVA) revealed significant influences of dose (p < 0.05) and duration (p < 0.01) on the activity of SOD. The activity of SOD was also significantly (p < 0.01) affected by the interaction of dose and duration.
Effect of imidacloprid on the activities of a superoxide dismutase (SOD), b catalase (CAT), c peroxidase (POD), and cellulase in E. fetida. Each bar represents the mean of three replicates, and the error bars represent the standard deviation (SD). *p < 0.05 or **p < 0.01 compared with the control group at the same exposure time
The activity of CAT in the 0.2 and 0.66 mg kg−1 imidacloprid treatments was significantly increased (p < 0.05) compared with the control only at a 14-day exposure (Fig. 1b). Over the three exposure times, the activity of CAT was significantly (p < 0.05) increased at the dose of 2 mg kg−1 imidacloprid. Although a slight decline in CAT activity was observed when E. fetida was treated with 4 mg kg−1 imidacloprid after 1–14 days of exposure, the activity was not significantly different compared with the control (p > 0.05). The duration of exposure played a crucial role in affecting the activity of CAT in E. fetida (Table 1).
The activity of POD in E. fetida at a dose of 0.2 mg kg−1 imidacloprid was significantly (p < 0.05) stimulated after a 7-day exposure (Fig. 1c). With the increase of concentration of imidacloprid, the activity of POD was still significantly (p < 0.05) increased after exposure to 0.66, 2, and 4 mg kg−1 imidacloprid after 1-day of exposure, and the activity reached approximately 112, 121, and 113 % of the value in the control, respectively. Noticeably, the activity of POD at a dose of 0.66 mg kg−1 imidacloprid was significantly (p < 0.01) increased after 7 and 14 days of exposure. The concentration of imidacloprid was the most important factor influencing the activity of POD in E. fetida (Table 1).
The activity of cellulase was significantly (p < 0.05) increased when E. fetida was treated with 0.2 and 0.66 mg kg−1 imidacloprid after a 1-day exposure (Fig. 1d). However, the activity of cellulase was significantly (p < 0.05) inhibited by 2 and 4 mg kg−1 imidacloprid over the entire exposure time (14 days). Dose as well as dose and duration interactions together affected the activity of cellulase in E. fetida (Table 1).
ROS production induced by imidacloprid
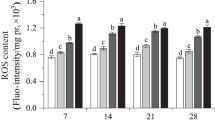
Compared to the controls, there were no significant (p > 0.05) changes in ROS levels at a dose of 0.2 mg kg−1 imidacloprid during the exposure period of 14 days (Fig. 2). After 1- and 7-day exposures, ROS were significantly (p < 0.05) induced by 0.66 mg kg−1 imidacloprid. With increasing concentrations of imidacloprid, the content of ROS became higher (p < 0.05 and p < 0.01) than that of the control at 2 and 4 mg kg−1 over 14 days of exposure. Dose, duration, and their interaction were regarded as having the same effect on the production of ROS (Table 1).
Effects of imidacloprid on lipid peroxidation in E. fetida
Compared with the controls, there were no significant (p > 0.05) changes in MDA content at a dose of 0.2 mg kg−1 imidacloprid (Fig. 3). After 7- and 14-day exposures, the content of MDA was significantly higher (p < 0.05) than that of the control at a dose of 0.66 mg kg−1 imidacloprid. After exposure to 2 mg kg−1 imidacloprid, the content of MDA was always significantly (p < 0.05) higher than that of the control on days 1, 7, and 14. Moreover, when imidacloprid was applied at a dose of 4 mg kg−1, the content of MDA was significantly (p < 0.05 and p < 0.01) higher than that of the control; an approximate dose-dependent relationship was observed with increasing exposure time, and the content of MDA increased by 17, 38, and 40 % above the values in the controls on days 1, 7, and 14, respectively. The results of ANOVA showed that dose (p < 0.05), duration (p < 0.01), and the interaction of dose and duration (p < 0.01) all played crucial roles in affecting the content of MDA induced by imidacloprid (Table 1).
Discussion
In the present study, toxic effects of imidacloprid on E. fetida were detected at the biochemical level. The data presented in this work showed that activities of the enzymes as well as ROS and MDA levels varied according to the tested doses and exposure duration, although these differences were not always significant. There were significant toxic effects when the concentration of imidacloprid exceeded 0.66 mg kg−1. The concentrations used in the present study represent environmentally relevant scenarios (the predicted imidacloprid concentration can be as high as 0.66 mg kg−1 dry weight in soil) (Oi 1999). And since imidacloprid is frequently used in agriculture in recent years, therefore, our results deserve further attention.
ROS are a series of free radicals including mainly hydroxyl radicals (·OH), superoxide (O2 −), nitric oxide (NO·), and peroxyl (RO2·), which are formed and degraded by all aerobic organisms and have important physiological roles in redox homeostasis and cell antioxidant signaling. Usually, ROS do little harm to the cell if the intracellular mechanisms that reduce their damaging effects work properly. However, excess ROS can be induced by numerous toxic environmental chemicals such as heavy metals, pesticides, ozone, and other xenobiotics. Once the balance between the generation of ROS and the activity of the antioxidant is disrupted, severe oxidative stress can occur and cause cell damage or death (Valko et al. 2007; Poljšak and Dahmane 2012). In the present study, ROS were not excessively produced compared with the control at 0.2 mg kg−1 imidacloprid (Fig. 2), suggesting that the antioxidant system in E. fetida had sufficient capacity for scavenging excess ROS to protect itself from oxidative damage at this dose. With the increase of dose, the ROS level in E. fetida was increased significantly at 0.66, 2, and 4 mg kg−1 imidacloprid (Fig. 2), showing a greater degree of oxidative damage. According to previous studies, the reason for this increase in ROS is that antioxidant enzymes lack the ability to dispose of the excess ROS completely.
The antioxidant system plays an effective role in preventing damage to the biological system from ROS. Antioxidant enzymes constitute a mutually supportive group of defenders against ROS. In the antioxidant system of the earthworm, SOD, CAT, and POD are usually the first line of defense for eliminating excess ROS. Some studies have shown that these antioxidant enzymes can be induced by slight oxidative stress. However, the activity of these enzymes will be inhibited if they are subjected to a severe oxidative stress due to the loss of compensatory mechanisms in the organisms (Nordberg and Arner 2001; Lin et al. 2010). The three antioxidant enzymes investigated in this study play different roles in the defense against oxidative stress. SOD, an important antioxidant enzyme, catalyzes the transformation of O2 − into H2O2. The results of the present study show that the activity of SOD was significantly increased at 0.66 and 2 mg kg−1 imidacloprid over the 14 days of exposure (Fig. 1a). This phenomenon indicated that two doses of imidacloprid could induce the formation of O2 − and then stimulate the biosynthesis of SOD in earthworm to protect the cells from oxidant damage. The increase in SOD activity also suggests that SOD has the capacity to scavenge ROS under the stress of a lower concentration of imidacloprid. However, our result was contrary to the findings of Luo et al. (1999), who used filter paper contact toxicity test and found that a lower concentration of imidacloprid (0.1 mg L−1) could suppress SOD activity. This inconsistency may be because E. fetida is more sensitive to imidacloprid when supplied in filter paper than in the artificial soil (Chen et al. 2014). Although SOD activity was still markedly stimulated on the first day, the activity was significantly inhibited at 7- and 14-day exposures when E. fetida were exposed to the 4 mg kg−1 imidacloprid (Fig. 1a), indicating that overt oxidative stress had occurred. SOD apparently did not have the capacity to eliminate the overproduction of ROS (Fig. 2) at relatively high concentrations of imidacloprid with increasing exposure time. According to Sun et al. (2007), the decline in SOD activity is caused by the ability of the excess O2 − to suppress the activity of SOD and inactivate it. From the results for SOD in the present study, we conclude that E. fetida is likely to suffer significant oxidative stress from the effects of 4 mg kg−1 imidacloprid over extended exposure times.
After conversion of O2 − to H2O2 by the process of SOD catalysis, H2O2 and other free radicals are further scavenged by other antioxidant enzymes such as CAT and POD. CAT, an important antioxidant enzyme, exists in peroxisomes, the cytosol, and mitochondria, which can degrade H2O2 to H2O and O2 (Zhang et al. 2007). In the present study, the trend of change in the activity of CAT was generally consistent with the activity of SOD at 0.66 and 2 mg kg−1 imidacloprid (Fig. 1b). The increase of CAT activity could be explained by increases in the contents of its substrate to maintain the level of H2O2 as an adaptive mechanism (Zhang et al. 2013). When exposed to 4 mg kg−1 imidacloprid, the activity of CAT was slightly inhibited. This inhibition could be attributed to the H2O2 accumulation and indicated that the H2O2 scavenging function of CAT was impaired (Geret et al. 2002; Liu et al. 2010). POD also has the capacity to scavenge H2O2 through the oxidation of cosubstrates such as guaiacol and ascorbate. In the present study, the activity of POD was significantly increased at 0.66 mg kg−1 imidacloprid during the entire exposure period. However, at concentrations of 2 and 4 mg kg−1 imidacloprid, POD activity was significantly induced only on the first day (Fig. 1c), indicating that POD plays a crucial role in protecting E. fetida from antioxidant stress at a relatively low concentration of imidacloprid over short-term exposures. The results of the present study indicated that the activity of the three antioxidant enzymes had different responses to the toxicity of different concentrations of imidacloprid for different exposure periods. This result also supported the opinions of (Holovská et al. 1998), who have reported that the sensitivity of SOD, SAT, and POD to various levels of oxidative stress varies greatly and that organisms might respond to oxidative stress in different ways. Moreover, the three antioxidant enzymes cooperatively play an important role in the O2 − and H2O2 elimination process. The activity of SOD was the most sensitive to imidacloprid according to the present results. The concentration threshold of response for the three enzymes was approximately 2 mg kg−1, but further studies are required to confirm this threshold concentration.
Compared with the antioxidant enzymes, cellulase is a very important digestive enzyme in the earthworm. The activity of cellulase in the gut of E. fetida is 7-fold higher than in other earthworm species such as Metaphire guillelmi (Zhang et al. 2000). Therefore, in previous studies, E. fetida was the most frequently chosen species for the investigation of the effects of pollutants on earthworms by detecting the activity of cellulase. In the present study, the activity of cellulase was significantly increased at 0.2 and 0.66 mg kg−1 imidacloprid on the first day. The increase in cellulase activity may be attributed to the physiological functions in E. fetida strengthening against the oxidative attack (Xiao et al. 2006). However, the activity of cellulase was markedly inhibited at 2 and 4 mg kg−1 imidacloprid, and the inhibition became increasingly severe with the extension of the exposure time (Fig. 1d). The inhibitory effects indicated that 2 and 4 mg kg−1 imidacloprid could cause harmful damage to the biochemical metabolism of E. fetida. The result was consistent with the report of Luo et al. (1999), who found that imidacloprid could inhibit the activity of cellulase in E. fetida. Xiao et al. (2006) also obtained a similar result after measuring the cellulase activity of E. fetida when exposed to the herbicide acetochlor. According to the data from the present study, the effect of imidacloprid on cellulase in E. fetida should be subjected to further investigation.
Many studies have successfully proven that MDA is a reliable biomarker for evaluating the effects of pollutants on lipid peroxidation in living organisms. In the present study, the content of MDA did not change in response to 0.2 mg kg−1 imidacloprid. However, it was significantly induced by 0.66, 2, and 4 mg kg−1 imidacloprid. Moreover, the content of MDA was elevated after exposure to increasing imidacloprid concentrations and extending the exposure time (Fig. 3). The increase in the MDA content indicated that lipid peroxidation could be caused by relatively high concentrations of imidacloprid in E. fetida. The reason for lipid peroxidation has been previously suggested by some related studies, who reported that ROS can induce membrane lipid peroxidation and other negative effects if they cannot be scavenged in a timely manner (Shalata and Tal 1998; Fazeli et al. 2007; Lin et al. 2010). The results of the present study also confirm this point. Additionally, the correlation analysis showed that dose, duration, and the interaction between the dose of imidacloprid and the duration of exposure significantly influenced (p < 0.05) the content of MDA and ROS as well as the activity of SOD (Table 1). These results revealed that the biomarkers adopted in the present study were correlative and showed that imidacloprid could induce oxidative stress and lipid peroxidation in E. fetida in dose- and duration-dependent manners.
The aim of this study was to investigate the effects of imidacloprid on earthworm. To this end, soil toxicity test with E. fetida was performed using OECD artificial soil. However, the toxicity data obtained from OECD artificial soil may be different from those using native soil due to the differences between artificial and native soil with respect to pH, soil moisture, organic matter content, temperature, etc. Previous studies have also found that the sensitivity of earthworm to pollutants (e.g., heavy metal and pesticide) was different with the changes of soil environment (Bradham et al. 2006; Garcia et al. 2008). With respect to the degradation and transformation of imidacloprid in soil, some studies showed that abiotic processes including sorption, photodegradation, and hydrolysis were mainly responsible for imidacloprid degradation (Krohn J. Hellpointner E 2002; Liu et al. 2006). In addition, microbial degradation also plays an important role in the transformation of imidacloprid to a certain extent (Liu et al. 2011). All those indicate that the bioavailability of imidacloprid to earthworm may vary in different soil surrogates. Therefore, the future direction would be to study the toxic effects of imidacloprid on earthworm in native soils.
Conclusion
Based on the present work, imidacloprid exhibited a potentially harmful effect on E. fetida at the biochemical level. Although SOD was the most sensitive among the three antioxidant enzymes, all enzymes play a collaborative role in protecting against oxidation when subjected to imidacloprid stress. With the increase in imidacloprid concentration (>0.66 mg kg−1), the balance between antioxidant enzyme activity and ROS content was disrupted, and lipid peroxidation was induced. At the same time, the activity of cellulase was also significantly inhibited. The data also indicate that the sensitivity of E. fetida exposed to imidacloprid was enhanced with increasing exposure time. The information may be helpful for assessing the risk of imidacloprid in the soil ecosystem. However, further work is necessary to investigate the toxicity of imidacloprid on earthworm in native soils for obtaining more comprehensive toxicity data.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bradham KD, Dayton EA, Basta NT, Schroder J, Payton M, Lanno RP (2006) Effect of soil properties on lead bioavailability and toxicity to earthworms. Environ Toxicol Chem 25:769–775
Capowiez Y, Rault M, Mazzia C, Belzunces L (2003) Earthworm behaviour as a biomarker—a case study using imidacloprid: The 7th international symposium on earthworm ecology Cardiff Wales 2002. Pedobiologia 47:542–547
Capowiez Y, Bastardie F, Costagliola G (2006) Sublethal effects of imidacloprid on the burrowing behaviour of two earthworm species: modifications of the 3D burrow systems in artificial cores and consequences on gas diffusion in soil. Soil Biol Biochem 38:285–293
Chen C, Wang YH, Zhao XP, Wang Q, Qian YZ (2014) Comparative and combined acute toxicity of butachlor, imidacloprid and chlorpyrifos on earthworm, Eisenia fetida. Chemosphere 100:111–115
Dittbrenner N, Moser I, Triebskorn R, Capowiez Y (2011) Assessment of short and long-term effects of imidacloprid on the burrowing behaviour of two earthworm species (Aporrectodea caliginosa and Lumbricus terrestris) by using 2D and 3D post-exposure techniques. Chemosphere 84:1349–1355
Durak I, Yurtarslanl Z, Canbolat O, Akyol Ö (1993) A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta 214:103–104
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plantarum 51:98–103
Felsot A, Cone W, Yu J, Ruppert J (1998) Distribution of imidacloprid in soil following subsurface drip chemigation. Bull Environ Contam Toxicol 60:363–370
Fitzgerald DG, Warner KA, Lanno RP, Dixon DG (1996) Assessing the effects of modifying factors on pentachlorophenol toxicity to earthworms: applications of body residues. Environ Toxicol Chem 15:2299–2304
Garcia M, Römbke J, de Brito MT, Scheffczyk A (2008) Effects of three pesticides on the avoidance behavior of earthworms in laboratory tests performed under temperate and tropical conditions. Environ Pollut 153:450–456
Geret F, Serafim A, Barreira L, Bebianno MJ (2002) Effect of cadmium on antioxidant enzyme activities and lipid peroxidation in the gills of the clam Ruditapes decussates. Biomarkers 7:242–256
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Holovská K, Lenartova V, Rosival I, Kičinková M, Majerčiaková A, Legáth J (1998) Antioxidant and detoxifying enzymes in the liver and kidney of pheasants after intoxication by herbicides MCPA and ANITEN. J Biochem Mol Toxicol 12:235–244
Jain M, Mathur G, Koul S, Sarin N (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep 20:463–468
Jemec A, Tišler T, Drobne D, Sepčić K, Fournier D, Trebše P (2007) Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere 68:1408–1418
Juraske R, Castells F, Vijay A, Muñoz P, Antón A (2009) Uptake and persistence of pesticides in plants: Measurements and model estimates for imidacloprid after foliar and soil application. J Hazard Mater 165:683–689
Kobori Y, Amano H (2004) Effects of agrochemicals on life-history parameters of Aphidius gifuensis Ashmead (Hymenoptera: Braconidae). Appl Entomol Zool 39:255–261
Krohn J. Hellpointner E (2002) Environmental fate of imidacloprid. Pflanzenschutz Nachrichten Bayer, Special Edition, 55:1-26
Lal O, Palta R, Srivastava Y (2001) Impact of imidacloprid and carbofuran on earthworm castings in okra field. Ann Plant Prot Sci 9:137–138
Laurent FM, Rathahao E (2003) Distribution of [14C] imidacloprid in sunflowers (Helianthus annuus L.) following seed treatment. J Agric Food Chem 51:8005–8010
Lawler JM, Song W, Demaree SR (2003) Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Bio Med 35:9–16
Laycock I, Lenthall KM, Barratt AT, Cresswell JE (2012) Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21:1937–1945
Lin DS, Zhou QX, Xie XJ, Liu Y (2010) Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida). Chemosphere 81:1328–1333
Liu W, Zheng W, Ma Y, Liu KK (2006) Sorption and degradation of imidacloprid in soil and water. J Environ Sci Health B 41:623–634
Liu Y, Zhou QX, Xie XJ, Lin DS, Dong LX (2010) Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology 19:1551–1559
Liu ZH, Dai YJ, Huang GD, Gu YY, Ni J, Wei H, Yuan S (2011) Soil microbial degradation of neonicotinoid insecticides imidacloprid, acetamiprid, thiacloprid and imidaclothiz and its effect on the persistence of bioefficacy against horsebean aphid Aphis craccivora Koch after soil application. Pest Manag Sci 67:1245–1252
Lukkari T, Taavitsainen M, Soimasuo M, Oikari A, Haimi J (2004) Biomarker responses of the earthworm Aporrectodea tuberculata to copper and zinc exposure: differences between populations with and without earlier metal exposure. Environ Pollut 129:377–386
Luo Y, Zang Y, Zhong Y, Kong ZM (1999) Toxicological study of two novel pesticides on earthworm Eisenia foetida. Chemosphere 39:2347–2356
Ma WC, Bodt J (1993) Differences in toxicity of the insecticide chlorpyrifos to six species of earthworms (Oligochaeta, Lumbricidae) in standardized soil tests. Bull Environ Contam Toxicol 50:864–870
Maity S, Roy S, Chaudhury S, Bhattacharya S (2008) Antioxidant responses of the earthworm Lampito mauritii exposed to Pb and Zn contaminated soil. Environ Pollut 151:1–7
Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22:573–580
Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Bio Med 31:1287–1312
OECD (2004) Guidelines for testing of chemicals no. 222: earthworm reproduction tests (Eisenia fetida/Eisenia andrei). Organization for economic co-operation and development (OECD), Paris
Oi M (1999) Time-dependent sorption of imidacloprid in two different soils. J Agric Food Chem 47:327–332
Phillips PJ, Bode RW (2002) Concentrations of pesticides and pesticide degradates in the Croton River watershed in southeastern New York, July-September 2000. US Department of the Interior, US Geological Survey
Poljšak B, Dahmane R (2012) Free radicals and extrinsic skin aging. Dermatol Res Pract Article ID 135206, 4 pages
Sardo A, Soares A (2010) Assessment of the effects of the pesticide imidacloprid on the behaviour of the aquatic oligochaete Lumbriculus variegatus. Arch Environ Contam Toxicol 58:648–656
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:169–174
Shi YJ, Shi YJ, Wang X, Lu YL, Yan SF (2007) Comparative effects of lindane and deltamethrin on mortality, growth, and cellulase activity in earthworms (Eisenia fetida). Pestic Biochem Physiol 89:31–38
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41:905–909
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20:2482–2486
Sun YY, Yin Y, Zhang JF, Yu HX, Wang XR (2007) Bioaccumulation and ROS generation in liver of freshwater fish, goldfish Carassius auratus under HC Orange No. 1 exposure. Environ Toxicol 22:256–263
Sur R, Stork A (2003) Uptake, translocation and metabolism of imidacloprid in plants. B Insectol 56:35–40
Tejada M, Gómez I, Hernández T, García C (2010) Response of Eisenia fetida to the application of different organic wastes in an aluminium-contaminated soil. Ecotoxicol Environ Saf 73:1944–1949
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Xiang R, Wang DN (1990) The improvement of lipid peroxidation thiobarbituric acid spectrophotometry. Prog Biochem Biophys 17:241–243
Xiao NW, Jing B, Ge F, Liu XH (2006) The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere 62:1366–1373
Xu JB, Yuan XF, Lang PZ (1997) Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. Environ Chem 16:73–76 (in chinese)
Yasmin S, D’Souza D (2007) Effect of pesticides on the reproductive output of Eisenia fetida. Bull Environ Contam Toxicol 79:529–532
Zang Y, Zhong Y, Luo Y, Kong ZM (2000) Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida. Environ Pollut 108:271–278
Zhang BG, Li GT, Shen TS, Wang JK, Sun Z (2000) Changes in microbial biomass C, N, and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol Biochem 32:2055–2062
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhang QM, Zhu LS, Wang J, Xie H, Wang JH, Han YN, Yang JH (2013) Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ Sci Pollut Res 20:201–208
Acknowledgments
This research was supported by the National Science Foundation of China (Grant No. 41101488) and Tai-Shan Scholar Construction Foundation of Shandong Province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, B. & Wang, C. Ecotoxicological effects on the earthworm Eisenia fetida following exposure to soil contaminated with imidacloprid. Environ Sci Pollut Res 21, 12345–12353 (2014). https://doi.org/10.1007/s11356-014-3178-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3178-z