Abstract
The dairy industry is among the most polluting industries as it produces large volume of wastewater that may adversely affect the environment if discharged untreated. Dairy wastewater is characterized by high COD, BOD and nutrient levels. In this study, water treatment sludge was used as a coagulant for the treatment of synthetic dairy wastewater in the pH range of 4–10. Turbidity, COD, BOD, TSS and TDS removals from the synthetic dairy wastewater were found to be around 93, 65, 67, 84 and 85%, respectively, at the optimum conditions. Water treatment sludge was found to perform even better than other conventional coagulants used for the same. Results showed that it has the potential to substitute the conventional coagulants partially or fully in the primary treatment of dairy wastewater. The utilization of water treatment sludge at dairy wastewater treatment plants would provide sustainable sludge management and cost-effective dairy wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The dairy industry is crucially important for every nation of the world as it provides essential nutrition to human and generates heavy revenue too. Increasing demand of milk and milk products has led to the growth of dairy industries in most of the countries of the world. India is also among the leading producers of milk and other dairy products in the world and contributes about 35% of the total Asian milk (Gupta 1997; Qasim and Mane 2013). Dairy industry produces a variety of milk products such as milk powder, flavoured milk, condensed milk, fluid milk, butter, cheese, yogurt and ice cream through various manufacturing processes (Kushwaha et al. 2011). Large volume of wastewater is generated as by-products of whey, cheese and ice cream making processes and during cleaning and washing of milk containers, equipments and floors (Karadag et al. 2015). Therefore, dairy industry is regarded as one of the most polluting industries. Vourch et al. (2008) reported that about 0.2–10 L of wastewater is produced while processing 1 L of milk. Dairy wastewater mainly contains proteins, fats, lactose, nutrients, including detergents and sanitizers. It is generally characterized by high COD (80–95,000 mg/L) and BOD (40–48,000 mg/L), relatively large load of suspended solids (24–45,000 mg/L) and significant variation in pH (4.2–9.4) (Rico Gutierrez et al. 1991; Kushwaha et al. 2011). Hence, discharging such wastewater without prior treatment will cause serious environmental problems.
Dairy wastewater treatment methods commonly involve biological process such as activated sludge process (ASP), sequencing batch reactor (SBR), trickling filters, anaerobic filters, aerated lagoons and anaerobic sludge blanket (UASB) reactor. (Demirel et al. 2005). High energy requirements during aerobic biological treatment and poor nutrients removal in case of anaerobic biological treatment methods are the main drawbacks of these processes. Therefore, Hamdani et al. (2004), Kushwaha et al. (2010a, b, c), Rao and Bhole (2002), Sarkar et al. (2006), Sengil and Ozacar (2006), Tchamango et al. (2010) investigated the physicochemical methods such as adsorption, electrocoagulation and coagulation–flocculation for the treatment of dairy wastewater. Significant removal of COD, BOD, TS, TN, etc., from the dairy wastewater was achieved in those studies.
Conventional water treatment plants also involve the process of coagulation–flocculation for removing the colloidal impurities from the raw water. Large volume of waste or residue known as water treatment sludge (WTS) is generated during the coagulation–flocculation process at these plants. Aluminium salts are commonly used as coagulants during the coagulation process; therefore, WTS contains considerable part of aluminium hydroxide precipitate. In many developing countries, WTS is discharged directly into nearby water bodies which cause significant damage to the environment. Safe and sustainable disposal of WTS through constructive utilization is encouraged nowadays. In this regard, sincere efforts have been made globally, and several constructive utilization methods have been identified and investigated (Ahmad et al. 2016a). Application of the WTS in wastewater treatment has been reported in many previous studies (Guan et al. 2005; Nair and Ahammed 2015; Parsons and Daniels 1999; Xu et al. 2009). However, in most of the previous studies, WTS was used as a coagulant for the treatment of municipal wastewater. As per the knowledge, utilization of WTS for treatment of dairy wastewater has not been investigated so far and reported in any open literature.
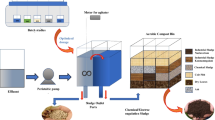
In the present study, a novel treatment approach has been investigated to utilize waste/residue of water treatment plants for treating wastewater discharged from dairy industry. WTS was used as a coagulant to treat the synthetic dairy wastewater (SDW) by coagulation–flocculation method. Its efficiency in removing turbidity, COD, BOD, TSS and TDS from the SDW was evaluated and compared with the efficiency of conventional coagulants such as alum, ferric chloride and ferrous sulphate.
2 Materials and methods
2.1 Wastewater and its characteristics
SDW was generated by adding 3 mL full cream milk (Mother Dairy brand, manufactured by Mother Dairy Fruits and Vegetable Ltd.) in 1 L distilled water and 1 g of laboratory-grade kaolin (H2Al2Si2O8H2O) powder was also mixed to get a uniform solution. The SDW composition was kept constant throughout the study and was prepared freshly whenever required. SDW was prepared as per the similar methods described in previously published literatures (Kushwaha et al. 2010a; Ramasamy et al. 2004). The prepared SDW samples have been analysed for pH, turbidity, BOD and COD as per the standard procedure (APHA 1998). TSS and TDS were measured gravimetrically using Whatman Grade 1 filter paper. Table 1 presents the characteristics of SDW prepared in the laboratory for the present study.
2.2 Sludge collection and its conditioning
WTS was collected from the Chandrawal Water Treatment Plant, Delhi, India, which is treating Yamuna river water. The plant is using PACl as coagulant. Major compounds present in the WTS were determined using energy-dispersive X-ray fluorescence spectroscopy (ED-XRF), and trace metals were analysed using wavelength-dispersive X-ray fluorescence spectroscopy (WD-XRF) technique. The collected WTS was treated with variable normality of H2SO4 (1.0, 1.5, 2.0, 2.5, 3, 3.5 and 4 N) at a uniform rate of 50 mL/L of sludge. The optimum normality and dose of H2SO4 found in this process were selected for preparing conditioned water treatment sludge (CWTS) through acid conditioning.
2.3 Experimental methodology
Alum, ferric chloride, ferrous sulphate and CWTS were used as a coagulant, and their efficiency in treating dairy wastewater was evaluated by measuring the turbidity, BOD, COD, TSS and TDS removal from SDW. Laboratory-grade alum, ferric chloride and ferrous sulphate manufactured by Central drug house, India were used in this study. Alum had 97% purity, whereas ferric chloride and ferrous sulphate were 96% pure. Stock solutions (1000 mg/L, i.e. 1 mL = 1 mg coagulant) of coagulants were prepared, and variable doses of 10–40 mL/L with an increment of 5 mL/L were applied to SDW. Jar tests were carried out to simulate the conventional coagulation–flocculation process. Six beakers containing 1000 mL SDW sample were placed on a standard jar test apparatus. A flash/rapid mixing at 100 rpm for 2 min was carried out after adding the coagulant dose; then the slow mixing was carried out at 20 rpm for 25 min. Thereafter, jars were kept standstill for 20 min to settle down the flocs. A series of jar tests were performed to determine the effect of coagulant nature, coagulant dose and initial pH on turbidity removal from SDW. Removal of colloidal suspension from the SDW was investigated over a wide pH range of 4–10. Initial pH of SDW was maintained to the required level using H2SO4 or NaOH before adding different coagulants. Batch experiments were carried out at each pH condition for variable dosage of conventional coagulants and CWTS. The supernatant from each jar was withdrawn and analysed for colloidal suspension removal in terms of turbidity through Nephelo turbidity meter. The supernatants were also analysed for residual COD, BOD, TSS and TDS at the optimum condition for each of the coagulants used.
3 Results and discussion
3.1 SDW characteristics
SDW had slightly acidic pH of 6.2–6.8 and high COD value of 1250 mg/L. Turbidity and TSS values were 372 NTU and 348 mg/L, respectively, with high TDS value of 1187 mg/L; thus, total solids present in the SDW was about 1535 mg/L. Preliminary experiments showed that higher coagulant doses were required to settle down the colloidal suspension. Kushwaha et al. (2010c) also reported higher optimum dose of different coagulants in their study. Therefore, to make the solution uniform and economize the required coagulant dose, kaolin was added while preparing the SDW. Addition of kaolin lowered the optimum coagulant dose in each of the coagulants applied in this study. It may due to adsorption of colloids onto the surface of kaolin particles.
3.2 WTS characteristics
Chemical composition of the WTS is shown in Table 2. SiO2 (67.75%) and Al2O3 (16.76%) were the major oxides present in the WTS; however, Fe2O3 was also found in small quantity. Since PACl is used at the water treatment plant, WTS had higher percentage of aluminium in the dried mass of WTS; therefore, conditioning of WTS would regenerate the aluminium and iron present in the sludge. The regenerated aluminium and iron work as coagulant in the treatment of dairy wastewater. Some trace elements listed in Table 3 were also found in the WTS. These metals are either present in the raw water or present as impurities in the coagulants and get accumulated into the small mass of sludge (Ahmad et al. 2016a). Some of these metals are toxic in nature and require careful attention. Hence, safe disposal of such waste is warranted for sustainable development.
3.3 Conditioning of WTS
Turbidity removal at variable dosage of WTS acidified with the different normality of H2SO4 is shown in Fig. 1. It is evident from Fig. 1 that, as the normality of H2SO4 was increased from 1 to 3 N, turbidity removal was also increased, whereas it decreased when WTS was acidified with H2SO4 of normality above 3 N. Conditioning of WTS with acids releases the aluminium and iron metals from the sludge matrix which act as coagulating agent. Xu et al. (2009) recovered coagulants from WTS through the acidification process and applied in the wastewater treatment. It was reported in the previous studies that the alum recovery is favoured at lower pH (Keeley et al. 2012; Xu et al. 2009). Dissolution of aluminium at too low pH condition negatively affects the coagulation process (Kim et al. 2002) and was also observed in the experiments conducted in this study for removing the colloidal suspension from SDW by acidifying WTS with H2SO4 above 3 N. Therefore, WTS acidified with H2SO4 of normality above 3 N was found less efficient in removing colloidal suspension from SDW. The optimum normality of H2SO4 was found to be 3 N, gave highest turbidity removal at all the dosage of acidified WTS. Hence, the collected WTS was acidified with 3 N H2SO4 at the rate of 50 mL/L of sludge to prepare CWTS and was used as a coagulant in the treatment of SDW at variable condition.
3.4 Effect of initial pH and coagulant dose
Turbidity removal from SDW in the pH range of 4–10 at variable dosage of alum, ferric chloride, ferrous sulphate and CWTS is shown in Fig. 2a–d, respectively. It could be observed from Fig. 2a that as pH was increased, turbidity removal also increased up to pH 7. It could be further inferred that the turbidity removal increased with alum dose and attained the maxima at about 25 mL/L alum dose for all pH conditions. Maximum turbidity removal of 93% was achieved at the alum dose of 25 mL/L when the initial pH of SDW was kept at pH 7. Figure 2b, c presents the turbidity removal from SDW at variable dose of ferric chloride and ferrous sulphate at different initial pH of SDW. Turbidity removal was found to increase with increasing pH for both the iron-based coagulant. Optimum pH was found to be 7 which gave maximum turbidity removal of 95% at the optimum dose of 35 mL/L in case of ferric chloride. However, ferrous sulphate was found to be more effective in the higher pH range of 7–10. Turbidity removal of 94% was achieved at pH 7 for the coagulant dose of 35 mL/L, whereas about same 94 and 93% turbidity was removed at pH 9 and 10 at the coagulant doses of 30 and 25 mL/L, respectively. The optimum condition for ferrous sulphate was selected as pH 7 and 35 mL/L dose because the initial pH of the SDW was around this value. Figure 2d shows the effect of initial pH of the SDW and CWTS dose on the turbidity removal from SDW. In case of CWTS also the turbidity removal increased with pH and had increased up to pH 7, after that it decreased rapidly as the CWTS dose was increased from 10 to 40 mL/L. At pH 5, 6 and 7 turbidity removal had increasing trend with CWTS dose and attained maximum removal at the dose of 25 mL/L and then decreased. CWTS was found to be more effective at neutral pH, gave maximum turbidity removal of 93% from SDW at the optimum dose of 25 mL/L.
In fact, the colloidal removal or the coagulation mechanism is determined by the interrelations existing between coagulant dose, pH and colloidal concentration. Formations of metallic cations are dominant at lower pH and favours colloidal removal through adsorption by charge neutralization. However, at higher pH and increasing the dose, formation of hydroxide precipitate leads to colloidal solids removal mainly by sweep floc mechanism and physical adsorption (Ahmad et al. 2016b; Guan et al. 2005; Kim et al. 2007; Lee et al. 2000). However, Amirtharajah and O’Melia (1990) observed that the coagulation mechanisms of hydrolysing metal salts are complex and do not necessarily involve charge neutralization. The casein (milk protein) present in the dairy wastewater mainly imparts colloidal nature to the wastewater (Herrington 1948). Selmer-Olsen et al. (1996) reported that dairy wastewater has the isoelectric point (pHiso) around 4.2. At pH > pHiso milk proteins present in the dairy wastewater carry negative charge and hence, can be removed through charge neutralization by positively charged coagulant species. Therefore, in case of alum and CWTS, Al3+ caused higher turbidity removal between the pH range of 6–8, and reduction in colloidal removal efficiency above pH 8 could be explained due to formation of Al(OH)4− species in water (Duan and Gregory 2003). The electrostatic repulsion between negatively charged colloidal particles and Al(OH)4− ions reduced the turbidity removal and the associate pollutant removal from the SDW. Iron-based coagulants showed higher turbidity removal even at pH > 8. It could be attributed to the findings that at pH > 8, formation of positively charged Fe2+ and Fe(OH)+ and neutral Fe(OH)2 hydrolysis species caused better colloidal removal as compared to other coagulants used in this study. However, formation of negatively charged Fe(OH) −3 ions occurs when pH > 10.5, causing lower turbidity removal (Benjamin 2002). CWTS was found efficient in removing colloidal suspensions from SDW at neutral or acidic pH conditions; however, dissolution of Al into solution at pH < 4 negatively affected the turbidity removal. At pH > 8, formation of Al(OH)4− reduces the colloidal removal efficiency (Duan and Gregory 2003), and it is evident from Fig. 2d that CWTS was effective at 10 mL/L dose but removal efficiency decreases with increasing the dose. Therefore, CWTS worked efficiently in the SDW having initial pH around 7 and the results were comparable with the efficiency of conventional coagulants in this pH range.
3.5 Quality of treated SDW at optimum condition
During the coagulation–flocculation process, apart from turbidity other pollutants associated with the colloidal particles also get removed. Therefore, SDW obtained after the coagulation–flocculation process at the optimum conditions in case of each coagulant was analysed for COD, BOD, TSS and TDS removals. COD, BOD, TSS and TDS removal from the SDW after physicochemical treatment is shown in Table 4. CWTS had the highest COD removal per cent (65%) when compared with alum (63%), ferric chloride (64%) and ferrous sulphate (64%). BOD removal by CWTS was also comparable to that of BOD removal by other coagulants under investigation. BOD removal of 67% was achieved with CWTS, whereas alum removed 67% BOD; ferric chloride and ferrous sulphate both removed 68% BOD from the SDW. TSS and TDS removals were also higher than that of conventional coagulants. TSS and TDS removal from the SDW was 84 and 85%, respectively, at the optimum condition of pH 7 and 25 mL/L CWTS dose. However, the TSS and TDS removals were 80 and 81%, respectively, for alum, 84 and 83%, respectively, for ferric chloride and 84 and 83%, respectively, in case of ferrous sulphate. Unlike other pollutants, TDS removal efficiency seems to be unexpectedly high in case of all the coagulants. Such higher removal efficiency values were observed may be due to the constraint of gravimetric method used to determine the TDS removal. The cation–anions along with the ultrafine flocs were removed on the filter paper giving higher TDS removal efficiency in all the cases. However, it was a comparative study and except for BOD, CWTS was found to perform even better than the conventional coagulants in removing COD, TSS and TDS from SDW. BOD removal result was also very close to that of conventional coagulants. It is evident from the results that the CWTS is efficient in removing pollution load from the dairy wastewater. Hence, from the above discussion, it can be inferred that WTS can be applied in the primary treatment of dairy wastewater and could substitute the conventional coagulants partially or fully in the dairy wastewater treatment plants. WTS application would provide safe and sustainable sludge disposal option. At the same time, it could provide cost-effective physicochemical treatment to dairy wastewater by reducing the requirements of chemical coagulants.
4 Conclusions
Physicochemical treatment of SDW by coagulation/flocculation process using CWTS was successfully achieved in this study. CWTS prepared from WTS acidified with 3 N H2SO4 at the rate of 50 mL/L sludge performed even better than the conventional coagulants for the treatment of SDW. Maximum turbidity removal of 93% was achieved at pH 7 and CWTS dose of 25 mL/L. Along with the turbidity, pollutants associated with the colloidal particles also get removed. Significant removal of COD (65%), BOD (67%), TSS (84%) and TDS (85%) from the SDW was achieved at the optimum condition. Removal of all the pollutants from SDW by CWTS was comparable to that of conventional coagulants such as alum, ferric chloride and ferrous sulphate. Hence, it can be concluded from the present study that CWTS could be potentially utilized to treat the dairy wastewater. Recycling of waste from water treatment plants into dairy wastewater treatment plants would provide constructive utilization and sustainable disposal of WTS, at the same time providing cost-effective physicochemical treatment of dairy wastewater.
References
Ahmad, T., Ahmad, K., Ahad, A., & Alam, M. (2016a). Characterization of water treatment sludge and its reuse as coagulant. Journal of Environmental Management, 182, 606–611.
Ahmad, T., Ahmad, K., & Alam, M. (2016b). Sustainable management of water treatment sludge through 3 ‘R’ concept. Journal of Cleaner Production, 124, 1–13.
American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environmental Federation (WEF). (1998). In L. Clesceri, A. Greenberg, & A. Eaton (Eds.), Standard methods for the examination of water and wastewater (20th ed.). Washington, DC: American Public Health Association.
Amirtharajah, A., & O’Melia, C. R. (1990). Coagulation processes: Destabilization, mixing, and flocculation, water quality and treatment (4th ed., pp. 269–371). New York, N.Y.: McGraw Hill.
Benjamin, M. M. (2002). Water chemistry. New York: McGraw Hill International Edition.
Demirel, B., Yenigun, O., & Onay, T. T. (2005). Anaerobic treatment of dairy wastewaters: A review. Process Biochemistry, 40, 2583–2595.
Duan, J., & Gregory, J. (2003). Coagulation by hydrolyzing metal salts. Advances in Colloid and Interface Science, 100–102, 475–502.
Guan, X.-H., Chen, G.-H., & Shang, C. (2005). Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Research, 39, 3433–3440.
Gupta, P. R. (1997). Dairy India (5th ed.). Anand: National Dairy Development Board.
Hamdani, A., Chennaoui, M., Assobhei, O., & Mountadar, M. (2004). Dairy effluent characterization and treatment by coagulation decantation. Le Lait, 84(3), 317–328.
Herrington, B. L. (1948). Milk and milk processing. Toronto: McGraw-Hill Book Company.
Karadag, D., Köroglu, O. E., Ozkaya, B., & Cakmakci, M. (2015). A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochemistry, 50, 262–271.
Keeley, J., Jarvis, P., & Judd, S. J. (2012). An economic assessment of coagulant recovery from water treatment residuals. Desalination, 287, 132–137.
Kim, J. G., Kim, J. H., Moon, H., Chon, C., & Ahn, J. S. (2002). Removal capacity of water plant alum sludge for phosphorus in aqueous solution. Chemical Speciation & Bioavailability, 14, 67–73.
Kim, S., Park, N., Kim, T., & Park, H. (2007). Reaggregation of flocs in coagulation-cross-flow microfiltration. Journal of Environmental Engineering, 133(5), 507–514.
Kushwaha, J. P., Srivastava, V. C., & Mall, I. D. (2010a). Treatment of dairy wastewater by commercial activated carbon and bagasse fly ash: Parametric, kinetic and equilibrium modelling, disposal studies. Bioresource technology, 101, 3474–3483.
Kushwaha, J. P., Srivastava, V. C., & Mall, I. D. (2010b). Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Separation and Purification Technology, 76, 198–205.
Kushwaha, J. P., Srivastava, V. C., & Mall, I. D. (2010c). Treatment of dairy wastewater by inorganic coagulants: Parametric and disposal studies. Water Research, 44, 5867–5874.
Kushwaha, J. P., Srivastava, V. C., & Mall, I. D. (2011). An overview of various technologies for the treatment of dairy wastewaters. Critical Reviews in Food Science and Nutrition, 51, 442–452.
Lee, J. D., Lee, S. H., Jo, M. H., Park, P. K., Lee, J. H., & Kwak, J. W. (2000). Effect of coagulation conditions on membrane filtration characteristics in coagulation-microfiltration process for water treatment. Environmental Science and Technology, 34, 3780–3788.
Nair, A. T., & Ahammed, M. M. (2015). The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. Journal of Cleaner Production, 96, 272–281.
Qasim, W., & Mane, A. V. (2013). Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resources and Industry, 4, 1–12.
Ramasamy, E. V., Gajalakshmi, S., Sanjeevi, R., Jithesh, M. N., & Abbasi, S. A. (2004). Feasibility studies on the treatment of dairy wastewaters with upflow anaerobic sludge blanket reactors. Bioresource technology, 93, 209–212.
Rao, M., & Bhole, A. G. (2002). Removal of organic matter from dairy industry wastewater using low-cost adsorbents. Journal of Indian Chemical Engineering Section A, 44(1), 25–28.
Rico Gutierrez, J. L., Garcia Encina, P. A., & Fdz-Polanco, F. (1991). Anaerobic treatment of cheese-production wastewater using a UASB reactor. Bioresource Technology, 37, 271–276.
Sarkar, B., Chakrabarti, P. P., Vijaykumar, A., & Kale, V. (2006). Wastewater treatment in dairy industries—Possibility of reuse. Desalination, 195, 141–152.
Selmer-Olsen, E., Ratanweera, H. C., & Pehrson, R. (1996). A novel treatment process for dairy wastewater with chitosan produced from shrimp-shell waste. Water Science and Technology, 11, 33–40.
Sengil, A., & Ozacar, M. (2006). Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. Journal of Hazardous Materials, 137, 1197–1205.
Tchamango, S., Nanseu-Njiki, C. P., Ngameni, E., Hadjiev, D., & Darchen, A. (2010). Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Science of the Total Environment, 408, 947–952.
Vourch, M., Balannec, B., Chaufer, B., & Dorange, G. (2008). Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination, 219, 190–202.
Xu, G. R., Yan, Z. C., Wang, Y. C., & Wang, N. (2009). Recycle of Alum recovered from water treatment sludge in chemically enhanced primary treatment. Journal of Hazardous Materials, 161, 663–669.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suman, A., Ahmad, T. & Ahmad, K. Dairy wastewater treatment using water treatment sludge as coagulant: a novel treatment approach. Environ Dev Sustain 20, 1615–1625 (2018). https://doi.org/10.1007/s10668-017-9956-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-017-9956-2