Abstract
The production of sago starch from cassava at an industrial scale in Salem, Tamil Nadu, India results in discharge of starch/carbon-rich fibrous waste and effluents, contributing to a major environmental problem. The present study aimed at exploring the potential of an extremely halophilic archaeon, Halogeometricum borinquense strain E3 to utilize starch and cassava waste (CW) as carbon substrate and synthesis of polyhydroxyalkanoates (PHAs). The culture E3 was able to grow and utilize both starch and CW with maximum PHA concentration of 4.6 g L−1 and 1.52 g L−1, respectively. When grown in starch, the cells of the strain E3 appeared bright orange due to produced carotenoids, whereas, when grown in CW the culture cells appeared light brown due to masking of the pigment by the impurities from CW hydrolysate. The polymer obtained from starch and CW hydrolysate was characterized using UV–Visible spectrophotometry, differential scanning calorimetry, fourier transform infrared spectroscopy, nuclear magnetic resonance spectroscopy, and was reported to be a co-polymer of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] comprising of 13.11% and 19.65% 3HV units, respectively. The present investigation is supportive of our previous studies which indicated Hgm. borinquense strain E3 as an attractive candidate for production of co-polymer of P(3HB-co-3HV) when fed with commercial substrate such as glucose and agro-industrial waste such as sugarcane bagasse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cassava or tapioca (Manihot esculenta) is a tuber crop cultivated in tropical and subtropical countries world-wide and is known for thriving in low-nutrient, low rainfall, high temperature and in various soil types. Cassava is a high-starch producer with the dry roots containing starch content of 90%, making it a cheap source of starch for the low-income countries of Asia, Africa and South America [1]. Apart from being used as a staple food product, cassava is also used in the animal feed and other food and non-food applications such as in textile industry and adhesive industry [2]. The wide application of cassava along with its ability to grow in demanding conditions makes it as a favorite crop among the small-scale farmers.
The main objective of cassava processing is the release of the starch granules from the tubers and this is initiated by washing and peeling the outer skin and the rind of the cassava tubers. Following this, the tubers are subjected to rasping, a process during which the tubers are crushed and starch entrapped within the cellular constituents is released. Screening is carried out for the separation of the starch granules from the pulp obtained after rasping and is achieved by mixing the grated tubers with water resulting in slurry. This slurry is subjected to sieve of various pore sizes resulting in the separation of crude pulp and starch milk. The pulp is dried and used as fodder and the starch milk is allowed to standstill for the sedimentation of starch and insoluble pulp/impurities/suspended solid waste. The sedimented starch cake is (a) crumbled into small clumps, granulated, partially gelatinized and dried for 24–120 h, to obtain sago, or (b) dried and pulverized, to obtain starch. Majority of sago produced is for human consumption whereas the starch produced has a profound role in the industries [3]. Though the production of starch and sago appears lucrative especially for a small-scale industry, the waste that is being generated in the process are enormous and hazardous [4]. The runoff water during rasping and sedimentation along with unprocessed pulp pose a serious environmental issue. In the world, India is the 5th largest producer of cassava, with an annual production of 7.2 million tons (MT) in 305 thousand hectares of cultivation [5].
In India, southern state of Tamil Nadu alone has 109 thousand hectares of area under cassava cultivation and a production capacity of 4.2 MT which represents 60% of total production in the country. Though, cassava forms an important culinary item, almost entire cassava produced in the state is utilized for sago production. Salem district in Tamil Nadu state of India, has 650 units which constitutes 89% of country’s sago production. In Salem alone, 150 thousand farmers are involved in cassava cultivation which is 25 ton/hectare as compared to worldwide cultivation of 10 ton/hectare, thereby making Salem the supreme cassava production area in the world [6, 7]. Apart from the farmers, thousands of other people are involved directly or indirectly in sago production. The average cassava processed by these plants is 5 tons per day while generating 0.45 ton of processed pulp as a waste. Further on daily average water required for processing the pulp amounts to 150–200 × 103 m3 with entire water being pumped out of the cassava processing plants. The runoff water further contains hydrogen cyanide, a respiratory inhibitor apart from rich nutrients which acts as an excellent media for the microorganisms to generate further foul odor producing metabolites. The waste generated from these sago processing units has rendered about 1000 ha of agricultural land in the Salem region uncultivable apart from posing a health hazard for the residents located surrounding the sago processing plants [8]. This has threatened the very existence of the cassava processing units in the Salem district with tussles between the local populations and the processing units. Therefore, development of sustainable processes for the bioconversion of this crude cassava waste into value added products remains a challenge to be pursued.
Polyhydroxyalkanoates (PHAs) are a group of natural polyesters which are intracellularly accumulated by a range of microorganisms in presence of excess of carbon and a limited supply of a growth-essential nutrient. The major pollutants originating from sago starch industries are the fibrous material (cassava peels), wet solid waste (cassava bagasse) and the effluent from the setting tanks (liquid waste/cassava pulp), which are carbon-rich materials and therefore could be an attractive and economical source for PHA production. Poomipuk et al. isolated Cupriavidus sp. KKU38 a Gram-negative bacteria, from cassava starch wastewater and reported it to synthesize poly(3-hydroxybutyrate) which is a homopolymer of HB, by using cassava starch hydrolysate as substrate [9]. Another study by Silva et al., reported the potential of Pseudomonas oleovorans ATCC29347 to synthesize co-polymers of polyhydroxyalkanoate (PHAs), when cultivated on cassava extracts (sugary) with andiroba oil supplements [10]. Though the strain ATCC29347 was an attractive candidate for PHA production, conditions of high stress was reported to favor the intracellular depolymerization of the polymer.
Halophilic archaea are polyextremophilic organisms thriving in hypersaline regions, experiencing myriad of stresses such as UV radiation, fluctuating salinity, pH and temperatures. These organisms offer a huge number of potential application in various fields of microbial technology and are known to produce novel biomolecules [11]. Recent reports on halophilic archaea have proven their potential in bioconversion of highly polluting industrial waste to biodegradable plastic, i.e., PHAs. To the best of our knowledge, till date, Haloferax and Haloarcula are the only two genera among 48 genera of the family Halobacteriaceae in the domain Archaea [12], reported to synthesize substential amount of PHAs from industrial waste materials. Studies on bioconversion of waste/byproducts from various industries such as dairy (whey), ethanol (stillage and vinasse), biodiesel (petrochemical wastewater and crude glycerol phase), agriculture (cornstarch/ rice bran) to PHAs by haloarchaea have been documented [13,14,15,16,17,18]. We have recently reported the potential of Halogeometricum borinquense strain E3 to synthesize PHAs from sugarcane bagasse [19].
Though various alternate approaches for managing cassava bagasse such as generation of biogas, use as an animal fodder has been proposed, so far nothing has materialized. Hence, in the present study, a process for the conversion of this cassava waste to PHAs by halophilic archaeon, Hgm. borinquense strain E3 is proposed. Till date, and to the best of our knowledge, there has been no study on the bioconversion of cassava waste to PHAs by members of the class Halobacteria, domain Archaea. This approach may provide a value added product from a challanging agro-industrial waste, simultaneously managing the wastes generated. Employing halophilic archaea for PHA synthesis is advantageous because of the less cost involved in downstream processing of the polymer produced. The intracellular polymer synthesized was extracted and characterized using various analytical techniques.
Materials and Methods
Chemicals, Microorganism and Culture Conditions
All chemicals and solvents used were of analytical grade. The standard Poly[(R)-3-hydroxybutyric acid], natural origin (CAS Number 29435-48-1) and Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) natural origin, with 12 mol% PHV content (CAS Number 80181-31-3) were purchased from Sigma-Aldrich, India.
Halogeometricum borinquense strain E3 was isolated from the solar salterns of Marakkanam in Tamil Nadu, India. The culture was maintained on high salts containing nutrient-rich complex, Extremely Halophilic Medium (EHM). The starter culture was prepared by inoculating loopful of the culture from EHM agar plate into 25 mL EHM broth contained in 100 mL Erlenmeyer flask [20]. The seed culture was prepared by inoculating 2 mL of the starter culture into 100 mL of Norberg and Hofstein (NH) medium comprising of (g L−1) NaCl 200.0, MgSO4·6H2O 10.0, KCl 5.0, CaCl2·2H2O 0.2, Yeast Extract 1.0, and supplemented with starch 2 g L−1 as substrate, with pH adjusted to 7.0–7.2 using NaOH [21]. The starter and seed culture flasks were maintained on a rotary shaker at 37 °C, 110 rpm (Skylab Instruments, India).
Characterization of the Cassava Waste
Cassava waste (CW) was collected from sago industry discharge in Salem, Tamil Nadu, India. CW was dried for 5–7 days under sunlight for complete removal of moisture and with the help of blender, the CW clumps were pulverized. Physico-chemical characterization of the CW powder was done as follows: (i) the American Public Health Association (APHA) protocols were used to estimate Total solids (TS) and volatile solids (VS) [22]; (ii) CHNS Analyzer (Elementar, Germany) was used to determine the carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) contents; (iii) protocol from Raposo et al. was used to determine the chemical oxygen demand (COD) [23].
Cassava waste hydrolysate was obtained by subjecting CW powder to dilute acid hydrolysis using sulfuric acid (0.75% v/v) and neutralized to pH 7.0–7.4 using NaOH [19, 24]. Phenol sulphuric acid method was used to estimate the total carbohydrate content of the CW hydrolysate [25]. Total Kjeldahl nitrogen (TKN) was estimated as per the protocol by Labconco [26]. All the analysis was done in triplicate to determine mean and standard deviations.
Growth and Screening for PHA Production using Starch and Cassava Waste
Halogeometricum borinquense strain E3 was screened for PHA production by colony staining method using Nile Red dye. Colony staining was done by incorporating Nile Red dye (50 µL) [Nile red stock, 0.01% (w/v) in DMSO] in 100 mL minimal (NH) medium containing 0.5% (w/v) soluble starch and/or CW as substrates [27]. The culture was streaked and the plates were incubated at 37 °C for 3–10 days. PHA accumulation by strain E3 was confirmed by fluorescence (bright orange) after irradiation of the culture with ultraviolet (UV) light using gel documentation system (BIO-RAD Laboratories, USA).The growth of the halophilic archaeon in medium containing dye permitted the detection of PHA in viable culture during various time intervals of the growth.
PHA Production by Batch Culture
Batch culture studies for PHA production by strain E3 was done in NH medium containing commercial starch (soluble) and CW hydrolysate, separately. Two percent of the seed culture was inoculated in each of the two 500 mL NH medium containing 2% (w/v) soluble starch and 10% (v/v) CW hydrolysate, respectively and contained in 1000 mL Erlenmeyer flask. The volume of the medium was adjusted such that after adding 10% CW hydrolysate, the final volume remained 500 mL. The flasks were incubated at 37 °C, 110 rpm for 10 days on a rotary shaker (Skylab Instruments, India).
Determination of Cell Dry Mass (CDM)
The growth of the culture was measured by determining the biomass as CDM gravimetrically in units of g L−1. Briefly, 5 mL of the culture broth was centrifuged at 10,000 rpm for 15 min, the pellet of the biomass was washed with d/w (distilled water), recentrifuged at 12,000 rpm for 20 min, and dried until a constant weight at 60 °C using oven. Five mL of plain medium (without culture) was processed in similar way and subtracted from the biomass (CDM) to avoid medium error.
Determination of PHA
The intracellular PHA was extracted using sodium hypochlorite (NaOCl) and then quantified gravimetrically (in units of g L−1) [28]. Briefly, 20 mL of the culture broth was centrifuged at 10,000 rpm for 15 min, the pellet of biomass was washed with d/w (distilled water), and recentrifuged at 12,000 rpm for 20 min. The biomass pellet was suspended in 40 mL of NaOCl containing 4% active chlorine and incubated at 37 °C for 30 min on shaker. After incubation, the NaOCl-cell suspension was centrifuged at 12,000 rpm for 20 min, the supernatant was discarded. The pellet containing PHAs was washed with diethyl ether and dried using oven, until constant weight.
Extraction and Characterization of PHA
Strain E3 was grown in NH medium containing starch and CW as substrate. The cell biomass was harvested by centrifugation, dried (60 °C for 12 h) in an oven, and PHAs extracted using chloroform in a soxhlet extractor for 6 h at 60 °C as described by Sánchez et al. and Salgaonkar and Bragança [19, 29]. Using rotary evaporator (Rotavapor R-210, Switzerland) major part of chloroform was recovered and the concentrated polymer in chloroform was casted into clean glass Petri dish to obtain polymer film.
Polymer obtained using starch and CW as substrates was characterized using (i) UV–Visible spectrophotometry (UV-2450, Shimadzu, Japan) after converting the PHA to crotonic acid, on hydrolysis with concentrated sulphuric acid (H2SO4) [30], (ii) differential scanning calorimeter (DSC) analysis using model DSC-60 (Shimadzu, Japan) which determined the melting temperature (Tm) and degradation temperature (Td), (iii) Fourier transform infrared (FT-IR) spectroscopy using single bounce Attenuated Total Reflectance (ATR) accessory which determined the functional groups and (iv) proton nuclear magnetic resonance (1H-NMR) spectroscopy using a model Bruker Avance III, 400 MHz which determined the monomeric units of the polymer. The analysis was done as described by Salgaonkar and Bragança [20] and the spectrum obtained were compared with standard P(3HB) and P(3HB-co-3HV) which were analyzed in similar way.
Results and Discussion
Processing of Cassava and Analysis of Cassava Waste
Cassava/cassava tubers (Manihot esculenta) is a high-starch producer with the dry roots containing 90% starch content, thereby making cassava based industry as one of the major employment provider for a large proportion of population in the developing and under developed countries. However, generation of huge amount of tough-to-degrade wastes is threatening the existence of the industry while all other alternate approaches for waste management so far has proved difficult to implement for the small-scale cassava industries [6, 7].
The process of extracting starch and sago from cassava tubers is represented in Fig. 1. The entire process of extraction of starch from the cassava tubers requires tons of water and generated huge amount of waste in form of fibrous material (cassava peels), wet solid waste (cassava bagasse) and liquid waste/ cassava pulp [3]. This waste from sago processing units is dumped on landfills and has created environmental havoc. The cassava waste used in the present study was in the form of brown, semi sticky with bad stinky/ foul odor, it was transformed into granular form upon drying under sunlight. It had total solids (TS), volatile solids (VS) and chemical oxygen demand (COD) of 92.3 ± 0.21%, 78.8 ± 0.14% and 1.33 ± 0.32 g/Kg, respectively. The CHNS content was found to be carbon (C) = 35.775 ± 0.4%, hydrogen (H) = 6.12 ± 0.08%, nitrogen (N) = 0.755 ± 0.007%, and sulfur (S) = 0.158 ± 0.008%. The dilute sulfuric acid hydrolysis of CW yielded total carbohydrates and total Kjeldahl nitrogen (TKN) of 24.78 ± 1.7 g L−1 and 0.56 g L−1 with the C/N ratio (%) of 44.25.
Cassava waste (CW) is a high organic content waste rich in polysaccharide starch. Starch comprises of amylose and amylopectin which are made up of homopolymer of glucose units. Therefore, starch hydrolysis results in readily available reducing sugar, glucose. Woiciechowski et al., investigated acid and enzymatic hydrolysis methods for recovering, reducing sugars from cassava waste/bagasse and observed that the acid hydrolysis was much economical and efficient as compared to enzymatic hydrolysis which was time extensive and costly [24].
Screening for PHA Production using Starch and Cassava Waste
Halogeometricum borinquense strain E3 grew well on NH plates supplemented with starch and CW as substrate. Upon exposure of the culture to UV light, the strain E3 exhibited bright orange fluorescence due to the uptake and binding of Nile Red dye present in the medium to PHA granules inside the cells. The screening confirmed PHA synthesis by the culture Hgm. borinquense strain E3 using starch and CW as substrates. The growth and fluorescence intensity exhibited by the culture E3 was equivalent when grown in starch as well as CW. Our previous studies have proved, halophilic archaeon Hgm. borinquense strain E3 as potential accumulator of PHA in a minimal medium supplemented with glucose as well as sugarcane bagasse [19].
Nile red stain for polyhydroxyalkanoates (PHAs) is a sensitive and rapid method effectively distinguishes non-PHA producing microbial biomass from PHA-positive cultures [27]. Though the method was extensively employed for swift identification of PHA-positive bacteria, Legat et al. proved the compatibility of the method for halophilic archaeal strains grown in media containing high salt concentration [31]. This was further confirmed by our previous studies which supported the efficiency of Nile Red stability and uptake by halophilic archaea grown in salt saturated media supplemented with commercial substrate as well as agro-industrial waste such as glucose and sugarcane bagasse [19]. PHA-positive bacteria are reported from almost all possible ecological niches. Till date there is a constant search for novel PHA-positive wild type organisms. Among halophilic archaea, to date, Haloferax mediterranei is the highly explored representative and is considered as the best PHA accumulator. However, for the past decade, research has focused on exploring the potential of new genera of halophilic archaea to synthesize PHA [32] with Halogeometricum being one of the potential genus.
PHA Production by Strain E3 using Starch and CW
Raw materials for PHA biosynthesis play a crucial role in determining the overall cost of the final product. Cost of commonly used substrates, such as pure sugars, fatty acids, or noble oils, is known to account for about 50% of the total production cost of PHAs [32]. Researchers are constantly working on exploring inexpensive substrates to reduce the production cost and thereby making PHA more economical. Keeping this in mind, a variety of potential carbon-rich substrates, such as agro-industrial byproducts and waste effluents, has been investigated. Starch could be used as an ideal substrate/carbon source due to its abundance in nature, origin from renewable resources and low cost [33]. To the best of authors knowledge, till date, there are only four reports on PHA production by halophilic archaea using starch as substrates. However, there are no reports of synthesis of PHA using cassava waste by halophilic archaea. In the present study, we investigated and compared the potential of halophilic archaeon, Hgm. borinquense strain E3 to utilize starch and CW as carbon-rich substrates for the synthesis of PHAs. It was observed that the culture E3 was able to grow and utilize both starch and CW and reached maximum PHA concentration of 4.6 g L−1 and 1.52 g L−1, with PHA of 74.19% and 44.7% (wt/wt) of CDM, respectively (Table 1).
Lillo and Rodriguez-Valera, reported maximum concentration of 6 g L−1 of poly(3-hydroxybutyrate) with 60% (wt/wt) P(3HB) of the CDM, by Hfx. mediterranei ATCC 33500 by utilizing 2 g L−1 starch as carbon source [33]. A recent study by Danis and coworkers reported, corn starch to positively influence PHA production in various halophilic archaeal isolates, with 53.14% (wt/wt) PHA of the CDM was accumulated by Natrineam pallidum 1KYS1 and was reported as the highest PHA-producing strain [34]. Maximum PHA concentration of 1.74 g L−1 with 24.88% (wt/wt) PHA of the CDM was obtained for wild type halophilic archaeal isolate, Hfx. mediterranei when grown in minimal medium (MST) supplemented with 1% starch as substrate [35]. Interestingly, Huang et al. employed substrate from renewable resources such as starch and extruded cornstarch (ECS) which are economically advantageous at industrial level and yielded Maximum PHA of 24.2 g L−1 by Hfx. mediterranei ATCC 33500 with PHA of 38.7% (wt/wt) of the CDM [18] (Table 1).
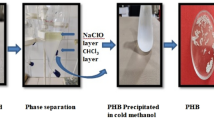
The polymer from the biomass of Hgm. borinquense strain E3 cultivated in NH medium containing starch and CW as substrates was isolated using Soxhlet extraction. The polymer film obtained from starch as substrate appeared orange due to the co-extracted carotenoids and was treated with acetone for 10 min to remove the carotenoids from the polymer. This phenomenon is supportive of our previous studies which indicated production of carotenoids by strain E3 when fed with glucose and sugarcane bagasse as substrates [19]. However, in presence of CW the culture cells appeared light brown which might be due to masking of the carotenoid pigment by the impurities from CW hydrolysate and therefore, the polymer film obtained appeared light brown (Fig. 2).
Characterization of PHA
The polymers were characterized using a UV–Visible spectrophotometry, differential scanning calorimetry (DSC), fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy, analysis.
Spectrophotometric Analysis
The UV–Visible spectra of the polymers hydrolyzed by concentrated H2SO4 gave characteristic absorption maxima at 235 nm corresponding to crotonic acid [30]. The crotonic acid spectrum resembled well with the standard P(3HB)/ P(3HB-co-3HV) (Sigma-Aldrich, USA) and also with the copolymer of P(3HB-co-3HV) synthesized by halophilic archaeon Natrinema sp. 1KYS1, using corn starch as a substrate [34].
Differential Scanning Calorimetry (DSC) Analysis
Figure 3 represents comparison of the thermograms derived from DSC analysis, for the PHAs obtained using starch, CW, standard P(3HB) and P(3HB-co-3HV). The PHAs obtained from starch and CW exhibited two melting endotherms, first one at Tm1 = 149.8/140 °C and second one at Tm2 = 160/151.1 °C, respectively and matched well with results obtained for the standard copolymer of P(3HB-co-3HV)with Tm1 = 146.6 °C and Tm2 = 158.4 °C (Fig. 3C). The standard homopolymer of P(3HB) exhibited only one melting endotherm at Tm = 169.3 °C (Fig. 3D). The degradation temperature (Td) endotherms for the starch, CW, standard P(3HB) and P(3HB-co-3HV) were at 270.5 °C, 272 °C, 273.9 °C and 280 °C, respectively (Table 1). Halophilic archaea are known to synthesize PHAs with myriad monomer units such as hydroxybutyrate (HB) and hydroxyvalerate (HV), and PHAs with different monomeric units exhibit multiple melting endotherms [17]. Chen et al. reported the copolymer P(3HB-co-3HV) synthesized by H. mediterranei ATCC 33500 from extruded cornstarch, to exhibit two melting endotherms at Tm1 = 129.1 °C and Tm2 = 144.0 °C [36].
Fourier Transform Infrared (FT-IR) Analysis
The functional groups of the polymer obtained from starch, CW hydrolysate and standard P(3HB) were determined using FTIR analysis and IR spectra of polymer is represented in Fig. 4. All the polymers exhibited one intense absorption band at 1724 cm−1, distinctive of ester carbonyl group (C=O) stretching [37]. Another band between region 1281–1284 cm−1 and 3100–2800 cm−1 represents C–O–C and C–H stretching, respectively. Polymer obtained from starch exhibited one prominent band at 1628 cm−1 which could be of the cellular protein amide (–CO–N) from the microbial biomass [38]. Other major bands observed could be as a result of the hydroxyl and carbonyl group interactions of the PHA, which results in a shift of the stretching. The IR spectra of the polymers obtained from starch and CW matched well with the standard P(3HB).
Nuclear Magnetic Resonance (1H-NMR) Analysis
The proton NMR scans of the polymers obtained from strain E3 using starch and CW are represented in Fig. 5. The monomeric composition of the PHA was determined by 1H NMR spectrum, and the signal intensity ratios were used to quantize the monomeric units in the PHA. Signals between 0.85–0.91 ppm and 1.26–1.28 ppm are characteristic of methyl (CH3) from 3-hydroxyvalerate (3HV) and 3-hydroxybutyrate (3HB) units, respectively. 1H NMR spectrum of homopolymer of HB [P(3HB)] exhibits single major peak of CH3 group at 1.25 ppm. The 1H-NMR spectrum signals matched well with the results obtained for those of co-polymer of [P(3HB-co-3HV)] synthesized by Hfx. mediterranei strain ATCC 33500 by utilization of cornstarch [36]. Hence, the PHAs obtained using starch and CW hydrolysate was confirmed to be a co-polymer of [P(3HB-co-3HV)]. The copolymer composition, i.e., monomeric units (mol%) of HV in the polymer of P(3HB-co-3HV) was estimated as the ratio of peak areas, due to the CH3 group resonance of HV and the sum of the CH3 group resonances of HB and HV, as described by Salgaonkar and Bragança [20] and is represented in Table 2.
Studies on members of halophilic archaea, such as Hfx. mediterranei, Natrinema sp. 1KYS1, Har. hispanica, have proved their potential to synthesize copolymer of P(HB-co-HV), without addition of propionic and/valeric acid as the substrates precursors. Lu et al. reported accumulation of P(3HB-co-3HV) by Hfx. mediterranei comprising of 13.37 mol% 3HV fraction when grown in nutrient-limited MST medium with starch substrate [35]. In the present study, the polymer produced by Hgm. borinquense strain E3 using soluble starch and CW, comprised of 13.11% and 19.65% 3HV units, respectively, and matched well with the results by Lu et al. [35]. This is also in correlation with our previous studies on production of P(3HB-co-3HV) by Hgm. borinquense strain E3, with 21.47% and 13.29% of 3HV units when fed with glucose and sugarcane bagasse, respectively [19, 20]. The utilization of crude and myriad type of agro-industrial waste by Hgm. borinquense strain E3 makes it an attractive candidate with promising potential for PHA production.
Conclusion
The sago industry at Salem, a district in Tamil Nadu, India constitutes 89% of entire country’s production. As any other industry, the production of sago starch from cassava results in waste products such as fibrous material and the effluent from the setting tanks, causing major environmental hazard. For this reason, treatment of this waste has become a major issue and needs immediate attention. The present study aimed at exploring the potential of the extremely halophilic archaeon, Hgm. borinquense strain E3 to utilize cassava waste as carbon substrate in comparison with starch. The culture E3 was able to grow and utilize both starch and CW with maximum PHA concentration of 4.6 g L−1 and 1.52 g L−1, with PHA of 74.19% and 44.7% (wt/wt) of CDM, respectively. When grown in CW the culture appeared brown in colour due to masking of the bacterioruberin pigment by the impurities from CW hydrolysate while the PHA polymer appeared light brown. The polymer obtained from both starch and CW was characterized as a co-polymer of P(3HB-co-3HV) with 13.11% and 19.65% 3HV units, respectively. The present investigation is supportive of our previous studies which indicated Hgm. borinquense strain E3 as an attractive candidates for production of co-polymer of P(3HB-co-3HV) when fed with commercial substrate such as glucose and agro-industrial waste such as sugarcane bagasse. Hgm. borinquense strain E3 has the potential to utilize CW for the production of PHAs thus helping with both recalcitrant waste management and reducing the substrate expense. Our present focus is to increase the biomass of strain E3 and the corresponding increase in polymer accumulation. For achieving this, we are optimizing the PHA production media, cultivation parameters, inoculum size and certain steps to detoxicify the waste hydrolysate.
Abbreviations
- PHA:
-
Polyhydroxyalkanoates
- P(3HB):
-
Poly-3-hydroxybutyrate
- P(3HB-co-3HV):
-
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
- CW:
-
Cassava waste
- Hgm:
-
Halogeometricum
- Har:
-
Haloarcula
- Nnm:
-
Natrinema
- Hfx:
-
Haloferax
- TS:
-
Total solids
- VS:
-
Volatile solids
- COD:
-
Chemical oxygen demand
- CDM:
-
Cell dry mass
- rpm:
-
Revolutions per minute
References
Shittu TA, Alimi BA, Wahab B, Sanni LO, Abass AB (2016) Tropical roots and tubers: production, processing and technology, Wiley, Hoboken, pp 415–450
Edhirej A, Sapuan SM, Jawaid M, Zahari NI (2017) Polym Compos 38:555–570
Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LP, Mohan R (2000) Bioresour Technol 74:81–87
Sánchez AS, Silva YL, Kalid RA, Cohim E, Torres EA (2017) Renew Sustain Energ Rev 73:1265–1275
Rajakumari D, Gomath V (2017) Ph.D. Dissertation, Bharathidasan University, Tamil Nadu, India. https://scholar.google.com/scholar?um=1&ie=UTF-8&lr&q=related:orLqWf40tskBFM:scholar.google.com/
Thanuskodi S, Kalyani KS (2010) Lib Phil Pract e-journal:370. https://scholar.google.com/scholar?q=related:zLhNFQq7dDoJ:scholar.google.com/&scioq=&hl=en&as_sdt=0,5
Srinivas T (2007) Starch Stärke 59:477–481
Rajendran R, Soora M, Kandasamy S, Dananjeyan B, Krishnamurthy K, Benckiser G (2011) Int J Sustain Eng 4:348–358
Poomipuk N, Reungsang A, Plangklang P (2014) Int J Biol Macromol 65:51–64
Silva DAD, Antonio RV, Rossi JM, Pena RDS (2014) Food Sci Technol 34:738–745
Ma Y, Galinski EA, Grant WD, Oren A, Ventosa A (2010) Appl Environ Microbiol 76:6971–6981
Chen S, Xu Y, Liu HC, Yang AN, Ke LX (2017) Int J Syst Evol Microbiol 67:818–823
Koller M, Hesse P, Fasl H, Stelzer F, Braunegg G (2017) Appl Food Biotechnol 4:65–78
Bhattacharyya A, Saha J, Haldar S, Bhowmic A, Mukhopadhyay UK, Mukherjee J (2014) Extremophiles 18:463–470
Pramanik A, Mitra A, Arumugam M, Bhattacharyya A, Sadhukhan S, Ray A, Haldar S, Mukhopadhyay UK, Mukherjee J (2012) Folia Microbiol 57:71–79
Taran M (2011) J Hazard Mater 188:26–28
Hermann-Krauss C, Koller M, Muhr A, Fasl H, Stelzer F, Braunegg G (2013) Archaea 2013:129268
Huang TY, Duan KJ, Huang SY, Chen CW (2006) J Ind Microbiol Biotechnol 33:701–706
Salgaonkar BB, Bragança JM (2017) Bioengineering 4:50
Salgaonkar BB, Bragança JM (2015) Int J Biol Macromol 78:339–346
Norberg P, Hofstein BV (1969) J Gen Microbiol 55:251–256
American Public Health Association (1981) American Water Works Association. Standard methods for the examination of water and wastewater: selected analytical methods approved and cited by the United States Environmental Protection Agency, 20th edn. American Public Health Association, Washington, DC
Raposo F, De la Rubia MA, Borja R, Alaiz M (2008) Talanta 76:448–453
Woiciechowski AL, Nitsche S, Pandey A, Socco CR (2002) Braz Arch Biol Technol 45:393–400
Dubois M, Gilles KA, Hamilton JK, Rebers PAT, Smith F (1956) Anal Chem 28:350–356
Labconco CA (1998) Guide to kjeldahl nitrogen determination methods and apparatus. Labconco Corporation, Houston, TX
Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbüchel A (1999) Arch Microbiol 171:73–80
Salgaonkar BB, Mani K, Braganca JM (2013) J Appl Microbiol 114:1347–1356
Sánchez RJ, Schripsema J, da Silva LF, Taciro MK, Pradella JG, Gomez JGC (2003) Eur Polym J 39:1385–1394
Law JH, Slepecky RA (1961) J Bacterial 82:33–36
Legat A, Gruber C, Zangger K, Wanner G, Stan-Lotter H (2010) Appl Microbiol Biotechnol 87:1119–1127
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G (2017) New Biotechnol 37:24–38
Lillo JG, Rodriguez-Valera F (1990) Appl Environ Microbiol 56:2517–2521
Danis O, Ogan A, Tatlican P, Attar A, Cakmakci E, Mertoglu B, Birbir M (2015) Extremophiles 19:515–524
Lu Q, Han J, Zhou L, Zhou J, Xiang H (2008) J Bacteriol 190:4173–4180
Chen CW, Don TM, Yen HF (2006) Process Biochem 41:2289–2296
Hong K, Sun S, Tian W, Chen GQ, Huang W (1999) Appl Microbiol Biotechnol 51:523–526
Gumel AM, Annuar MSM, Heidelberg T (2012) PLoS ONE 7:e45214
Acknowledgements
B. B. Salgaonkar thank Council of Scientific and Industrial Research (CSIR), India for Research Associateship (RA) (09/919(0030)/2016-EMR-I). Authors acknowledge the Sophisticated Instrumentation Facility (SIF), Chemistry Division, VIT University, Vellore for FT-IR and 1H NMR analysis. Authors are grateful to Prof. Narendra Nath Ghosh, Dept. of Chemistry, BITS Pilani Goa Campus, for DSC analysis.
Author information
Authors and Affiliations
Contributions
BBS, KM and JMB conceived the idea, designed the experiments and analyzed the data. BBS performed the experiments and drafted the manuscript, which was reviewed and edited by KM and JMB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Salgaonkar, B.B., Mani, K. & Bragança, J.M. Sustainable Bioconversion of Cassava Waste to Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense Strain E3. J Polym Environ 27, 299–308 (2019). https://doi.org/10.1007/s10924-018-1346-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1346-9