Abstract
Plastics of petrochemical origin are materials difficult to degrade, and they have caused environmental impacts. The development of new biotechnological strategies for the production of bioplastics has attracted the attention of researchers all over the world, enabling the production of economically more viable, biodegradable materials with different possibilities of application in various industrial sectors. The polyhydroxyalkanoates are biopolymers with physicochemical properties similar to those of polypropylene, polystyrene, and polyethylene commonly used by the industry. These bioplastics can be biosynthesized by various microorganisms and accumulate polyhydroxyalkanoates granules intracellularly. The high cost of polyhydroxyalkanoates production is still a limiting factor for its large-scale production, and the costs associated with the carbon source used are one of the reasons that increase the price of the product. This review discusses the main factors associated with polyhydroxyalkanoates production, providing an overview of the different attempts to produce the biopolymer from the use of low-cost substrates and the development of different fermentation strategies for the production of these polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics of petrochemical origin are essential commodities in society, being widely used in a variety of products that provide comfort and quality of life to people. Due to their physicochemical properties, synthetic plastics have been an extremely relevant material from agriculture to the biomedical sector. However, its excessive use and its non-biodegradable composition generate major environmental problems, since the degradation rate of these materials is prolonged, with a half-life up to 500 years. In addition, plastics occupy large volumes in landfills, making it difficult for other organic materials to decompose (Vigneswari et al. 2021). In 2018, there was a disposal of approximately 396 million tons of plastic, with an estimate for the year 2050 of 810 million tons (Sharma et al. 2021).
Contamination by microplastics (plastics smaller than 5 mm) is also a frequent concern among researchers. These materials are present everywhere contaminating the soil, air, and the entire aquatic environment. Fish and other marine animals can consume the microplastics and affect the digestive process, leading to their death from malnutrition (Watteau et al. 2018). An alternative to replace traditional plastics and, consequently, reduce the problems caused by the inappropriate disposal of these polymers is the development of biodegradable materials. The polyhydroxyalkanoates (PHA) are highlighted as a class of polyesters biosynthesized by several microorganisms, when exposed to culture media with excess carbon and limitation of other nutrients such as nitrogen and magnesium. The bioplastics are produced intracellularly and accumulated in the form of hydrophobic granules of 0.2–0.5 μm, surrounded by a boundary layer of proteins (Albuquerque and Malafaia 2018).
This review explores the use of alternative carbon sources for the production of PHA by different groups of microorganisms, presenting an overview of the opportunities and challenges inherent in the sustainable production and economic viability of polyhydroxyalkanoates.
Polyhydroxyalkanoates (PHA)
PHA is a class of biodegradable polyesters, consisting of hydroxyalkanoic acid monomers, produced by a wide variety of microorganisms (Alcaligenes latus, Azobacter vinelandii, Bacillus megaterium, Cupriavidus necator, Pseudomonas oleovorans e Escherischia coli) (Kumar et al. 2020). PHA biosynthesis happens when there is an excess of carbon and a limitation of essential nutrients for cell multiplication, such as phosphorus, nitrogen, and magnesium. Under these conditions, there is an accumulation of the biopolymer intracellularly in the form of granules as a source of carbon and energy reserve (Shahid et al. 2021). The versatility of its properties has made PHA widely applicable in biomedical devices, electronics, civil construction, automotive sector, packaging and agriculture, increasing its importance in the global market (Saratale et al. 2021; Bedade et al. 2021).
Polyhydroxyalkanoates were first reported by microbiologist Maurice Lemoigne in 1926, who observed PHA granules produced by Bacillus megaterium (Kumar et al. 2020). With the petroleum crisis, there was greater interest in research on the production of PHA. Researchers were attracted by the characteristics of the polymer such as resistance to UV radiation, biocompatibility, biodegradability, water insolubility, in addition to having a wide range of mechanical and thermal properties, making PHAs an excellent replacement for plastic of petrochemical origin (Bedade et al. 2021; Sharma et al. 2021).
The first PHA reported in the literature was polyhydroxybutyrate (PHB), a homopolymer that has four carbon atoms in its main chain, produced intracellularly by bacterium Cupriavidus necator (Saratale al. 2021). Among the more than three hundred species of PHA-producing microorganisms, the bacterium C. necator has been considered of great biotechnological interest due to its large-scale production capacity, accumulating up to 80% of its dry mass in PHB (Schmidt et al. 2016).
PHB is a thermoplastic polymer with a melting point around 180 °C capable of being molded and used in various industrial segments (Raza et al. 2018). The main limitation for its use is its high crystallinity (around 70%) and low elasticity, leaving the material fragile and brittle, requiring the addition of plasticizers, co-polymers, or modifying agents to minimize this limitation.
The mechanical properties of PHAs vary according to the number of carbon atoms that constitute the monomeric unit. The size of the carbon chain differentiates its mechanical characteristics, which can be rigid and fragile, for short-chain PHA (3 to 5 carbon atoms), up to more flexible and elastomeric polymers for medium-chain (6–14 carbon chains) and long (longer than 15 carbon chains) PHA. This difference in the chemical structure of the polymer is directly related to the species of microorganism, the carbon source used, and the growing conditions (Vigneswari et al. 2021; Bedade et al. 2021).
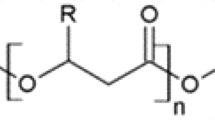
According to Shabina et al. (2015), there are about 150 types of hydroxyalkanoic acid monomers that are part of the chemical makeup of the polyhydroxyalkanoate family. These different chemical structures can be constituted by different functional groups such as halogens, epoxy, hydroxyls, carboxyls, enabling the formation of polymers with different properties (Chee et al. 2019). Figure 1 shows the general chemical structure of PHA.

adapted from Bedade et al. 2021)
General Chemical Structure of PHA (
Despite its excellent characteristics, PHA production is still limited due to its high production cost compared to synthetic plastics. The carbon sources used make production more expensive and can reach up to 40% of the total cost of the process (Carpine et al. 2020). Recent alternatives for sustainable PHA production involve the use of cheaper feedstock, and it includes agro-industrial by-products, as residual glycerol, agro-industrial residues, whey, vegetable oils from industrial processing/frying, and lignified biomass (Kumar et al. 2020; Saratale et al. 2021).
Bacterial mechanism for PHA biosynthesis
The understanding of the PHA synthesis pathway has been conducted since the 1920s. These pathways have already been elucidated, and some attempts to improve the efficiency of PHA biosynthesis have been made (Meng and Chen 2018). Recent advances are focused on systematic improvements in the biopolymer metabolic pathway, including changing the growth pattern for rapid proliferation, increasing cell size for greater PHA accumulation, and reprogramming biosynthetic pathways that redirect the metabolic flux (Chen and Jiang 2018; Meng and Chen 2018). Genome editing tools are being used for this purpose, such as CRISPR/Cas9 technology (Chen and Jiang 2018). In addition, the use of non-traditional bacteria, such as halophytes (Halomonas spp.), is also being explored to minimize the complexity in PHA production (Ye and Chen 2021). Rapid bacterial growth on simple media and the possibility of achieving a high cell density with high PHA content are important factors for a successful production process. E. coli is a widely studied bacteria with well-established technologies for genome manipulation, cultivation, and processing; many studies focus on using E. coli to effectively produce these biopolymers (Meng and Chen 2018). This bacterium is not a PHA producer; however, genes involved in the biosynthesis of C. necator H16 have already been transferred into E. coli, and production can be observed in this microorganism (Slater et al. 1998). Since then, many genetic modifications have been tested to improve PHA storage at low cost with high productivity, either by producing various copolymers using metabolic engineering and/or synthetic biology strategies (Wang et al. 2014). The details of the PHA biosynthetic pathway and its related enzymes are extensively studied (Wang et al. 2014; Chen and Hajnal 2015). Many of these studies have been conducted to enhance the metabolic flux of PHA synthesis (Kourmentza et al. 2017; Tan et al. 2020; Bedade et al. 2021), such as the limitation of essential elements (nitrogen, phosphorus, sulfur, or iron); oxygen limitation; repression of the beta-oxidation cycle; over-expression of NADH (or NADPH); synthesis enzymes and construction of new pathways for synthesis of non-3-hydroxybutyrate (3HB) monomers, such as 4-hydroxybutyrate (4HB) or 3-hydroxyvalerate (3HV), from glucose as a substrate (Fig. 2). CRISPR/Cas9 technology, especially CRISPRi (interference CRISPR), has been successfully used to manipulate genes related to PHA synthesis (Tao and Chen 2017; Chen and Jiang 2018; Lin et al. 2021). CRISPRi-based genomic editing was used in repressing competing pathways in 4-hydroxybutyrate (4HB) production, allowing the glucose-generated flux to be directed exclusively to 4HB production (Chen and Jiang 2018). Tao and Chen (2017) used CRISPRi to repress the propionate competition pathway and direct only to 3HV formation. In another study, the CRISPRi was able to regulate the metabolic pathways related to PHBV synthesis, thereby enhancing PHBV production (Lin et al. 2021). The downregulation by CRISPRi on the citrate synthase genes (citZ and gltA) improved the PHBV accumulation by 76.4% (from 1.78 to 3.14 g/L). They also further shorten the PHBV fermentation period and enhance PHA productivity by 165%.

adapted from Kumar et al. 2020)
Representation of metabolic pathways for microbial biosynthesis of PHAs (
There are three main pathways for PHA synthesis in microbial systems, involving different substrate sources and forms of fermentation: pathway I, it involves the conversion of acetyl-CoA to 3-hydroxybutyryl-CoA; pathway II, the degradation of fatty acids by the β-oxidation mechanism; and pathway III, through fatty acid biosynthesis. Pathways I and III employ fermentable sugars, while pathway II uses fatty acids for microbiological growth and PHA production (Saratale et al. 2021). Figure 2 shows the representation of different metabolic pathways for PHA biosynthesis. The scl-PHAs are synthesized in three main steps, starting with acetyl-CoA, while mcl-PHAs can be synthesized in two different ways. The first route consists of the β-oxidation of fatty acids before their incorporation into the polyester chain; the second consists of fatty acid biosynthesis (Arumugam et al. 2019).
The CRISPR/Cas9 approach operates on the three main PHA biosynthesis pathways. In Fig. 2, it is possible to observe the target region of CRISPRi in the different metabolic routes of PHA production. In route 1, sugar as a carbon source, the CRISPRi acts in the regulation of genes of the products involved in the TCA cycle (e.g., acetyl CoA). In route 2, the CRISPRi acts in the conversion of the products hydroxyacyl CoA and acyl CoA. Finally, it also acts directly in the conversion of hydroxyacyl ACP into hydroxyacyl CoA in route 3. The role in gene modulation played by crispr/cas9 system is already known. It is possible to repress and overexpress genes of interest.
Manipulated organisms carrying genes related to PHA synthesis, named "chassis," are one of the critical factors for biosynthesis. Contamination-resistant and easily manipulated bacteria become useful for biotechnology that has additional advantages in PHA production. Halomonas spp. are examples of feasibility and challenges to develop a New Generation Industrial Biotechnology (NGIB) in PHA production more competitively. NGIB depends on these microorganisms for continuous large-scale bioproduct production to occur (Chen and Jiang 2018). In recent years, biological evolution is observed to improve biosynthesis efficiency and optimize the industrial production of PHA. Strategies include redirecting metabolism for PHA synthesis, increasing cell size, accelerating cell growth, and reprogramming biosynthesis by CRISPR/Cas9. If successful, the reprogrammed organisms should be able to grow under open, continuous conditions for economic PHA production (Chen and Jiang 2018).
As shown in Fig. 2, PHA biosynthesis competes with other metabolic cycles and intermediaries, so regulation of competing pathways is critical so that more substrates are diverted into the PHA biosynthesis pathway. For example, PHB synthesis is regulated by the flux of acetyl-CoA, which can enter the tricarboxylic acid (CAT) cycle or serve as a precursor for PHB biosynthesis. The fate of acetyl-CoA can be induced by a nutrient limitation or microbial growth conditions (Sudesh et al. 2000). Under nutrient-limited conditions, there is an increase in intracellular NADH which inhibits key regulatory enzymes of the TCA cycle (citrate synthase and isocitrate dehydrogenase), resulting in downregulation of the TCA cycle. As a consequence, acetyl-CoA does not participate in the TCA cycle, accumulating inside cells. Accumulation of acetyl CoA occurs simultaneously with the reduction of free Co-A levels, activating the β-ketothiolase enzyme of the PHB synthetic pathway (Sudesh et al. 2000; Obruca, Sedlacek and Koller 2021).
Although the biosynthetic pathways of PHA are well investigated, there are still aspects that are not understood about the physiology of the microorganisms that produce this biopolymer. The optimization of cultivation conditions is another important factor in the metabolic regulation for PHA production, mainly for industrial-scale processes. Culture conditions include carbon, nitrogen, phosphate, dissolved oxygen, pH, carbon to nitrogen and phosphate ratios, trace elements, and supplement levels. Supplements are substances that are not essential for microbial growth but can trigger specific biosynthetic pathways and significantly increase PHA titer and productivity. Nutrient limitation and culture medium conditions can trigger PHA accumulation to higher intracellular levels (Li and Wilkins 2020).
Strategies for PHA production from agro-industrial residues
Currently, PHA production represents only 1.2% of total bioplastic production. The main cause of this low percentage is the cost associated with its production, about 2–3 times higher than the production of conventional plastics. The use of alternative carbon sources has been a strategy used to lower the price of the product, exerting a positive impact on production at the industrial level (Arumugam et al. 2019; Sehgal; Gupta 2020). The availability of renewable resources, mainly, agro-industrial by-products, is an excellent strategy to decrease the production costs of polyhydroxyalkanoates. In addition, the same by-product can be used by different bacteria to generate a PHA with different molecular compositions (Jian et al. 2016).
Polyhydroxyalkanoates are manufactured by several companies around the world: Metabolic (Woburn, MA, EUA), Procter & Gamble Co., Ltd. (Cincinnati, OH, EUA), Tianjin Green Bioscience Co., Ltd. (Tianjin, China) Bio on (Italy), Biocycle PHB Industry SA (São Paulo, Brazil) e Goodfellow Cambridge, Ltd. (UK), with variable annual production. According to the subtract used, it is possible to suggest which metabolic pathway the PHA was obtained, since each biosynthesis pathway depends on the source consumed by the microorganism (Fig. 2).
The substitution of expensive substrates such as glucose, mannitol, 1,4-butanediol, xylose, and fructose with cheaper carbon sources for the production of polyhydroxyalkanoates is extremely important to obtain economically viable products. Carbon sources from agro-industrial residues are organically rich materials and can be metabolized by a variety of microorganisms, such as: Cupriavidus necator, Alcaligenes eutrophus, Wautersia eutropha ou Ralstonia eutropha, Alcaligenes latus, Aeromonas hydrophila, Pseudomonas putida, and Escherichia coli (Albuquerque and Malafaia 2018). Table 1 lists the PHA-producing microorganisms and the wastes used to produce the bioplastic industrially.
Among the substrates used as a carbon source for PHA production, glycerol stands out. The rapid growth of the biodiesel industry has generated a significant amount of residual glycerol (RG), making it a promising carbon source for the production of polyhydroxyalkanoates by bacterial fermentation. Albuquerque et al. (2018) evaluated the production of the biopolymer from fermentation using the bacterium C. necator. During biomass production, the researchers verified a 35.75 and 45.08% consumption of pure glycerol (PG) and crude glycerol (CG), respectively, indicating higher PHA accumulation in PG.
Ntaikou et al. (2018) studied the accumulation efficiency of PHA produced from acidified residual glycerol (ARG) by pure microbial cultures (PMCs) and mixed cultures (MMCs). Maximum accumulation capacity occurred in MMCs with 40% PHA per dry cell mass. The lowest accumulation capacity was observed for residual non-acidified glycerol, which led to the formation of P(3HB), while in the presence of ARG and its derivatives (propionate or hexanoate) PHA with different monomeric compositions was produced. Other studies using commercial glycerol, residual glycerol, and substrate mixtures are presented in Table 2.
Residual sources of lipids such as cooking oil and residues from vegetable oil production have attracted the attention of researchers since the 1990s because of their low cost and wide availability (Chee et al. 2019). The peanut oil (Arachis hypogea) was reported by Pérez-Arauz et al. (2019) as a carbon source in PHA production using the bacterium C. necator. At the end of the fermentation process, 26.8% peanut oil was consumed with 51% PHA accumulation. Table 3 shows studies performed with lipid substrates with different microorganisms and the PHA yield obtained for each substrate.
Whey is one of the main by-products of the dairy industry, obtained during cheese production. According to Amaro et al. (2019), there is an annual production of 120 million tons of whey worldwide, and only 50% of its volume is used for food production. The large availability of this by-product and the amount of carbohydrates present in its constitution make cheese whey a promising substrate for PHA production.
The study conducted by Das et al. (2018) evaluated ultrafiltered whole cheese whey for P(3HB) production by B. megaterium NCIM 5472 with 75% P(3HB) accumulation. Raho et al. (2020a, b) used cheese whey for PHA production using the bacterium Haloferax mediterranei obtaining 1.18 g/L of PHA. Other research using whey-based waste and PHA-producing microorganisms is available in Table 4.
Agroindustrial residues have gained notoriety and are considered relevant substrates for the production of PHAs on an industrial scale (Chee et al. 2019). Rao et al. (2019) used different agricultural residues as the sole source of carbon and nitrogen for the production of PHAs by Bacillus subtilis MTCC 144. The concentrations of these sources were statistically optimized using response surface methodology (RSM), associated with the genetic algorithm approach. Among the residues used, watermelon rinds and legume peels were the most suitable showing recovery of 78.60% PHA.
The sugar cane molasses, vinasse, starch, and lignocellulosic materials are favorable feedstocks for PHA production. The use of molasses as a by-product for PHA production is an excellent production strategy, since molasses contains significant amounts of minerals, essential for biomass growth, and the use of this waste decreases the costs associated with its disposal. Other research using the above-mentioned substrates is present in Table 5.
According to Nielsen et al. (2017) many agro-industrial substrates despite having all the advantages mentioned above, have very complex chemical compositions requiring pretreatment before being used as alternative carbon sources during the production process. Acid or enzymatic hydrolysis is commonly used pretreatments to convert the different renewable carbon sources into fermentable sugars. During the sample pretreatment step, there is the production of growth inhibitors, and consequently, there is a decrease in the production yield, making it necessary to add new procedures to eliminate these inhibitors.
When crude glycerol is used as a substrate for bioplastic production, the number of inorganic salts, methanol, and organic acids can cause a decrease in production yield. The reduction of bacterial growth, an essential step during PHA production, is related to methanol in the substrate that can be removed from the alcohol evaporation (Cavalheiro et al 2012).
Acid hydrolysis of lignocellulosic materials produces microbial growth inhibitors such as phenolic and aromatic compounds are released specifically from lignin, and furans are produced by the dehydration of pentoses and hexoses and organic acids produced from the acetyl group cleavage of hemicellulose or thermochemical degradation products. These compounds need to be removed from the hydrolysate to increase the yield of the PHA, and this can be done using membrane filtration techniques, evaporation of volatile compounds, ion exchange resins, and even the use of microorganisms that are able to degrade the inhibitors before fermentation (Obruca et al. 2015).
Other wastes such as cooking oil and cheese whey have the advantage of not requiring pretreatment and can be added directly to the growth medium to produce the biopolymer. This strategy is an excellent alternative for the reuse of oil, minimizing the environmental pollution caused when this material is discarded in the environment (Nielsen et al. 2017). Currently, industries use agro-industrial residues for PHA production according to Table 1.
The use of photosynthesizing microorganisms as producers of polyhydroxyalkanoates
Algae have long been investigated as a plausible reserve of several compounds, attributed to their fast-growing characteristics and short doubling time. Compounds extracted from algae are being studied in various pharmaceutical, cosmetic, cancer biology, nanoscience, food, and environmental industries (Zhang et al. 2020). Algae produce a range of basic materials that can be used to assemble bioplastics. Poly-3-hydroxybutyrate (PHB) is a polymer that is a type of PHA that is widely explored commercially and has excellent potential as biodegradable plastics and bioderivatives (Mendhulkar and Shetye 2017; Abdo and Ali 2019). There are two types of algae: microalgae and cyanobacteria, the latter also known as blue green algae. These photosynthetic microorganisms are capable of synthesizing and accumulating polyhydroxyalkanoates under different cultivation conditions, including: photoautotrophic, heterotrophic, and mixotrophic (Roja et al. 2019). Under autotrophic conditions, cyanobacteria fix their carbon source in the Calvin–Benson–Bassham (CBB) cycle with the help of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO). RuBisCO has a higher efficiency for CO2 fixation than for O2 and is known to be responsible for the assimilation of a large amount of carbon present in the earth's biomass. Inorganic carbon (Ci) carriers present in the cell wall transfer atmospheric CO2, thus helping to maintain the carbon concentration for RuBisCO. The output of the Calvin cycle, glyceraldehyde-3-phosphate after its conversion to 3-phosphoglycerate (PGA), can then enter any of the three pathways for sugar metabolism, i.e., the Entner-Doudoroff (ED) pathway, glycolysis, pentose phosphate and be finally converted to acetyl-CoA for use in the synthetic PHA pathway according to Fig. 3 (Singh and Mallick 2017). Different cultivation strategies are used for the production of PHAs in algae, such as the use of standard media, deficiency or addition of some nutrients, modification of parameters such as salinity and gas exchange, including the use of effluents such as sewage. Table 6 shows different types of algae grown under different growing conditions and their respective PHA/biomass (w/w) yields.

adapted from Afreen et al. 2021)
PHA conversion from carbon accumulation and CO2 fixation in cyanobacteria (
Several cultivation models, extraction methods, and obtaining PHAs from algae are studied daily to obtain a better yield of the biopolymer. The table shows that the PHA yield can vary greatly depending on the form of cultivation. However, metabolic studies of the production and composition of these bioproducts in algae have not yet been achieved and extensive research is needed (Arias et al. 2020).
Conclusion
The use of petrochemical plastics causes tons of waste to accumulate all over the planet, causing serious environmental impacts. The development of biodegradable plastics is necessary and must unite biodegradability, economic viability, and the production of materials with desirable physicochemical properties for their application in industry.
The production of polyhydroxyalkanoates is an excellent alternative for replacing conventional plastics. These biopolymers are produced by a series of microorganisms that can metabolize various types of substrates, contributing to the reduction of waste and costs associated with the production of bioplastics.
References
Abdo SM, Ali GH (2019) Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull Natl Res Cent 43:1–4. https://doi.org/10.1186/s42269-019-0135-5
Abid S, Raza ZA, Hussain T (2016) Production kinetics of polyhydroxyalkanoates by using Pseudomonas aeruginosa gamma ray mutant strain EBN-8 cultured on soybean oil. 3 Biotech. https://doi.org/10.1007/s13205-016-0452-4
Afreen R et al (2021) Challenges and perspectives of polyhydroxyalkanoate production from Microalgae/Cyanobacteria and bacteria as microbial factories: an assessment of hybrid biological system. Front Bioeng Biotechnol 9:624885. https://doi.org/10.3389/fbioe.2021.624885
Ahn WS, Park SJ, Lee SY (2000) Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol 66:3624–3627. https://doi.org/10.1128/aem.66.8.3624-3627.2000
Albuquerque PBS, Malafaia CB (2018) Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int J Biol Macromol 107:615–625. https://doi.org/10.1016/j.ijbiomac.2017.09.026
Albuquerque PBS, Araújo KS, Silva KAA, Houllou LM, Locatelli GO, Malafaia CB (2018) Potential production of bioplastics polyhydroxyalkanoates using residual glycerol. J Environ Anal Prog. https://doi.org/10.24221/jeap.3.1.2018.1701.055-060
Alsafadi D, Al-Mashaqbeh OA (2017) one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol 34:47–53. https://doi.org/10.1016/j.nbt.2016.05.003
Amaro TMMM, Rosa D, Comi G, Iacumin L (2019) Prospects for the use of whey for polyhydroxyalkanoate (PHA) production. Front Microbiol 10:1–10. https://doi.org/10.3389/fmicb.2019.00992
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): Screening, optimization and characterization. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0158168
Arias DM, García J, Uggetti E (2020) Production of polymers by cyanobacteria grown in wastewater: current status, challenges and future perspectives. New Biotechnol 55:46–57. https://doi.org/10.1016/j.nbt.2019.09.001
Arumugam A, Anudakshaini TS, Shruthi R, Jeyavishnu K, Sundarra Harini S, Sharad JS (2019) Low-cost production of PHA using cashew apple (Anacardium occidentale L.) juice as potential substrate: optimization and characterization. Biomass Conv Bioref 10:1167–1178. https://doi.org/10.1007/s13399-019-00502-5
Basnett P, Marcello E, Lukasiewicz B, Panchal B, Nigmatullin R, Knowles JC, Roy I (2018) Biosynthesis and characterization of a novel, biocompatible medium chain length polyhydroxyalkanoate by Pseudomonas mendocina CH50 using coconut oil as the carbon source. J Mater Sci: Mater Med 29:179–190. https://doi.org/10.1007/s10856-018-6183-9
Bedade DK, Edson CB, Gross RA (2021) Emergent approaches to efficient and sustainable polyhydroxyalkanoate production. Mol 26:1–55. https://doi.org/10.3390/molecules26113463
Berwig KH, Baldasso C, Dettmer A (2016) Production and characterization of poly(3-hydroxybutyrate) generated by Alcaligenes latus using lactose and whey after acid protein precipitation process. Bioresour Technol 218:31–37. https://doi.org/10.1016/j.biortech.2016.06.067
Bhati R, Mallick N (2015) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: process optimization and polymer characterization. Algal Res 7:78–85. https://doi.org/10.1016/j.algal.2014.12.003
Carpine R, Olivieri G, Hellingwerf KJ, Pollio A, Marzocchella A (2020) Industrial production of poly-β-hydroxybutyrate from CO2: can cyanobacteria meet this challenge? Processes 8:1–23. https://doi.org/10.3390/pr8030323
Castro OV, Calderon JC, León E, Segura A, Arias M, Pérez L, Sobral PJA (2016) Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralstonia eutropha. J Biotechnol 231:232–238. https://doi.org/10.1016/j.jbiotec.2016.06.018
Cavalheiro JMBT et al (2012) Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol 111:391–397. https://doi.org/10.1016/j.biortech.2012.01.176
Chang YC et al (2021) Two-Stage Polyhydroxyalkanoates (PHA) Production from Cheese Whey Using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioeng 8:1–23. https://doi.org/10.3390/bioengineering8110157
Chee JY, Lakshmanan M, Jeepery IF, Hairudin NHM, Sudesh K (2019) The potential application of Cupriavidus necator as polyhydroxyalkanoates producer and single cell protein: a review on scientific, cultural and religious perspectives. Appl. Food Biotechnol. 6:19–34
Chen GQ, Hajnal I (2015) The ‘PHAome.’ Trends Biotechnol 33:559–564. https://doi.org/10.1016/j.tibtech.2015.07.006
Chen GQ, Jiang XR (2018) Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr Opin Biotechnol 53:20–25. https://doi.org/10.1016/j.copbio.2017.10.008
Coelho VC et al (2015) Polyhydroxybutyrate production by Spirulina sp. LEB 18 grown under different nutrient concentrations. Afr J Microbiol Res 9:1586–1594. https://doi.org/10.5897/AJMR2015.7530
Costa SS et al (2018) Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int J Biol Macromol 116:552–562. https://doi.org/10.1016/j.ijbiomac.2018.05.064
Cruz MV et al (2016) Valorization of fatty acids-containing wastes and byproducts into short and medium chain length polyhydroxyalkanoates. New Biotechnol 33:206–215. https://doi.org/10.1016/j.nbt.2015.05.005
Dalsasso RR, Pavan FA, Bordignon SE, Aragão GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem 85:12–18. https://doi.org/10.1016/j.procbio.2019.07.007
Das S, Majumder A, Shukla V, Suhazsini P, Radha P (2018) Biosynthesis of poly(3-hydroxybutyrate) from cheese whey by Bacillus megaterium NCIM 5472. J Polym Environ 26:4176–4187. https://doi.org/10.1007/s10924-018-1288-2
Dong T, Xiong W, Yu J, Pienkos PT (2018) Co-production of fully renewable medium chain α-olefins and bio-oil via hydrothermal liquefaction of biomass containing polyhydroxyalkanoic acid. RSC Adv 8:34380–34387. https://doi.org/10.1039/C8RA07359G
Fauzi AHM, Chua ASM, Yonn LW, Nittami T, Yeoh HK (2019) Enrichment of PHA-accumulators for sustainable PHA production from crude glycerol. Process Saf Environ Prot 122:200–208. https://doi.org/10.1016/j.psep.2018.12.002
Gahlawat G, Soni SK (2017) Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour Technol 243:492–501. https://doi.org/10.1016/j.biortech.2017.06.139
Gahlawat G, Soni SK (2019) Study on sustainable recovery and extraction of polyhydroxyalkanoates (PHAs) produced by Cupriavidus necator using waste glycerol for medical applications. Chem Biochem Eng Q 33:99–110
Haas R, Jin B, Zepf FT (2008) Production of poly(3-hydroxybutyrate) from waste potato starch. Biosci Biotechnol Biochem 72:253–256. https://doi.org/10.1271/bbb.70503
Hairudin NHBM, Ganesan S, Sudesh K (2021) Revalorization of adsorbed residual oil in spent bleaching clay as a sole carbon source for polyhydroxyalkanoate (PHA) accumulation in Cupriavidus necator Re2058/pCB113. Polym J 53:169–178. https://doi.org/10.1038/s41428-020-00418-2
Israni N, Venkatachalam P, Gajaraj B, Varalakshmi KN, Shivakumar S (2020) Whey valorization for sustainable polyhydroxyalkanoate production by Bacillus megaterium: Production, characterization and in vitro biocompatibility evaluation. J Environ Manage 255:1–10. https://doi.org/10.1016/j.jenvman.2019.109884
Jerez A et al (2007) Protein-based bioplastics: effect of thermo-mechanical processing. Rheol Acta 46:711–720. https://doi.org/10.1007/s00397-007-0165-z
Jiang G, Hill D, Kowalczuk M, Johnston B, Adamus G, Irorere V, Radecka I (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci 17:1–21. https://doi.org/10.3390/ijms17071157
Kachrimanidou V et al (2014) Sunflower-based biorefinery: poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour Technol 172:121–130. https://doi.org/10.1016/j.biortech.2014.08.044
Kavitha G et al (2016) Optimization of polyhydroxybutyrate production utilizing wastewater as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int J Biol Macromol 93:534–542. https://doi.org/10.1016/j.ijbiomac.2016.09.019
Khattab AM, Esmael ME, Farrag AA, Ibrahim MIA (2021) Structural assessment of the bioplastic (poly-3-hydroxybutyrate) produced by Bacillus flexus Azu-A2 through cheese whey valorization. Int J Biol Macromol 190:319–332. https://doi.org/10.1016/j.ijbiomac.2021.08.090
Kim SW, Kim P, Lee HS, Kim JH (1996) High production of poly-β-hydroxybutyrate (PHB) from Methylobacterium organophilum under potassium limitation. Biotechnol Lett 18:25–30. https://doi.org/10.1007/BF00137805
Koller M, Mukherjee A (2022) A new wave of industrialization of PHA biopolyesters. Bioenge. https://doi.org/10.3390/bioengineering9020074
Kourmentza C et al (2017) Recent Advances and challenges towards sustainable Polyhydroxyalkanoate (PHA) Production. Bioeng 11:55. https://doi.org/10.3390/bioengineering4020055
Kovalcik A et al (2017) Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int J Biol Macromol 102:497–504. https://doi.org/10.1016/j.ijbiomac.2017.04.054
Kumar P, Jun HB, Kim BS (2018) Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int J Biol Macromol 107:2552–2558. https://doi.org/10.1016/j.ijbiomac.2017.10.147
Kumar M et al (2020) Bacterial polyhydroxyalkanoates: opportunities, challenges, and prospects. J Clean Prod 263:1–91. https://doi.org/10.1016/j.jclepro.2020.121500
Li M, Wilkins MR (2020) Recent advances in polyhydroxyalkanoate production: feedstocks, strains and process developments. Int J Biol Macromol 156(691):703. https://doi.org/10.1016/j.ijbiomac.2020.04.082
Li T, Chen XB, Chen JC, Wu Q, Chen GQ (2014) Open and continuous fermentation: products, conditions and bioprocess economy. Biotechnol J 9:1503–1511. https://doi.org/10.1002/biot.201400084
Lin L et al (2021) Optimizing PHBV biopolymer production in haloarchaea via CRISPRi-mediated redirection of carbon flux. Commun Biol 4:1007. https://doi.org/10.1038/s42003-021-02541-z
Lopes MSG, Gosset G, Rocha RCS, Gomez JGC, Silva LF (2011) PHB biosynthesis in catabolite repression mutant of Burkholderia sacchari. Curr Microbiol 63:319–326. https://doi.org/10.1007/s00284-011-9981-6
López-Cuellar M, Alba-Flores J, Rodríguez JG, Pérez-Guevara F (2011) Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int J Biol Macromol 48:74–80. https://doi.org/10.1016/j.ijbiomac.2010.09.016
Lv L, Ren YL, Chen JC, Wu Q, Chen GQ (2015) Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: controllable P (3HB-co-4HB) biosynthesis. Metab Eng 29:160–168. https://doi.org/10.1016/j.ymben.2015.03.013
Martino L et al (2014) Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int J Biol Macromol 71:117–123. https://doi.org/10.1016/j.ijbiomac.2014.04.016
Mendhulkar VD, Shetye L (2017) Synthesis of biodegradable polymer polyhydroxyalkanoate (PHA) in cyanobacteria Synechococcus elongates under Mixotrophic nitrogen- and phosphate-mediated stress conditions. Ind Biotechnol 13:85–88. https://doi.org/10.1089/ind.2016.0021
Meng DC, Chen GQ (2018) Synthetic Biology of Polyhydroxyalkanoates (PHA). Adv Biochem Eng Biotechnol 162:147–174. https://doi.org/10.1007/10_2017_3
Morya R, Kumar M, Thakur IS (2018) Utilization of glycerol by Bacillus sp. ISTVK1 for production and characterization of polyhydroxyvalerate. Bioresour Technol Rep 2:1–6. https://doi.org/10.1016/j.biteb.2018.03.002
Narayanan A, Kumar VS, Ramana KV (2014) Production and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus mycoides DFC1 using rice husk hydrolyzate. Waste Biomass Valorization 5:109–118. https://doi.org/10.1007/s12649-013-9213-3
Nielsen C et al (2017) Food waste conversion to microbial polyhydroxyalkanoates. Microb Biotechnol 10:1338–1352. https://doi.org/10.1111/1751-7915.12776
Ntaikou I, Koumelis I, Tsitsilianis C, John P, Gerasimos L (2018) Comparison of yields and properties of microbial polyhydroxyalkanoates generated from waste glycerol-based substrates. Int J Biol Macromol 112:273–283. https://doi.org/10.1016/j.ijbiomac.2018.01.175
Obruca S, Marova I, Melusova S, Mravcova L (2011) Production of polyhydroxyalkanoates from cheese whey employing Bacillus megaterium CCM 2037. Ann Microbiol 61:947–953. https://doi.org/10.1007/s13213-011-0218-5
Oh YH et al (2015) Development of rice bran treatment process and its use for the synthesis of polyhydroxyalkanoates from rice bran hydrolysate solution. Bioresour Technol 181:283–290. https://doi.org/10.1016/j.biortech.2015.01.075
Oliveira CSS, Silva MOD, Silva CE, Carvalho G, Reis MAM (2018) Assessment of protein-rich cheese whey waste stream as a nutrients source for low-cost mixed microbial PHA production. Appl Sci 8:1–16. https://doi.org/10.3390/app8101817
Orliac O, Silvestre F (2003) Microwave esterification of sunflower proteins in solvent-free conditions. Bioresour Technol 87:63–68. https://doi.org/10.1016/s0960-8524(02)00200-6
Pais J, Serafim LS, Freitas F, Reis MA (2016) Conversion of cheese whey into poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. New Biotechnol 33:224–230. https://doi.org/10.1016/j.nbt.2015.06.001
Pérez-Arauz A et al (2019) Production and characterization of biodegradable films of a novel polyhydroxyalkanoate (PHA) synthesized from peanut oil. Food Packag Shelf Life 20:1–19. https://doi.org/10.1016/j.fpsl.2019.01.001
Phithakrotchanakoon C, Champreda V, Aiba SI, Pootanakit K, Tanapongpipat S (2015) Production of polyhydroxyalkanoates from crude glycerol using recombinant Escherichia coli. J Polym Environ 23:38–44. https://doi.org/10.1007/s10924-014-0681-8
Rahman A et al (2015) (2015) Polyhydroxybutyrate production using a wastewater microalgae-based media. Algal Res 8:95–98. https://doi.org/10.1016/j.algal.2015.01.009
Raho S et al (2020a) Production of the polyhydroxyalkanoate phbv from ricotta cheese exhausted whey by Haloferax mediterranei fermentation. Foods 9:1459. https://doi.org/10.3390/foods9101459
Raho S et al (2020b) Production of the Polyhydroxyalkanoate PHBV from Ricotta cheese exhausted whey by Haloferax mediterranei fermentation. Foods 9:1–21. https://doi.org/10.3390/foods9101459
Ramachandran H, Amirul AA (2013) Yellow-pigmented Cupriavidus sp., a novel bacterium capable of utilizing glycerine pitch for the sustainable production of P(3HB-co-4HB). J Chem Technol Biotechnol 88:1030–1038. https://doi.org/10.1002/jctb.3928
Rao A, Haque S, El-Enshasy HA, Singh V, Mishra BN (2019) RSM–GA based optimization of bacterial PHA production and in silico modulation of citrate synthase for enhancing PHA production. Biomol 9(872):1–16. https://doi.org/10.3390/biom9120872
Raza ZA, Riaz S, Banat IM (2018) Polyhydroxyalkanoates and chemical modification approaches for their functionalization. Biotechnol Progr 34:29–41. https://doi.org/10.1002/btpr.2565
Rebocho AT (2019) Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl Food Biotechnol 6:71–82
Roja K et al (2019) Extraction and characterization of polyhydroxyalkanoates from marine green algae and cyanobacteria. Biocatal Agric Biotechnol 22:101358. https://doi.org/10.1016/j.bcab.2019.101358
Sabapathy PC, Devaraj S, Parthiban A, Kathirvel P (2018) Bioprocess optimization of PHB homopolymer and copolymer P3(HB-co- HV) by Acinetobacter junii Bp25 utilizing rice mill effluent as sustainable substrate. Environ Technol 39:1441–1430. https://doi.org/10.1080/09593330.2017.1330902
Salgaonkar BB, Mani K, Bragança JM (2019) Sustainable bioconversion of cassava waste to poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense Strain E3. J Polym Environ 27:299–308. https://doi.org/10.1007/s10924-018-1346-9
Samantary S, Mallick N (2015) Impact of various stress conditions on poly-β-hydroxybutyrate (PHB) accumulation in Aulosira fertilissima CCC 444. Curr Opin Biotechnol 4:366–372. https://doi.org/10.2174/2211550104666150806000642
Saratale RG et al (2021) A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour Technol 325:124685. https://doi.org/10.1016/j.biortech.2021.124685
Schmidt M et al (2016) Poly(3-hydroxybutyrate) production by Cupriavidus necator supplemented with miniemulsified soybean oil. Braz J Chem Eng 33:13–20. https://doi.org/10.1016/j.biortech.2018.08.064
Sehgal R, Gupta R (2020) Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech 10:1–14
Shabina M, Afzal M, Hameed S (2015) Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem Lett Rev 08:56–77. https://doi.org/10.1080/17518253.2015.1109715
Shahid S, Razzaq S, Farooq R, Nazli ZH (2021) Polyhydroxyalkanoates: next generation natural biomolecules and a solution for the world’s future economy. Biol Macromol 166:297–321. https://doi.org/10.1016/j.ijbiomac.2020.10.187
Sharma V, Sehgal R, Gupta R (2021) Polyhydroxyalkanoate (PHA): properties and modifications. Polymer 212:123161. https://doi.org/10.1016/j.polymer.2020.123161
Singh AK, Mallick N (2017) Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol Lett 364:20. https://doi.org/10.1093/femsle/fnx189
Slater S et al (1998) Multiple beta-ketothiolases mediate poly(beta-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J Bacteriol 180:1979–1987. https://doi.org/10.1128/JB.180.8.1979-1987.1998
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 10(1503):1555. https://doi.org/10.1016/S0079-6700(00)00035-6
Tan HT et al (2020) Evaluation of BP-M-CPF4 polyhydroxyalkanoate (PHA) synthase on the production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from plant oil using Cupriavidus necator transformants. Int J Bio Macromol 159:250–257. https://doi.org/10.1016/j.ijbiomac.2020.05.064
Tao W, Lv L, Chen GQ (2017) Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb Cell Fact 16:1–10. https://doi.org/10.1186/s12934-017-0655-3
Thinagaran L, Sudesh K (2019) Evaluation of sludge palm oil as feedstock and development of efficient method for its utilization to produce Polyhydroxyalkanoate. Waste Biomass Valor 10:709–720. https://doi.org/10.1007/s12649-017-0078-8
Vigneswari S et al (2021) Recent advances in the biosynthesis of polyhydroxyalkanoates from lignocellulosic feedstocks. Life 11:1–25. https://doi.org/10.3390/life11080807
Wang Y, Yin J, Chen GQ (2014) Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol 30:59–65. https://doi.org/10.1016/j.copbio.2014.06.001
Watteau F, Dignac MF, Bouchard A, Revallier A, Hout S (2018) Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Front Sustain Food Syst 2:1–14. https://doi.org/10.3389/fsufs.2018.00081
Ye JW, Chen GQ (2021) Halomonas as a chassis. Essays Biochem 65:393–403. https://doi.org/10.1042/EBC20200159
Ye J et al (2018) scale-up of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production by halomonas bluephagenesis via cell growth adapted optimization process. Biotechnol J 13:1800074. https://doi.org/10.1002/biot.201800074
Zhang J et al (2020) Utilization of enzymatic cell disruption hydrolysate of Chlorella pyrenoidosa as potential carbon source in algae mixotrophic cultivation. Algal Res 45:101730. https://doi.org/10.1016/j.algal.2019.101730
Acknowledgements
The authors gratefully acknowledge the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Processes: 302143/2021-1; 302267/2021-2; 302284 /2021-4; 302170/2021-9) and Centro de Tecnologias Estratégicas do Nordeste (CETENE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Editorial responsibility: Maryam Shabani.
Rights and permissions
About this article
Cite this article
Alves, A.A., Siqueira, E.C., Barros, M.P.S. et al. Polyhydroxyalkanoates: a review of microbial production and technology application. Int. J. Environ. Sci. Technol. 20, 3409–3420 (2023). https://doi.org/10.1007/s13762-022-04213-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04213-9