Abstract
Coconut processing industries generate a huge amount of wastewater, mainly coconut water from mature coconuts and wash water. Due to the high concentration of organic compounds in waste coconut water, it produces the most pollution even in small quantities compared to the overall effluent generated. Many small/medium-scale processing units discharge waste coconut water directly into the soil or drain it without treatment, polluting soil, groundwater, and surface water. Microbial fuel cells (MFCs) are bioelectrochemical reactors that generate electricity by biodegradation of organic compounds. The present study examines the ability of a dual-chambered MFC, to reduce organic contamination in wasted coconut water. Given the importance for achieving COD removal and bioenergy output, the various influential factors such as KMnO4 concentration as catholyte, catholyte pH, electrode material, and anode configurations were studied. At the best KMnO4 concentration of 2000 mg/L at pH 5, MFC produced a COD removal efficiency of 51.85%, with a maximum power density of 5.5 W/m3 using aluminium electrodes within 102 h of detention period. When the study proceeded with spatially distributed carbon cloth electrodes in anolyte, COD removal efficiency had improved up to 62.72% with a maximum power density of 6.5 W/m3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of the global population has generated stress on water and energy resources that are vital for the development of society as well as for human survival. A clean environment and renewable energy are the essential factors in sustainable development. Unfortunately, most freshwater resources face dreadful pollution from domestic, agricultural, and industrial sources (Ballesteros et al. 2016; Li et al. 2020).
Agriculture is the backbone of the Indian economy, employing over 70% of the rural population and 10% of the urban population (Archana 2019). Coconut cultivation and related sectors contribute to tens of millions of employment for households in Asia and around the world (Prades et al. 2016). According to the Coconut Development Board (CDB), established under the Government of India's Ministry of Agriculture and Farmers Welfare, coconut cultivation in India has spread over 2096.72 thousand hectors, producing a total of 23,798.23 million nuts according to 2017–2018 statistics, being the world's largest coconut producer. The various value-added products of coconut fruit are copra, coconut oil, desiccated coconut, coconut milk, coconut milk powder, coconut cream, and virgin coconut oil, made from mature coconut. Nearly all of Asia's major coconut-growing countries consider kernel and copra products to be their main economic product, though tender coconut water is the principal economic product in Brazil (Perera et al. 2015).
According to the report of ‘Price Policy for Copra: 2019 Season’, by the Ministry of Agriculture and Farmers Welfare, Government of India, only 16% of the total nut production in India is tender coconut, and the remaining 84% of the nuts are utilized in the mature state for domestic use (31%), production of copra (39%), and other value-added products (14%). A large amount of coconut water is wasted (estimated at 2.4 billion litres per year) during copra processing alone, with very high COD ranging from 45,000 to 50,000 mg/L. At the tender stage (7–9 months), coconut water is at pH 4.5–5 in its sweetest form with maximum total soluble solids. But sugar and phenolic content in matured coconut water arise very low with a higher concentration of amino acids, proteins, vitamins, and minerals such as K, Na, and Ca, which decreases the taste and acceptability of mature coconut water in the commercial market. Since the pH of coconut water rises to 5.7 on maturity, its shelf life would also reduce after extraction from the shell (Tan et al. 2014). The major commercial product from matured coconut water is natural vinegar, which is receiving less preference from manufacturers due to the difficulty in availability of clean substrates and long fermentation time (6–8 weeks). Besides, synthetic vinegar is cheaper in the local market than natural vinegar (Othaman et al. 2014).
The traditional wastewater treatment system for the coconut industry involves the UASB reactor or anaerobic ponds/digesters supplemented by facultative ponds or mechanically aerated ponds, activated sludge process, or rotating biological contactors (Industrial Pollution Control Guidelines, Sri Lanka—No.3 1992). The main drawbacks of conventional processes are high energy requirements during aerobic biological treatment and poor nutrient removal in the case of anaerobic biological treatment methods (Suman et al. 2017). Chanakya et al. (2015) studied the anaerobic degradability of wastewater from the desiccated coconut industry and reported that anaerobic bacteria took 15 days to adapt and that biogas generation occurred only with diluted coconut water. The author also stated that COD remains above 5000 mg/L even after 30 days of detention in a conventional anaerobic digester due to the presence of inhibitors such as VFA and high lipid concentration. The author suggested primary treatments such as filtration, adsorption, and neutralization to extract suspended solids and lipids and also to balance the pH to enhance the biodegradability of wastewater. Soletti et al. (2005) studied dissolved air flotation using Fe3SO4 as a flocculent agent, for the treatment of synthetic coconut industry effluent of 2756 mg/L COD and the recorded COD removal efficiency of 85%. Research was conducted by Gomes et al. (2014) on the coconut industry effluent using synthetic wastewater (0.6% coconut milk mixed with distilled water based on volume) by separate and combined Fenton and electrochemical treatments. The article reported that electrochemical oxidation followed by Fenton reaction reduces COD by 95%, an equivalent efficiency by the conventional treatment method of biological treatment (duration 10 days) together with physicochemical treatments.
The major quantity of wastewater produced in the coconut industry is from cleaning water but will be mixed with coconut water that is rich in organic compounds, and generally thrown into the atmosphere without much treatment, especially from small-scale copra industries (Soletti et al. 2005). Therefore, if wasted coconut water can be isolated, the strength of a large quantity of effluent can be significantly reduced. The small quantity of coconut water that needs intense treatment can be treated effectively to reduce pollution problems.
Worldwide, numerous research has been underway to minimize water pollution by inventing technologies to purify wastewater into recyclable form, but most of them are energy-intensive. It is estimated that only wastewater treatment uses 1–3% of the total energy production (Capodaglio and Olsson 2020; Mccarty et al. 2011). Conventional treatment systems demand alternative treatment technology that is energy-efficient, making the process/operation sustainable. In the latest past, more focus is being paid to anaerobic technologies for handling wastewaters. In the present scenario, viable renewable energy sources with low carbon dioxide emissions are gaining focus due to global energy scarcity and environmental issues. Microbial fuel cell technology uses biodegradable organic matter as fuel for electricity generation (Pandey et al. 2016; Teli et al. 2016). MFCs consider wastewater as a resource for generating electricity from biodegradable organic pollutants with lower carbon emissions compared to conventional wastewater treatment systems, which use energy and produce excess sludge from wastewater for disposal (Sreedharan and Pawels 2016). Wastewater treatment and generation of electricity are taking place concurrently in an MFC, and thus, the cost of treatment is minimized.
A microbial fuel cell is a microbial system that creates electrical potentials with the aid of microorganisms during the oxidation of biodegradable organic compounds. A typical MFC consists of anode and cathode, separated by a proton/cation exchange membrane. In an anaerobic environment, microbes oxidize the organic compounds and create protons and electrons inside the anode chamber. Electroactive microorganisms transfer electrons to the anode, which are then transferred through the external circuit to the cathode. The protons produced are transferred into the cathode chamber through the membrane. Because of its high oxidation capacity, oxygen serves as an electron acceptor in the cathode chamber. Thus, oxygen combines with electrons and protons, forming H2O as a clean by-product (Palanisamy et al. 2019; Pandey et al. 2016). Bioelectricity is created by the passage of electrons through the external circuit.
Researches on MFC has typically focused on two directions: first focus is on improving MFC efficiency to extract maximum bioelectricity based on various operating and physical factors such as substrate strength, feeding mechanism, retention time, microorganism concentration and type, pH, temperature, reactor configuration, electrode material, the surface area of the electrodes, membrane type, and external resistance. Most of these studies are conducted on synthetic samples to maintain uniformity of the samples. The second research focus is to verify the suitability of MFC to treat wastewater from various sources, such as domestic, industrial, agricultural, and related sources, and to define the operational factors affecting treatment efficiency and the resulting bioelectricity production (Palanisamy et al. 2019; Pandey et al. 2016; Akman et al. 2014; Solanki et al. 2013). MFC systems using wastewater are investigated worldwide, addressing waste management problems, increasing energy demand, and limited fossil fuel resources (Palanisamy et al. 2019; Pandey et al. 2016). Table 1 shows some of the latest researches on MFC to treat different types of wastewater with bioenergy production.
MFCs have been shown to remove azo dyes (Solanki et al. 2013), chloramphenicol, an antibiotic for the treatment of bacterial infections (Zhang et al. 2017), fipronil, a broad-spectrum insecticide (Zhang et al. 2019), and mesotrione, a selective herbicide, used in maize (Zhao and Zhang, 2021). Based on the study by Das and Mishra (2019), complete biodegradation of Remazol navy blue is possible by MFC at moderate concentrations ranging from 25 to 100 mg/L. According to the literature, COD removal efficiency and bioenergy generation depend on MFC configuration, wastewater COD, catholyte used, microbial culture, detention period, and the pre/post-treatment systems associated with MFC.
The present study focuses on the performance of dual-chambered MFC using wasted coconut water from coconut industries under various operating conditions. Due to significant expense, system design, scaling up, and energy recovery problems, practical implementation of MFC has not been realized (Flimban et al. 2019). Hence, we need to re-examine the limitations and viability of this technology to recognize it as a safe treatment of wastewater and also to find out whether the envisaged benefits of this technology can potentially be accomplished in the treatment of coconut water. The major factors influencing the performance of MFC are type substrate, microbial culture, type of electron acceptor in the anode, ionic strength of the mediums, pH, temperature, material, construction of electrodes, and membrane (Jayashree et al. 2019; Shanmuganathan et al. 2018). Yeast is considered as an ideal electroactive microorganism or biocatalyst for microbial fuel cell applications as most strains are non-pathogens, can metabolize wide range of substrates, are robust, and are easily handled. It was reported that carbon paper anode was effective with yeast as biocatalyst (Sayed and Abdelkareem 2017). Jayashree et al. (2019) reported potassium permanganate as a better catholyte compared to dissolved oxygen and potassium ferricyanide. As per You et al. (2010), an increase in the concentration of the KMnO4 solution improves bioenergy from MFC but considered only very small concentrations up to 200 mg/L corresponding to the low strength of anolyte as 600 mg COD/L. KMnO4 has oxidizing power in acidic, basic, and neutral pH, but it was reported that the oxidation potential of KMnO4 solution improves with lowering of pH (Nandi 2015; Osunlaja et al. 2012). At the same time, the difference in pH of anode and cathode chambers influences the diffusion of the proton through the membrane (Reddy et al. 2010).
The effects of concentration of KMnO4 as an electron acceptor in the cathode chamber, best pH of catholyte (in the acidic range), electrode materials, and configuration of the anode on efficiency to treat coconut water using MFC and corresponding bioenergy production were evaluated in this study. This study was conducted as a part of PhD at Cochin University of Science and Technology, Kerala, India, during 2017–2019.
Materials and methods
Materials
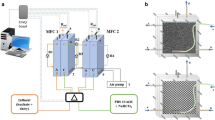
A two-chambered model of MFC was designed in acrylic. Both cathode and anode chambers have a working volume of 125 mL each. The cation exchange membrane (CEM: CMI-7000 Membranes International Inc., NJ, USA) physically separated the chambers through a hole of 30 mm in diameter. The chambers were coupled using nuts and bolts. Rubber gaskets were used as spacers to prevent leakage and sealed with silicone sealant. Figure 1a, b shows the arrangement of chambers separated by CEM and the experimental setup used for the investigation, respectively.
For each stage of the investigation, freshly collected clear coconut water was used. The collected sample was filtered into Erlenmeyer flask via the ordinary filter paper to remove the husk and kernel particles. Table 2 presents the characteristic properties of raw matured coconut water samples taken for investigation. Aluminium and conductive carbon cloth were used as electrode materials in different phases of the experiment, connected externally by means of light gauge copper wire and an LED bulb as external resistor. Potassium permanganate (KMnO4) was used in the cathode chamber as electron acceptor or chemical oxidizing agent, being the safest chemical (even used as a disinfectant for water), compared to many other toxic chemical oxidizing agents.
Methodology
The entire set of experiments were performed using freshly collected clear sample of coconut water, seeded Baker’s yeast, to accelerate the fermentation process. The seedling was prepared by adding 1 g of yeast into 150 mL of fresh coconut water. The seedling thus prepared was kept at ambient temperature in anoxic condition for 12 h and was then added to fresh coconut water to make a volume of 1 L, which was then used in the anode chambers. The entire study maintained the anoxic condition in the anode chamber under ambient temperature. Open circuit voltage (V) and current (mA) measurements were taken hourly over a period of 7 h daily using a digital multimeter manually (during laboratory hours from 9.30 a.m. to 4.30 p.m.). Based on the observations, power development and power density (normalized to the volume of anolyte) were calculated based on Eqs. 1 and 2, respectively.
where power was calculated in mW and power density in mW/m3. Experimental studies were performed in four phases using batch reactors of 102 h detention period. The changes in sample COD values were calculated after the detention period to indicate the efficiency of the treatment by MFC. The first phase of investigation was on the performance of KMnO4 as the electron acceptor/catholyte at various concentrations. In MFC modules, the various concentrations of KMnO4 solutions 0 mg/L, 500 mg/L, 1000 mg/L, 2000 mg/L, 3000 mg/L, and 3500 mg/L were compared using 50 mm × 50 mm aluminium sheets of 0.5 mm thickness as electrodes. The efficiency of MFC was represented by estimating the COD removal efficiency (standard methods of APHA, 1992) and power density (mW/m3) from cells. MFC’s performance was analysed at different pH conditions of KMnO4 solution in the second phase of the investigation. KMnO4 solutions were loaded to MFC at pH 3, 4, 5, and 6 using conjugate acid–base buffer (made of 0.1 M citric acid and 0.2 M sodium hydrogen orthophosphate) as shown in Table 3. The results were compared with the non-buffered condition of phase 1.
The first two phases of the evaluation were conducted using an aluminium electrode, which is one of the cheaply available metals. The third evaluation phase was a comparison study for COD removal between conductive carbon cloth and aluminium as electrode material. Electrodes pairs of same material having size 50 mm × 50 mm were used as both cathode and anode. For the cathode chamber of MFCs, the best catholyte conditions from the first two phases were utilized. The fourth phase of the assessment was the effect of various spatial configurations of carbon cloth in the anode chamber having a surface area (50 × 50 mm2) on MFC performance. As the first configuration, a single electrode of 50 mm × 50 mm was used (Conf. 1), two similar triangular pieces of carbon cloth were used as the second configuration by cutting a piece of 50 mm × 50 mm diagonally (Conf. 2), three pieces of size 16.7 mm × 50 mm were the third configuration (Conf. 3) , and four pieces of 25 mm × 25 mm were the fourth configuration (Conf. 4).
Results and discussion
Effect of KMnO4 concentration on performance of MFC
Catholyte plays a vital role in the treatment efficiency and bioenergy production of MFC. Figure 2 represents the effect of KMnO4 concentration on the efficiency of COD removal compared to the control sample. The control sample was a portion of the seeded anolyte, kept in a bottle at ambient temperature in anoxic condition. The efficiency of COD removal at the best KMnO4 concentration of 2000 mg/L was 36.68% compared to the control sample having COD removal of 9.42%. Even for KMnO4-free catholyte, natural aeration and dissolved oxygen present in water have effectively improved H+ ion transfer to enhance biodegradability in the anode chamber with a 14.70% reduction in COD.
Figure 3a shows the power density generated from MFC, at different concentrations of KMnO4 solution in the cathodic chamber. The power density was calculated based on the recorded voltage and current from the cells. The low power density in the absence of oxidizing agent indicates the importance of KMnO4 in completing the circuit of electron transfer. The average power density generated from the cell for the detention period as well as the maximum daily average power density shows a similar trend with respect to variation in KMnO4 concentration. The maximum value of power density generation was corresponding to the MFC having the KMnO4 concentration of 2000 mg/L. Figure 3b shows the detailed representation of daily average power density generated from MFC at the KMnO4 concentration of 2000 mg/L. A maximum daily average power density of 2257 mW/m3 was obtained on day 4. The potential from the cell was considerably reduced on day 5 indicated by the lower power density with a wider standard deviation from the average value as shown. The results show that the effective transfer of H+ ions from the anode to the cathode chamber indicated by better power generation in the cell is directly related to the enhanced biodegradability and hence improved treatment efficiency. The current study reveals that electron/proton transfer efficiency was negatively affected at very high concentrations of KMnO4 which reduces the power generation and treatment efficiency of MFC.
Effect of pH of KMnO4 solution on performance of MFC
The second assessment phase was performed by maintaining the constant catholyte concentration (2000 mg/L KMnO4 solution) in all batch reactors and varying the catholyte pH values. Figure 4 reflects the influence of catholyte pH on the efficiency of COD removal when compared to a control sample. At pH 5, MFC showed a maximum COD removal of 51.85%. Non-buffered KMnO4 solution was found to have pH in the alkaline range. Even though the oxidation potential of KMnO4 solution improves with lowering of pH, since anolyte also maintained acidic state by microbial metabolism, too lower pH value of catholyte reduced the proton transfer from anode chamber. Movement of protons directly provides a more suitable environment for biodegradability in the anode chamber, and thus increasing the efficiency of treatment.
Figure 5a shows cell average and maximum daily average power density based on voltage and current measurements from MFCs with different catholyte pH statuses. The maximum daily average power density of 5521 mW/m3 and cell average power density of 3187 mW/m3 were developed in MFC, with pH 5 buffered oxidizing agent. Figure 5b represents the daily average power density from the MFC with KMnO4 solution 2000 mg/L under pH 5. Even with higher electron acceptance of KMnO4 solution at lower pH ranges, reduction in proton transfer due to the equilibrium of ions between anode and cathode reduces the power density from MFC at lower pH ranges.
Effect of electrode material on performance of MFC
Aluminium was used as electrode material in both cathode and anode chambers, in the first two phases of the experimental investigation. In the third phase, the aluminium electrode was compared with the carbon cloth electrode. The anode chamber of MFC showed clear white settling after 102 h of detention due to bioflocculation and long stagnation time. Figure 6 demonstrates better COD removal efficiency for MFC using carbon cloth as electrode with 2000 mg/L KMnO4 solution having pH 5 as catholyte. In MFC with carbon cloth electrode, 59.08% of COD removal was achieved. No physical difference was observed for the aluminium electrode after using in cell as the anode. Carbon-based materials are reported with higher affinity to attach biofilm over the surface compared to metals. Hence, microbial retention in the substrate bulk volume is more effective while using carbon cloth by preventing the yeast floc from settling completely at the bottom of the anode chamber. This improves the COD removal efficiency while using carbon cloth as electrode compared to aluminium.
Figure 7a shows the average and maximum power density generated from MFCs with different electrodes. The maximum daily average power density of 6080 mW/m3 and cell average power density of 3622 mW/m3 were developed in MFC, using carbon cloth electrode. Figure 7b represents the daily average power density generated from the MFC using carbon cloth electrode with KMnO4 solution 2000 mg/L having pH 5. Attachment of the bacterial layer over the electrode surface could be the key factor in enhancing total power generation from MFC using carbon cloth. The tendency of yeast to attach over the carbon cloth improved the electron transfer from microbe to electrode, for better treatment efficiency and higher power generation from the cell.
Effect of anode configuration on performance of MFC
Spatial coverage of the anode reduces the distance the microbe travels to transfer an electron to the electrode. Because carbon cloth is not a stiff material like aluminium, it tends to sag down in anolyte. When in all MFC reactors, a single piece of 50 mm × 50 mm anode was divided into smaller pieces of equal surface, it gave spread through the volume. Figure 8 shows the effect of the carbon cloth anode configuration on the efficiency of COD removal as compared to the control sample. MFC showed a maximum COD removal efficiency of 62.72% with configuration 3. When the anode was split and spread spatially through the volume, due to the occurrence of biofilm attached to the anode, a slight increase in treatment efficiency was achieved. For conf. 4, a slight reduction in efficiency is due to smaller coverage of electrode throughout the depth of anolyte.
Figure 9a shows the variation in power density generated in each configuration of the anode. All configurations show a similar pattern of power density, with a steady increase from conf. 1 to conf. 3. For conf. 4, a slight reduction in potential is due to lower coverage of anode through the depth of anolyte, even with better horizontal coverage. The highest daily average power density of 6519 mW/m3 was observed in Conf. 3, such as three rectangular pieces of electrode material placed parallel inside the anode chamber covering the full depth of anolyte. Figure 9b shows the daily average power density generated from MFC with conf. 3 carbon cloth electrode. Even with the settling of bioflocculated organics by long detention periods, the third configuration could cover the anolyte to full depth and capture microbial metabolism-generated power effectively.
Conclusion
The two major concerns of present-day research are zero energy technologies and sustainable energy production. Microbial fuel cells (MFCs) are bioreactors which, through biodegradation, extract chemical energy stored in organic compounds into electrical energy. A large number of small-scale, coconut-based industrial units produce value-added products with excellent nutritional quality. It is not economically feasible to install complex wastewater treatment plants to treat their effluents. Conventional aerobic treatment systems are energy-intensive creating higher treatment costs, while anaerobic reactors are very slow. MFC, being a zero energy system with power generation during treatment, is a more sustainable way to treat wastewater. Therefore, separating coconut water, with higher COD strength, from washing water and treating it with MFC is a feasible and economical solution for reducing pollution issues. The current study revealed that at ambient temperature, the MFC batch reactor using 2000 mg/L of KMnO4 buffered at pH 5 as catholyte provides more than 50% of the COD removal efficiency while using an aluminium electrode. At the same time, nearly 60% of COD removal from the MFC was obtained by changing the electrode material to carbon cloth with an improved generation of bioenergy. By changing the spatial distribution of carbon cloth electrode in anolyte, the maximum COD removal efficiency of 62.72% was achieved with an average power density generation of 4.1 W/m3 from the cell. It is easy to provide a detention period of 5–7 days in most of the small-scale coconut processing industries which operate on batch mode for better treatment. MFC can be used as a primary treatment for small-to-medium-scale coconut industry wastewater to reduce high organic pollution. Since bioflocculation takes place in MFC, filtration or sedimentation with coagulants of the treated effluent further reduces COD. The treated effluent could be safely used for irrigation by combining with wash water. The best operating conditions of MFC batch reactor produce maximum energy output with zero energy usage, and they will minimize the fuel consumption if this energy can be channelized efficiently for the production processes.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Akman D, Ozdemir S, Cirik K, Ozkaya B, Cinar O (2014) Effect of some operational parameters in fed-batch microbial fuel cells for electricity generation. Res J Chem Environ 18(3):87–93
Ali AH, Mussawy HAA, Hussein MJ, Hamadi NJ (2019) Comparison between conventional and modified microbial fuel cell for wastewater treatment and electricity generation. Int J Environ Sci Technol 16:8141–8150. https://doi.org/10.1007/s13762-019-02355-x
Archana P (2019) An analytical study of Indian agriculture crop production and export with reference to wheat. Adv Manag 12(1):75–78
Ballesteros F, Vuong TH, Secondes MF, Tuan PD (2016) Removal efficiencies of constructed wetland and efficacy of plant on treating benzene. Sustain Environ Res 26(2):93–96. https://doi.org/10.1016/j.serj.2015.10.002
Capodaglio AG, Olsson G (2020) Energy issues in sustainable urban wastewater management: use, demand reduction and recovery in the urban water cycle. Sustainability 12(1):1–17. https://doi.org/10.3390/su12010266
Chanakya HN, Khuntia HK, Mukherjee N, Aniruddha R, Mudakavi JR, Thimmaraju P (2015) The physicochemical characteristics and anaerobic degradability of desiccated coconut industry waste water. Environ Monit Assess 187:772. https://doi.org/10.1007/s10661-015-4991-7
Chen F, Zeng S, Luo Z, Ma J, Zhu Q, Zhang S (2019) A novel MBBR–MFC integrated system for high-strength pulp/paper wastewater treatment and bioelectricity generation. Sep Sci Technol 55(14):2490–2499. https://doi.org/10.1080/01496395.2019.1641519
Das A, Mishra S (2019) Complete biodegradation of azo dye in an integrated microbial fuel cell-aerobic system using novel bacterial consortium. Int J Environ Sci Technol 16:1069–1078. https://doi.org/10.1007/s13762-018-1703-1
Das I, Ghangrekar M, Satyakam R, Srivastava P, Khan S, Pandey H (2020) On-site sanitary wastewater treatment system using 720-L stacked microbial fuel cell: case study. J Hazard Toxic Radioact Waste 24(3):04020025. https://doi.org/10.1061/%28ASCE%29HZ.2153-5515.0000518
Dong Y, Qu Y, He W, Du Y, Liu J, Ha X, Feng Y (2015) A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Biores Technol 192:66–72. https://doi.org/10.1016/j.biortech.2015.06.026
Firdous S, Jin W, Shahid N, Bhatti Z, Iqbal A, Abbasi U, Ali A (2018) The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ Technol Innov 10:143–151. https://doi.org/10.1016/j.eti.2018.02.006
Flimban SGA, Ismail IMI, Kim T, Oh SE (2019) Overview of recent advancements in the microbial fuel cell from fundamentals to applications: design, major elements, and scalability. Energies 12(17):3390. https://doi.org/10.3390/en12173390
Gomes LM, Duarte JLS, Pereira NM, Huitle CAM, Tonholo J, Zanta CLPS (2014) Development of a system for treatment of coconut industry wastewater using electrochemical processes followed by Fenton reaction. Water Sci Technol 69(11):2258–2264. https://doi.org/10.2166/wst.2014.129
Jayashree C, Tamilarasan K, Rajkumar M, Arulazhagan P, Yogalakshmi K, Srikanth M, Banu J (2016) Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J Environ Manag 180:351–358. https://doi.org/10.1016/j.jenvman.2016.05.050
Jayashree S, Ramesh ST, Lavanya A, Gandhimathi R, Nidheesh PV (2019) Wastewater treatment by microbial fuel cell coupled with peroxicoagulation process. Clean Technol Environ Policy 21:2033–2045. https://doi.org/10.1007/s10098-019-01759-0
Li T, Cai Y, Yang XL, Wu Y, Yang YL, Song HL (2020) Microbial fuel cell-membrane bioreactor integrated system for wastewater treatment and bioelectricity production: overview. J Environ Eng. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001608
Mansoorian HJ, Mahvi AH, Jafari AJ, Khanjani N (2016) Evaluation of dairy industry wastewater treatment and simultaneous bioelectricity generation in a catalyst-less and mediator-less membrane microbial fuel cell. J Saudi Chem Soc 20:88–100. https://doi.org/10.1016/j.jscs.2014.08.002
Mccarty PL, Bae J, Kim J (2011) Domestic wastewater treatment as a net energy producer–can this be achieved? Environ Sci Technol 45(17):7100–7106. https://doi.org/10.1021/es2014264
Nandi P (2015) A report on partial oxidation of sucrose. Int J Curr R Chem Pharm Sci 2(1):14–17
Osunlaja AA, Idris SO, Iyun JF (2012) Kinetics and mechanism of the methylene blue-permanganate ion reaction in acidic medium. Arch Appl Sci R 4(2):772–780
Othaman MA, Sharifudin SA, Mansor A, Kahar AA, Long K (2014) Coconut water vinegar: new alternative with improved processing technique. J Eng Sci Technol 9(3):293–302
Palanisamy G, Jung HY, Sadhasivam T, Kurkuri MD, Kim SC, Roh SH (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621. https://doi.org/10.1016/j.jclepro.2019.02.172
Pandey P, Shinde VN, Deopurkar RL, Kale SP, Patil SA, Pant D (2016) Review: recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Perera SACN, Ekanayake GK, Herath HMNB (2015) An investigation of the tender nut potential of diverse coconut (Cocos nucifera L.) varieties/forms in Sri Lanka. Int J Coconut R & D 31(1):34–45
Prades A, Salum UN, Pioch D (2016) New era for the coconut sector. What prospects for research? Oilseeds Fats Crops Lipids 23(6):D607. https://doi.org/10.1051/ocl/2016048
Reddy LV, Kumar SP, Wee YJ (2010) Microbial fuel cells (MFCs)—a novel source of energy for new millennium. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol 2(13):956–964
Sayed ET, Abdelkareem MA (2017) Yeast as a biocatalyst in microbial fuel cell. In: Lucas C, Pais C (eds) Old yeasts—new questions. Intech Open, London, pp 41–65
Shanmuganathan P, Rajasulochana P, Murthy RA (2018) Factors affecting the performance of microbial fuel cells. Int J Mech Eng Technol 9(9):137–148
Sivakumar D (2020) Wastewater treatment and bioelectricity production in microbial fuel cell: salt bridge configurations. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-020-02864-0
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Biores Technol 131:564–571. https://doi.org/10.1016/j.biortech.2012.12.063
Soletti JI, Carvalho SHV, Quintela PHL, Salles WFDL (2005) Coconut industry wastewater treatment using dissolved air flotation. In: 2nd mercosur congress on chemical engineering and 4th mercosur congress on process systems engineering, Rio de Janeiro.
Sreedharan S, Pawels R (2016) Microbial fuel cell (MFC) technology for household waste reduction and bioenergy production. Civ Eng Urban Plan Int J (CiVEJ) 3(2):119–126. https://doi.org/10.5121/civej.2016.3210
Suman A, Ahmad T, Ahmad K (2017) Dairy wastewater treatment using water treatment sludge as coagulant: a novel treatment approach. Environ Dev Sustain. https://doi.org/10.1007/s10668-017-9956-2
Tan TC, Cheng LH, Bhat R, Rasul G, Easa AM (2014) Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem 142:121–128. https://doi.org/10.1016/j.foodchem.2013.07.040
Tan SP, Kong HF, Bashir MJ, Lo PK, Ho CD, Ng CA (2017) Treatment of palm oil mill effluent using combination system of microbial fuel cell and anaerobic membrane bioreactor. Biores Technol 245:916–924. https://doi.org/10.1016/j.biortech.2017.08.202
Teli NC, Bhalerao SA, Didwana VS, Verma DR (2016) Microbial fuel cell: a source of sustainable energy. BIOVISTAS Int J Biol Res 5(6):1–12
Thung WE, Ong SA, Ho LN, Wong YS, RidwanF OYL, Oon YS, Leh HK (2019) Enhancement of mass and charge transport in scaled-up microbial fuel cell by using innovative configuration of bioanode. Int J Environ Sci Technol 16:8175–8184. https://doi.org/10.1007/s13762-019-02390-8
You SJ, Zhang JN, Yuan YX, Ren NQ, Wang XH (2010) Development of microbial fuel cell with anoxic/oxic design for treatment of saline seafood wastewater and biological electricity generation. J Chem Technol Biotechnol 85(8):1077–1083. https://doi.org/10.1002/jctb.2400
Zhang Q, Zhang Y, Li D (2017) Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community. Biores Technol 229:104–110. https://doi.org/10.1016/j.biortech.2017.01.026
Zhang Q, Zhanga L, Lia Z, Zhanga L, Li D (2019) Enhancement of fipronil degradation with eliminating its toxicity in a microbial fuel cell and the catabolic versatility of anodic biofilm. Biores Technol 290:121723. https://doi.org/10.1016/j.biortech.2019.121723
Zhao H, Zhang Q (2021) Performance of electro-Fenton process coupling with microbial fuel cell for simultaneous removal of herbicide mesotrione. Biores Technol 319:124244. https://doi.org/10.1016/j.biortech.2020.124244
Online document
Coconut Development Board (CDB), Ministry of Agriculture and Farmers Welfare, Government of India. https://www.coconutboard.gov.in/Statistics.aspx
Price Policy for Copra: 2019 Season. Ministry of Agriculture and Farmers Welfare, Government of India. http://cacp.dacnet.nic.in/ViewQuestionare.aspx?Input=2&DocId=1&PageId=37&KeyId=638
Industrial Pollution Control Guidelines, Sri Lanka—No.3 1992, Central Environmental Authority, Sri Lanka. http://dl.nsf.ac.lk/ohs/cea/04425.pdf
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Funding
There was no funding from any agencies; however, the ‘School of Engineering, CUSAT’ and ‘SCMS School of Engineering Technology’ in Kochi, Kerala, India, provided all laboratory equipment and chemical reagents for the experimental investigations.
Author information
Authors and Affiliations
Contributions
Conceptualization, visualization, methodology, formal research investigation, resources management, original draft writing, and correction after first review were all completed by SS. RP was in charge of supervision, project administration, methodology evaluation, and experimental investigation, as well as being a key contributor in modifying the initial draft of the manuscript before its first submission and revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Nil. Effluent pollution control for coconut-based industries, primarily copra processing units (small and medium scale—batch production basis), operating in rural areas, is purely academic institution-based research work. The authors declare that they have no established conflicting financial interests or personal relationships that may have influenced the research presented in this paper.
Ethics approval
All procedures performed in studies were in accordance with the ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors. For this study, formal consent is not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Editorial responsibility: Ta Yeong Wu.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Sreedharan, S., Pawels, R. Feasibility study on treatment of coconut industry wastewater and bioenergy production using microbial fuel cell (MFC). Int. J. Environ. Sci. Technol. 19, 5333–5342 (2022). https://doi.org/10.1007/s13762-021-03408-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03408-w