Abstract
A novel cationic surfactant type of N'alkyl N,'N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium iodide (10a–12a) and N'alkyl N', N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium iodide (10b–12b) of quaternary hydrazinium moieties in hydrophilic parts was synthesized. These quaternary hydrazinium surfactants were obtained using a two-step reaction scheme, starting from ring opening of succinic anhydride with a base (morpholine, piperidine), followed by ammonolysis with hydrazine hydrate, then alkylation of the amino group with alkyl bromides (RBr) that have different hydrophobic chain lengths (R, C12H25, C14H29, and C16H37), and ending with the quaternarization of the secondary amino group by two moles of methyl iodide. The chemical composition of the surfactants was analyzed by FTIR, 1HNMR, mass spectroscopy, and elemental analysis. A variety of surface-active characteristics were achieved by surface calculations, including CMC, γcmc, CMC/C20, Γmax, pC20, Amin, and Πcmc. These surface characteristics and foam stability rely on the nature of the hydrophobic chain. Preliminary results showed that an upgrade throughout the CH2 group in the fatty chain and the morpholine or piperidine ring lowers the CMC and increases the foaming capacity and stability of the quaternary hydrazinium surfactants. Anionic dye (Acid BG) interactions with 12b surfactant (as an example) were studied using the spectrophotometric technique and the binding constant (170.64 dm3.mol−1) was determined. The results indicate solubilization and binding took place at a large scale. Furthermore, considerable biodegradation of cationic surfactants was observed (68–87%). The antimicrobial activity of these surfactants has also been observed with the minimum inhibitory concentration (MIC) and the size of inhibited growth zone. The smallest MICs were found in 12a (64 µg/mL) and 12b (32 µg/mL) surfactants, indicating the highest antimicrobial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cationic surfactants are substances that have one or more hydrophobic groups linked directly or indirectly to positively charged atoms, frequently nitrogen (QAC quaternary ammonium compounds) [1, 2], and few phosphorous (QPC quaternary phosphonium compounds) [3]. Due to its broad synthesis and unrestricted use in a variety of industrial areas, cationic surfactants have long been the object of attention in chemistry [4, 5]. Cationic surfactants/amphiphiles are widely used in high-tech applications as well as by smooth models for mesoporous material synthesis [6], corrosion inhibitors [7], capping agents for nanoparticle synthesis [8], and biomedical applications such as gene and drug delivery [9], and antimicrobial activity [11]. The production of these surfactants should be environmentally acceptable allowing these components to be soluble and biodegradable. Cationic surfactants have been given a real consideration because the increased use of cationic surfactants in industrial, and household goods have caused ecological difficulties due to low solubility and biodegradability [12,13,14,15,16,17,18]. The configuration of the compounds is the main reason influencing the adsorption and micellization properties of cationic surfactants since adsorption is the major factor for the different applications [19, 20]. Consequently, structural modifications at the molecular stage may enhance surfactant properties. Recently, several reports analyzed and investigated several different forms of imidazolium [21], pyridinium [22], piperidineium [23], and pyrrolidinium cationic compounds [24]. So far, there are few syntheses and studies on cationic surfactants containing morpholine and piperidine rings attached to the hydrophilic head regarding their properties and potential use in many applications [25,26,27]. Asadov. et al. [28] have shown that morpholine amphiphile has petro-collecting and dispersing properties. Biocompatibility and sustainability considerations also have slowly allowed the production and design of several different forms of biocompatible surfactants consisting of biodegradable functional molecules, such as ester [29, 30] and amide groups [31, 32], which are deemed eco-friendly, to be easily broken down after usage. Hydrazinium derivatives have a wide range of applications, such as sourcing anhydrous hydrazine, propellant additives, antineoplastic agents, cancer and Hodgkin 's disease drugs, explosives, and ligands for the preparation of hydrazinium/hydrazine metal complexes[33,34,35]. Also, due to their industrial and biological uses, the knowledge of dye interaction with the surfactants is of great importance to the scientific and academic community [36]. Surfactants serve as solubilizers for water-insoluble dyes, breaking down dye aggregates to improve processes of adsorption on fiber, as auxiliaries for enhancing dye adsorption and leveling or diffusing agents [37, 38].
The substantial interest in cationic surfactants has encouraged us to prepare six new cationic surfactants including morpholine or piperidine nucleus attached to 4-oxobutanehydrazinium with terminal N-alkyl substitutes (C10–C16) (10a–12b).
The objective of this research is to determine surface activity by studying surface tension and study the interaction of anionic dye (Acid BG) with 12b surfactant by the spectrophotometric technique. Biodegradability and the effect of the introduction of amide units on their chemical nature are also analyzed. Additionally, microbial activity against both gram-positive and gram-negative bacteria was examined by identifying a minimum inhibitory concentration (MIC).
Materials and methods
Materials
Dodecanyl, tetradecyl, and hexadecyl bromide were bought from Aldrich (USA) and used without additional purification. Schuchardt chemically pure products were hydrazine hydrate (85%), piperidine, morpholine, and methyl iodide. Single-azo dye (Acid Red BG) was purchased from Sigma-Aldrich Chemical Company (Molecular Formula: C18H14N4Na2O8S2). All other chemicals were reagent grade unless stated to be otherwise. Water used for experiments was deionized water.
Structural characterization
Characterizations of cationic surfactants (10a–12b) were performed with the assistance of FTIR, 1HNMR, and MALDI MS techniques. Using a Bruker Tensor 27 spectrometer, Fourier transform infrared (FTIR) spectra were obtained within the range of 4000–400 cm-1. 1HNMR was performed with a Bruker DRX300 NMR spectrometer. With TMS as an internal standard, the samples were dissolved in CDCl3. The MALDI mass spectrum was acquired from the Bruker SolariX XR device in the dithranol matrix in dichloromethane.
Synthesis
Synthesis of N'alkyl-4-morpholino-4-oxobutanehydrazide (7a-9a) and N'alkyl-4-oxo-4-piperidinobutanehydrazide (7b-9b)
Succinic anhydride (5.0 g, 0.05 mol) was applied to a base solution (morpholine, piperidine) (0.05 mol) in methanol after full addition and stirring. The reaction mixture was heated under reflux, and the acid value of hemi amide acid was measured at different reaction time instances. The ester compound was fully formed when the reactants' acid value was less than 1.5mgKOH/gm. Hydrazine hydrate (3.20 g, 0.064 mol) was added to the flask and the mixture continued to be refluxed until the peak of the ester group disappeared from the IR spectra. Then, an alkyl bromide (dodecyl, tetradecyl, hexadecyl bromide) equivalent mole was introduced to the reaction solution. The experiment proceeded for another five hours under the same experimental conditions. The solvent was collected under reduced pressure; the residual was treated with water and purified by ethanol for recrystallization. (7a–9b).
N'-dodecyl-4-morpholino-4-oxobutanehydrazide (7a)
White semisolid (yield 87%) IR (KBr, cm−1)3181 (NH) and 1641, (C=O).
1HNMR of 7a(CDCl3,400 MHz) 0.96(3H,t,CH3CH2),1.29[20H,m,CH3(CH2)10], 1.59(2H,t,CH2CH2N)2.38[2H,t,NCOCH2], 2.71[2H,t,CH2CON],3.27(1H,s,CH2NH),
3.50[4H,t, CH2NCH2],3.77[4H,m, CH2OCH2] 9.81(1H,s,CONH),. 7a Calcd. for C20H39N3O3: C, 65.00; H, 10.64; N, 11.37. Found: C, 65.10; H, 10.66; N, 11.38 8a.Calcd. for C22H43 N3O3: C, 66.46; H, 10.9; N, 10.57. Found: C, 66.49; H, 10.93; N, 10.59 9a.Calcd. for C24H47 N3O3: C, 67.72; H, 11.13; N, 9.87. Found: C, 67.74; H, 11.15; N, 11.89, %.
N'-tetradecyl-4-oxo-4-piperidinobutanehydrazide (8b)
Yellowish white (yield 89%) IR (KBr, cm−1)3168 (NH) and 1639, (C=O). 1HNMR of 8b (CDCl3,400 MHz) 0.87(3H,t,CH3CH2),1.24–1.45[24H,m,CH3(CH2)12], 1.61[6H,m, CH2 CH2CH2] 2.29[4H,m,CO(CH2)2CO], 2.55(2H,t,CH2N), 3.21(1H,s,CH2NH) 3.32[4H,t,CH2NCH2]. 9.51(1H, s, CONH).Elemental analysis:0.7b Calcd. for C21H41N3O2: C, 68.62; H, 11.24; N, 11.43. Found: C, 68.64; H, 11.26; N, 11.45 8b Calcd. for C23H45N3O2: C, 69.83; H, 11.46; N, 10.62. Found: C, 69.84; H, 11.49; N, 10.649b Calcd. for C25H49 N3O2: C, 70.87; H, 11.66; N, 9.92. Found: C, 70.89; H, 11.68; N, 9.94, %.
Synthesis of (N'-alkyl N',N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium) iodide (10a-12a) and (N'-alkylN',N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium) iodide (10b-12b)
A sequence of six quaternary hydrazinium surfactants was produced by quaternaization of N'alkyl-4-(morpholino or piperidino-4-oxobutanehydrazide (7a,b–9a,b) (0.01 mol) with two moles of methyl iodide in ethanol as a solvent under reflux over eight hours. The mixtures were left to cool down, and the products obtained were further filtered by recrystallization from acetone/isopropanol [18:2 (vol/vol)] to get (10a–12b).
N'-Dodecyl N',N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium iodide (10a)
IR (KBr, cm−1)3178–3197 (NH) and 1631–1644 (C = O-N). Their yields were 86, 88, 91, 83, 87, and 94% for 10a–12b, respectively.
1HNMR of 10a (CDCl3,400 MHz)0.95(3H,t,CH3CH2),1.27[20H,m,CH3(CH2)10], 2.31[4H,m,CO(CH2)2CO],2.77[6H,s,N + (CH3)2],3.17[2H,t,CH2N+],3.35[4H,t,CH2NCH2],3.55 [4H,m, CH2OCH2], 5.89[1H,s, NHCO]. 10a Calcd. for C22H44IN3O3: C, 50.28; H, 8.44; N, 8.00. Found: C, 50.33; H, 8.45; N, 8.01%. MALDI-TOF MS m/z: 398 for [M-I]+.
N'-Tetradecyl N',N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium iodide (11a)
1HNMR of 11a (CDCl3,400 MHz)0.90(3H,t,CH3CH2),1.26[20H,m,CH3(CH2)12], 2.51[4H,m,CO(CH2)2CO],2.80[6H,s,N + (CH3)2],3.21[2H,t,CH2N+],3.41[4H,t,CH2NCH2],3.61[4H,m, CH2OCH2], 5.87[1H,s, NHCO]. 11a.Calcd. for C24H48 IN3O3: C, 52.07; H, 8.74; N, 7.59. Found: C, 52.12; H, 8.75; N, 7.60%. MALDI-TOF MS m/z: 426 for [M-I]+.
N'-Hexadecyl N',N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium iodide (12a)
1HNMR of 12a (CDCl3,400 MHz)0.93(3H,t,CH3CH2),1.33[20H,m,CH3(CH2)14], 2.42[4H,m,CO(CH2)2CO],2.67[6H,s,N + (CH3)2],3.27[2H,t,CH2N+],3.47[4H,t,CH2NCH2],3.66[4H,m, CH2OCH2], 5.74[1H,s, NHCO]. 12a.Calcd. for C26H52 IN3O3: C, 53.69; H, 9.01; N, 7.22. Found: C, 53.75; H, 9.02; N, 7.23%.MALDI-TOF MS m/z: 454 for [M-I]+.
N'-DodecylN',N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium iodide (10b)
1HNMR of 10b(CDCl3,400 MHz)0.95(3H,t,CH3CH2),1.23[20H,m,CH3(CH2)10], 1.41[6H,m, CH2CH2CH2],2.40[4H,m,CO(CH2)2CO],2.71[6H,s,N + (CH3)2],3.13[2H,t,CH2N+],3.37[4H,t,CH2NCH2], 7.40[1H,s, NHCO]10b.Calcd. for C23H46 IN3O2: C, 52.77; H, 8.86; N, 8.03. Found: C, 52.85; H, 8.93; N, 8.07%. MALDI-TOF MS m/z: 396 for [M-I]+.
N'-TetradecylN',N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium iodide (11b)
1HNMR of 11b (CDCl3,400 MHz)0.97(3H,t,CH3CH2),1.30[20H,m,CH3(CH2)12], 1.43[6H,m, CH2CH2CH2],2.44[4H,m,CO(CH2)2CO],2.75[6H,s,N + (CH3)2],3.17[2H,t,CH2N+],3.41[4H,t,CH2NCH2], 7.26[1H,s, NHCO]. 11b.Calcd. for C25H50 IN3O2: C, 54.44; H, 9.14; N, 7.62. Found: C, 54.56; H, 9.21; N, 7.71%. MALDI-TOF MS MALDI-TOF MS m/z: 424 for [M-I]+.
N'-Hexadecyl N',N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium iodide (12b)
1HNMR of 12b(CDCl3,400 MHz)0.99(3H,t,CH3CH2),1.26[H,m,CH3(CH2)14], 1.45[6H,m, CH2CH2CH2],2.47[4H,m,CO(CH2)2CO],2.77[6H,s,N + (CH3)2],3.19[2H,t,CH2N+],3.39[4H,t,CH2NCH2], 7.14[1H,s, NHCO]0.12b.Calcd. for C27H54 IN3O2: C, 55.95; H, 9.39; N, 7.25. Found: C, 56.07; H, 9.46; N, 7.31%. MALDI-TOF MS m/z: 452 for [M-I]+.
Measurement of surface-active properties
Surface tension measurements with a Krüss K 6 Tensiometer were performed at 25 ± 0.2 °C through a Du Nouy platinum ring technique. Measurements were performed at the initial concentration of 50 mM for the aqueous solution; other solutions were collected using the serial dilution process. CMC (critical micellular concentration) and γCMC (surface tension at critical micellular concentration) were measured as the surface tension breakpoint vs. concentration plot values (semilogarithmic scale). Foaming characteristics were measured at 2 g/dm3 and 25 °C. Foam stability was calculated by contrasting the level in the Ross–Miles method after ten minutes with the original foam point.
Spectrophotometric measurements
Hitachi UV-2800 spectrophotometer was used to measure the UV–visible spectrophotometric values inside the UV–visible range. The cells used were square cuvettes with a thickness of 1.0 cm. Distilled water was kept at the reference side in simple UV/visible absorption spectra, while the dye solution was adopted as a reference in differential UV/visible spectroscopy. A dye-surfactant-water ternary solution was taken into the sample cell in both situations. All the tests occurred at room temperature.
The synthesized surfactants biodegradability
Biodegradation assessments of the generated surfactants of cationic hydrazinium were carried out using the river water die-away technique [39]. River water had been collected from the Nile River for research. For this procedure, a stirring mixture including the surfactant examined (1000 ppm) was incubated at 25 °C. Samples were taken regularly, filtered using Whatman filter paper and had their surface tension measured via a Du-Nouy tensiometer (Kruss type K6). The procedure was replicated for seven days. The biodegradation percentage D % was determined in terms of the surface tension measured as shown by Eq. 1:
where γt surface tension exists at t, period zero is the surface tension γ0 (initial surface tension), and γbt is the blank experiment surface tension at period t.
Measurement of antimicrobial activity
Cationic hydrazinium surfactants were examined for antimicrobial activity against the reference of the following bacterial strains: Escherichia coli (NCTC-10416), Staphylococcus aureus (NCTC-7447), Pseudomonas aeruginosa (NCIB-9016), and Bacillus subtilis (NCIB-3610) using the agar diffusion method, which is a sterilized disc of filter paper saturated with a determined sample quantity that is put on a plate containing a solid bacterial nutrient agar broth, which has been heavily seeded with the spore suspension of the organism examined. Upon incubation, the width of the clear inhibition zone around the sample is taken as a measure of the inhibitory capacity of the sample against the specifically tested organism [40]. Minimum inhibitory concentration is determined by tube dilution method with inoculum to give 105 microorganisms per mL. The tested compounds in distilled water were diluted to provide final concentrations from 512 to 2 μg/mL. As the MIC, the lowest concentration of the antibacterial agent needed to inhibit the variable growth of the bacteria. This investigation was carried out at Fermentation Biotechnology and Applied Microbiology lab (FBAM, Al Azhar University, Cairo, Egypt).
Results and discussion
Preparation of (N'-alkyl N',N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium) iodide (10a–12a) and (N'-alkylN',N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium) bromide (10b–12b)
Cationic surfactants containing amide and hydrazinium groups and carrying morpholine or piperidine rings have been synthesized by opening the ring of succinic anhydride with a base (morpholine or piperidine) using methanol as solvent. Morpholino or piperidino oxobutanoate, when reacting with hydrazine hydrazide, produced corresponding 4-morpholino-4-oxobutane hydrazide and 4-oxo-4-piperidinobutane hydrazide which, when reacting with fatty alkyl bromide (dodecyl, tetradecyl, and hexadecyl), produced analogous N'alkyl-4-morpholino-4-oxobutane hydrazide and N'alkyl-4-oxo-4-piperidinobutane hydrazide, respectively. Subsequent quaternarization of the respective N'alkyl (4-morpholino or 4-piperidino) oxobutane hydrazide (7a–9b) with methyl iodide gave the corresponding cationic surfactants: (N'-alkyl N, N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium) iodide (10a–12a) and (N'-alkyl N, N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium) iodide (10b–12b) (Scheme 1).
Chemical structure confirmation
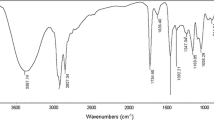
The progress in the reaction formation of hydrazide was detected by examining a small portion of the reaction mixture every 60 min until the rate of disappearance of the characteristic absorption band of the ester group increased. The FTIR spectrum was used to confirm the progress of the hydrazide formation reaction (Fig. 1a).
The structural verification is exemplified for N'-hexadecyl-4-morpholino-4-oxobutanehydrazide and N'-dodecyl-4-piperidine-4-oxobutanehydrazide derivatives (Fig. 2a, b).
In the study, 1HNMR was developed according to the chemical structures of the modern cationic surfactants. Chemical shifts for methyl protons of the fatty alkyl chain of hydrazinium surfactants occurred as a triplet between 0.90 and 0.99 ppm. The four butane protons of the two methylenes among carbonyl groups were found as a triplet of δ 2.63–2.68 ppm for, 10b, and 10a, respectively. The resonance of the two methyl protons immediately bound to the positive charge quaternary nitrogen that was detected as a singlet δ within 2.79–2.85 ppm for the cationic surfactants. A typical resonance signal for the hydrogen protons of the methylene group was directly bound to the positively charged quaternary nitrogen, which is a part of the fatty chain, and was within δ 3.13–3.21 ppm for the cationic amphiphiles. The methylene proton’s signals were next to the oxygen of the morpholine group, which are part of the hydrophilic part and were observed between δ 3.55 and 3.67 ppm for cationic surfactants. The resonance of one proton attached to nitrogen attached to the carbonyl group was shifted to the downfield affected by the adjacent quaternary nitrogen atom, which was observed for morpholino and piperidino cationic surfactants as a singlet at 5.71–7.19 ppm (Fig. 3a and b). The MS data obtained for all these current cationic surfactants confirmed a chemical construction. The parent-ion value for the surfactants was identified for both the positive ion without iodide ion for each surfactant atom. The calculated MALDI-TOF MS m/z values for morpholino surfactants 10a–12a were 398.338, 426.369, and 454. 401 and piperidino surfactants 10b–12b were 396.358, 424.390, and 452.421 for M-I which strictly matched the practical values for morpholino surfactants 10a–12a values of 398, 426, and 454 and for pipredino surfactants 10a–12b values of 396,424, and 452.
Surface activity
The surface properties of the new quaternary hydrazinium surfactant derived from morpholine and piperidine were examined via measuring the surface tension. CMC refers to the stage where a significant shift in slope happens on the curve. After the slope shift, the resulting lines and the corresponding slope lines indicate a decrease in the surface tension intersecting after a definite point, showing the CMC quantities for the surfactants (Fig. 4).
Variation in the surface tension of surfactants 10a–12a, 10b–12b vs. the concentration at 25 °C. For configurations, it includes scheme 1
It became clear that the molecular design of these quaternary hydrazinium surfactants should have a significant effect upon their surface behaviors. A CMC value for such cationic surfactants was estimated to rely on the size of the hydrophobic chain and the nature of the cationic head unit. Based on the data seen in Table 1, an increase in the fatty chain length (nonpolar tail) reduces the CMC of an investigated amphiphiles at 25 °C. The CMC values obtained for the quaternary hydrazinium piperidine series of 10b–12b at the same length of the hydrocarbon chain were lower than those obtained for the quaternary hydrazinium morpholine series of 10a–12a described here. Besides, the CMC values for these new quaternary hydrazinium-related cationic surfactants contradict with some other surfactants that depend on morpholine, piperidine, and traditional quaternary ammonium surfactants, which are equally efficient as other cationic compounds [25, 26]. γCMC is the highest surfactant capable of reducing water surface tension to be equal to the CMC value. Lowering the surface tension of the water happens as the surfactant monomers adsorb the air–water interface. That after a certain stage, the whole interface is filled by the monomers of the surfactant; therefore, there is no further decrease in the surface tension of the aqueous solution that will result from increasing the concentration of the surfactant. During this particular stage, the trend of the surface tension graph varies rapidly and is constant, and the resulting value is known as γmin. Values of γCMC were 40.15, 38.12, and 36.50 mN/m for the quaternary hydrazinium morpholine series 10a–12a and in the case of quaternary hydrazinium piperidine series 10b–12b, 38.91, 36.45, and 34.60 mN/m. The small variations in the γCMC values for the two series may be related to the design of the head group.
Certain surface parameters, i.e., surface excess concentration (Γmax), air–water interface molecular surface area (Amin), and surface tension decrease efficiency of 20 mN m−1 (C20) were derived from the quaternary hydrazinium surfactants surface tension graph (Table 1). Using the Gibbs adsorption isotherm Eq. 2, the maximum surface excess concentration at the air/water interface, i.e., Γmax, is calculated as follows:
From the previous equation, the gas constant R (8.31 J mol − 1 K‐1), the absolute temp of T, the surfactant C, and (dγ/d log C)T the slope of surface tension sections are below the CMC. The n is a fixed value determined by the number of singular ions which make up the adsorbed surfactant at the interface. The value of n would then be taken for cationic surfactants 2 as there is one counter ion linked to the ionic head group. Consistent adsorption of surfactant particles at the air–water interface with increasing surfactant concentrations induces a progressive reduction of the aqueous solution's surface tension. Continuous surfactant adsorption leads to high interface aggregation, and the surfactant monomer concentration at an interface is more concentrated than at the bulk solution. Γmax determines the region associated with the air–water interface concentration of quaternary hydrazinium surfactants and their value is dependent on the length of the hydrophobic chains and the size of the cationic head groups.
Using Eq. 3, the minimal area at the air–water interface occupied per surfactant molecule (Amin) has been calculated [41]:
where N is the number for Avogadro and where Amin is in nm2 (Table 1). Amin values for both the quaternary hydrazinium surfactants (10a–12b) synthesized throughout this study were found to be similar to those reported earlier for morpholine and piperidine surfactants [42]. A higher value of Γmax and a lower value of Amin indicate a denser structure of the surfactant particles on the surface of the solution [43]. It is evident from Table 1 that quaternary hydrazinium surfactants piperidine series 10b–12b have a significantly higher Γmax value and a prominent smaller Amin value than quaternary hydrazinium surfactants morpholine series 10a–12a, suggesting a denser structure of quaternary hydrazinium surfactants piperidine series 10b–12b at the air–water interface. This can be clarified by the conformational changes in the air–water interface owing to the repulsive force between the two lone pairs of electrons on the oxygen atom in the morpholine ring. A contrast between the values of Amin in both series shows that changes in the slopes are primarily due to the nature of the base ring influenced by the hydrazinuim head [41]. This was well recognized that the hydrophilic head group was a major inducer throughout the influence of the Γmax and Amin values of surfactants.
Certain significant surface-active parameters, like adsorption efficiency (pC20) and surface tension reduction efficiency (ΠCMC), also can be calculated from the surface tension chart. The pC20 is measured using Eq. 4:
In the equation, C is the surfactant's molar concentration and C20 is the surfactant concentration by which the pure solvent's surface tension is decreased by 20 mN m − 1. C20 is the lowest concentration arising from complete surface adsorption saturation [43]. Therefore, C20 can be used to test the adsorption potential of surfactant molecules at the air–water interface. Therefore, the higher the pC20 value, the higher the surfactant's adsorption performance. ΠCMC is the surface pressure at CMC, and this parameter measures the surfactant's effect on the surface tension of the pure solvent, i.e., water, as defined in Eq. 5:
(5) where γ0 is the surface tension of pure solvent and γCMC is the solution surface tension at CMC. ΠCMC establishes the maximum surface tension decrease and can be used to determine surfactant effectiveness to reduce water surface tension [44, 45]. So, the greater the surfactant's interest, the higher the surfactant's effectiveness. The pC20 and ΠCMC values are reported in Table 1. The variability of pC20 and ΠCMC suggested that both pC20 and ΠCMC increased as the hydrocarbon chain increased. The CMC/C20 ratio value is used to evaluate the main features of the adsorption and micellization process. A surfactant with a higher CMC/C20 ratio tends to adsorb more on the air–water interface than to form micelles in solutions. Table 1 shows that the surfactant with a longer fatty chain was easier to accumulate at the air–water interface than to self-assemble in a solution.
The data were consistent with the interpretation of the value pC20.
Study interaction of acid red BG with 12b cationic surfactant using the spectrophotometric technique
Figure 5a shows the UV/visible spectra of acid red BG in the absence (curve 1) and the presence of 12b (curves 2 to 13). In this case, (1.0 × 10 − 5 mol/L Red BG) the dye showed maximum absorption at 512.5 nm. The effect of various concentrations (0.1 to 4.5 × 10–4 mol/L) of 12b cationic surfactants (as an example) on Red BG fixed concentration absorption spectrum solubilization was analyzed and shown in Fig. 5a ( curves 2 to 13). Although the concentration of 12b slowly increased (0.1 to 0.17 × 10–4 mol/L), the size of the red BG band firstly decreases by the rise in the concentration of 12b well below the CMC (0.00019 mol/L) and reaches the minimum value and then increases for again with an additional rise in the surfactant beyond the CMC. Concentration is interpreted as CMC at the minimum observed (Fig. 5b). Red BG relates the initial decrease in intensity to the ion-pair interaction of red BG with the 12b. Once the 12b concentration rises to 0.00019 mol/L, a redshift with 548.7 nm bands is observed. This switch is likely a result of the red BG/12b micelles association [46]. A substantial increase in absorbance was found when the concentration of 12b (2.50 to 4.50 × 10–4 mol/L) was further added. The increase in absorbance in 12b above the CMC concentration can be due to the introduction of the red BG molecules into 12b micelles [47].
a Visible absorption spectra of acid red BG (1) in the presence of various 12b cationic surfactant concentrations (1 to 13). b The absorbance change of 1 × 10–5 mol/ L ( below and above the CMC) with the concentration of 12b surfactant. c The plot of DT/ A-Ao against 1/CM for acid red BG in 12b cationic surfactant
The penetration of dye particles into micelles is encouraged since cationic 12b attracts anionic dyes. Between the hydrated micelle surfaces up to the nonpolar heart, there is a continuum of climate. The solubilized components may be adsorbed to the surface and positioned there or become trapped in the center of hydrocarbons. Its polar and/or nonpolar groups are associated differently with the surfactant, based on their substituents. First, the dye-surfactant complex is produced and then adsorbed onto the surfaces of the micelle, gradually leading to the realignment of the dye molecules into the micelle. Long-range electrostatic forces and short-range hydrophobic forces work together to create a dye-surfactant matrix. The former are brought close together via dye and surfactant molecules, while the latter combine their hydrophobic elements in parallelly:
By time, these complexes undergo self-aggregation (as shown below), and subsequently, an increase in absorbance in the premicellar region is witnessed:
Once all the CMC dye molecules are interconnected as normal monomers in micelles, the absorbance reaches its limiting value:
In Eq. 8, DM is the reflection of dye monomers intercalated into the micelles. Solubilization is a dynamic process, and solubilization can lapse different instances of time of residence between core and surface at different rates. The hydrophilic and hydrophobic forces' imbalances make the solubilized components in the micelle somehow unpredictable, thus giving random absorbance values [48,49,50].
The dye–micelle complexes' binding constant Kb can be determined from the Benesi-Hildebrand equation (Eq. 9), as follows:
(9)
where DT is the dye concentration, ΔA = A-A0 is the difference between the absorption of the dye in the presence and the absence of the surfactant, \(\varepsilon_{M}\) is the molar extinguishing coefficient of dye that is completely bound to micelles (\(\varepsilon_{o} = 4126.32\) \({\text{L}}/ {\text{mol cm}})\),\(\varepsilon_{o}\) is the molar extinguishing coefficient of acid red BG (\(\varepsilon_{M} = 82100\;{\text{L}}/ {\text{mol }}\;{\text{cm}}\)), Kb is the binding constant, an \(C_{M }\) is the micellized surfactant concentration.
The binding constant (Kb) was calculated from the results of spectral measurements to be equal to 170.64 dm3 mol−1 (Fig. 5c) from the linear relationship between \(\frac{{D_{T} }}{\Delta A}\) against 1/CM.
Biodegradability
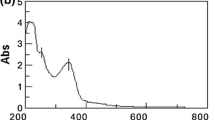
Biodegradation is the potential of living microorganisms for the biological degradation of organic materials. As surface-active agents are subject to biodegradation and to test their impact on the environment, the river die-away was used to investigate the biodegradability of the new quaternary hydrazinium–functionalized surfactants using the surface-tension technique as an analytical tool [39]. Surfactants can be classified as readily biodegradable if they can achieve 60% biodegradation under the Organization for Economic Co-operation and Development OECD guidelines [51]. The biodegradability results are described in Fig. 6. Under the experiment’s precision, all developed cationic surfactants tended to degrade quickly, and the level of biodegradation of various cationic surfactant solutions slowly increased by time, reaching maximum values in the river water after 14 days. The progressive increase in biodegradation is attributed to the reduction of the surface activity of the dissolved surfactants in river water. When biodegradation increased with hydrophobic chain lengths (fatty chains), the group showed increased linearity, and these data were consistent with the earlier study [52]. Furthermore, quaternary hydrazinium surfactants containing hydrazinium connection were investigated as amphiphiles containing a bond that breaks very easily, and these categories of surfactants were found to be partially or readily biodegradable when reviewed [53]. Thus, the results obtained show that these new cationic components can potentially be used to formulate large-performance cleaning products, stain remover, adhesives, fabric softeners, etc.
Biodegradation of cationic surfactants based on morpholine and piperidine investigated by the river die-away method using a surface-tension technique. For structures, see scheme 1
Antimicrobial activity of cationic hydrazinium surfactants
The cell membrane of microorganisms consists of various lipids (building units) and protein layers, in bilayer form, which imparts a hydrophobic character. Membrane permeability is the main function, namely the control of biological reactions in the cell. Any factor affecting the selective permeability of the cell membrane may significantly damage the microorganism. Cationic surfactants have a distinct ability to adsorb the most negatively charged cell membrane interface.
The potential antimicrobial activity of (N'-alkyl N', N'dimethyl-4-morpholino-4-oxobutanoylhydrazinium) iodide (10a–12a)and (N'-alkylN', N'dimethyl-4-piperidino-4-oxobutanoylhydrazinium) iodide (10b–12b) on gram-positive and gram-negative bacteria was evaluated in vitro by the determination of the inhibition zone (Fig. 7) and the minimum inhibitory concentration MIC (μg/mL) as shown in Table 2. The change in the biological activity (growth of the inhibition zone) cationic hydrazinium compounds depends on the nature of the hydrophobic tail connected to the quaternary nitrogen atom as well as the type of base (morpholine or piperidine) which forms the amide group [54]. The comparison of the sizes of inhibited growth zones calculated for the surfactants studied with those assessed for gentamycin used as control indicates that the newly developed cationic surfactants are highly active. Generally, compounds with long hydrocarbon chains and piperidine as a base showed lower MIC values against tested strains, similar to the piperidine derivatives study [55]. Moreover, it was shown that a compound with 16 carbon chains 12a and b has the strongest effect against S.aureus and B. Subtilis. Compared to conventional quaternary ammonium surfactants (QAS), these compounds display a medium degree of activity against bacteria with MIC values of 32–512 μg/mL[56]. Gram-negative P. aeruginosa was more resistant to the tested compounds, but by decreasing the chain length within compounds10a and b, an increase in their sensitivity was caused (MIC = 512). This could be explained by the different structure of the cell membrane of the two bacterial types. The external surface of the gram-negative bacteria is almost mainly composed of lipopolysaccharides and proteins which restrict the entrance of biocides as well as amphiphilic compounds [57]. The expected action of the synthetic cationic surfactant as an antibiotic focuses on the adhesion of the cell cytoplasmic membrane to the surfactants and on the contact between the positive head group of surfactants and the negatively charged membrane in which the fatty chain tail enters and interrupts the specific permeability of the cell wall resulting in cell damage [58, 59].
Bar graph for zone of inhibition vs. the synthesized cationic surfactants. For structures, see scheme 1
Conclusion
New cationic surfactants decrease the surface tension, interaction with dyes, are biodegradable, and inhibit the growth of microorganisms even when applied at low concentrations. The surface properties, as well as the antibacterial activity of the surfactants, depend on their structure and the length of the hydrophobic chain attached to the quaternary nitrogen atom. Surfactants, both morpholine and piperidine derivatives, with the highest carbon content in the alkyl chain (12a and b) were found to have the highest antimicrobial activity as well as the lowest CMC and the highest adsorption effectiveness among all surfactants considered. It is concluded that the anionic dyes acid red BG interacted with cationic surfactant 12b in aqueous media forming pairs of ions in the premicellar area despite the results obtained. The new complex was formed in the post micellar area of the surfactant due to the solubilization of the dye into a micelle. These categories of surfactants were found to be partially biodegradable and can be used to formulate high-performance cleaning products, stain removers, adhesives, softeners, etc.
References
J. Węgrzyńska, J. Chlebicki, I. Maliszewska, Preparation, surface-active properties and antimicrobial activities of bis(ester quaternary ammonium) salts. J. Surfactants Deterg. 10(2), 109–116 (2007)
L. Wang, H. Qin, L. Ding, S. Huo, Q. Deng, B. Zhao, L. Meng, T. Yan, Preparation of a novel class of cationic gemini imidazolium surfactants containing amide groups as the spacer: their surface properties and antimicrobial activity. J. Surfactants Deterg. 17(6), 1099–1106 (2014)
A. Kanazawa, T. Ikeda, T. Endo, Synthesis and antimicrobial activity of dimethyl- and trimethyl-substituted phosphonium salts with alkyl chains of various lengths. Antimicrob. Agents Chemother. 38(5), 945–952 (1994)
P. Foley, A. Kermanshahi Pour, E. Beach, J. Zimmerman, Derivation and synthesis of renewable surfactants. Chem. Soc. Rev. 41(4), 1499–1518 (2012)
L. Schramm, E. Stasiuk, D. Marangoni, 2-Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C: Phys. Chem. 99, 3–48 (2003)
Y. Guo, Y. Li, B. Zhi, D. Zhang, Y. Liu, Q. Huo, Effect of cationic surfactants on structure and morphology of mesostructured MOFs. RSC Adv. 2(12), 5424 (2012)
D. Asefi, M. Arami, N.M. Mahmoodi, Electrochemical effect of cationic gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid medium. Corros. Sci. 52, 794–800 (2010)
Q. Liu, M. Guo, Z. Nie, J. Yuan, J. Tan, S. Yao, Spacer-mediated synthesis of size-controlled gold nanoparticles using geminis as ligands. Langmuir 24, 1595–1599 (2008)
I. Badea, S. Wettig, R. Verrall, Foldvari, Topical non-invasive gene delivery using gemini nanoparticles in interferon-γ-deficient mice. M Eur. J. Pharm. Biopharm. 65, 414–422 (2007)
Y. Jiang, Y. Luan, F. Qin, L. Zhao, Z. Li, Catanionic vesicles from an amphiphilic prodrug molecule: a new concept for drug delivery systems. RSC Adv. 2, 6905–6912 (2012)
L. Perez, A. Pinazo, M.T. Garcia, M. Lozano, A. Manresa, M. Angelet, M.P. Vinardell, M. Mitjans, R. Pons, Infante, M. R. Cationic surfactants from lysine: synthesis, micellization and biological evaluation. Eur. J. Med. Chem. 44, 1884–1892 (2009)
C. Corless, G. Reynolds, N. Graham, R. Perry, T. Gibson, J. Haley, Aqueous ozonation of a quaternary ammonium surfactant. Water Res. 23(11), 1367–1371 (1989)
S. Giolando, R. Rapaport, R. Larson, T. Federle, M. Stalmans, P. Masscheleyn, Environmental fate and effects of DEEDMAC: a new rapidly biodegradable cationic surfactant for use in fabric softeners. Chemosphere 30(6), 1067–1083 (1995)
O. Kirk, F. Pedersen, C. Fuglsang, Preparation and properties of a new type of carbohydrate-based cationic surfactant. J. Surfactants Deterg. 1(1), 37–40 (1998)
T. Tatsumi, W. Zhang, T. Kida, Y. Nakatsuji, D. Ono, T. Takeda, I. Ikeda, Novel hydrolyzable and biodegradable cationic gemini surfactants: 1,3-bis[(acyloxyalkyl)-dimethylammonio]-2-hydroxypropane dichloride. J. Surfactants Deterg. 3(2), 167–172 (2000)
P.E. Hellberg, Ortho ester-based cleavable cationic surfactants. J. Surf. Deterg. 5, 217 (2002)
Z. Miao, J. Yang, L. Wang, Y. Liu, L. Zhang, X. Li, L. Peng, Synthesis of biodegradable lauric acid ester quaternary ammonium salt cationic surfactant and its utilization as calico softener. Mater. Lett. 62, 3450 (2008)
J. Lim, E. Kang, H. Lee, B. Lee, Synthesis and interfacial properties of ethoxylated cationic surfactants derived from n-dodecyl glycidyl ether. J. Ind. Eng. Chem. 22, 75–82 (2015)
A. Pinazo, M. Manresa, A. Marques, M. Bustelo, M. Espuny, L. Pérez, Amino acid–based surfactants: new antimicrobial agents. Adv. Coll. Interface. Sci. 228, 17–39 (2016)
E.M. Kandeel, M.R. El-Din, E.E. Badr, M.R. Mishrif, H.G. Nasr Mohamed, Synthesis and evaluation of new anionic Gemini dispersants as oil dispersants to treat crude oil spill pollution. J. Surfactants Deterg. 23(4), 753–770 (2020)
A. Bhadani, T. Misono, S. Singh, K. Sakai, H. Sakai, M. Abe, Structural diversity, physicochemical properties and application of imidazolium surfactants: recent advances. Adv. Coll. Interface. Sci. 231, 36–58 (2016)
P. Patial, A. Shaheen, I. Ahmad, Synthesis of ester based cationic Pyridinium Gemini surfactants and appraisal of their surface active properties. J. Surfactants Deterg. 16(1), 49–56 (2012)
A. Bhadani, T. Okano, T. Ogura, T. Misono, K. Sakai, M. Abe, H. Sakai, Structural features and surfactant properties of core–shell type micellar aggregates formed by Gemini piperidinium surfactants. Coll. Surf., A 494, 147–155 (2016)
B. Cai, X. Li, Y. Yang, J. Dong, Surface properties of gemini surfactants with pyrrolidinium head groups. J Coll Interface Sci 370, 111–116 (2012)
A. Bhadani, M. Tani, T. Endo, K. Sakai, M. Abe, H. Sakai, New ester based gemini surfactants: the effect of different cationic headgroups on micellization properties and viscosity of aqueous micellar solution. Phys. Chem. Chem. Phys. 17(29), 19474–19483 (2015)
E.E. Badr, Preparation, surface-active properties and antimicrobial activities of cationic surfactants based on morpholine and piperidine. Adv. Appl. Sci. Res. 8(2), 81–89 (2017)
R. Kamboj, S. Singh, V. Chauhan, Synthesis, characterization and surface properties of N-(2-hydroxyalkyl)-N-(2-hydroxyethyl)imidazolium surfactants. Coll Surf. A: Physicochem Eng. Asp. 441, 233–241 (2014)
Z.H. Asadov, A.H. Tantawy, I.A. Zarbaliyeva, R.A. Rahimov, Synthesis of new surface-active ammonium-type complexes based on palmitic acid for removing thin petroleum films from water surface. Egypt. J. Pet. 22(2), 261–267 (2013)
W.H. Ansari, N. Fatma, M. Panda, Kabir-ud-Din. , Solubilization of polycyclic aromatic hydrocarbons by novel biodegradable cationic gemini surfactant ethane-1,2-diyl bis(N, N-dimethyl-N-hexadecylammoniumacetoxy) dichloride and its binary mixtures with conventional surfactants. Soft Matter 9(5), 1478 (2013)
A.R. Tehrani-Bagha, K. Holmberg, Cationic ester-containing gemini surfactants: physical−chemical properties. Langmuir 26(12), 9276–9282 (2010)
J. Hoque, S. Gonuguntla, V. Yarlagadda, V.K. Aswal, J. Haldar, Effect of amide bonds on the self-assembly of gemini surfactants. Phys. Chem. Chem. Phys 16(23), 11279–11288 (2014)
M. Wang, Y. Han, F. Qiao, Y. Wang, Aggregation behavior of a gemini surfactant with a tripeptide spacer. Soft Matter 11(8), 1517–1524 (2015)
E.W. Schmidt, Hydrazine and Its Derivatives Preparation Properties and Applications (Wiley, New York, 1984).
B.N. Sivasankar, S. Govindarajan, Acetate and malonate complexes of cobalt(II), nickel(II) and zinc(II) with hydrazinium cation. J. Therm. Anal. 48(6), 1401–1413 (1997)
K.C. Patil, J.P. Vittal, C.C. Patel, Syntheses and thermal decompositions of hydrazinium salts. J. Therm. Anal. 26(2), 191–198 (1983)
A. Ali, F. Nabi, N.A. Malik, S. Tasneem, S. Uzair, Study of micellization of sodium dodecyl sulfate in non-aqueous media containing lauric acid and dimethylsulfoxide. J. Surfactants Deterg. 17(1), 151–160 (2013)
J. Ribe, J. Cegarra, L. Aizpurúa, The influence of ionic surface-active agents on the dyeing of hercosett wool with α-bromoacrylamide dyes. J. Soc. Dyers Colour. 99(12), 374–382 (2008)
A.M. Misselyn-Bauduin, A. Thibaut, J. Grandjean, G. Broze, R. Jérôme, Mixed micelles of anionic−nonionic and anionic−zwitterionic surfactants analyzed by pulsed field gradient NMR. Langmuir 16(10), 4430–4435 (2000)
J. Falbe, Surfactants in Consumer Products: Theory Technology and Application (Springer, Germany, 2012).
B. Brycki, Z. Dega-Szafran, I. Mirska, Synthesis and antimicrobial activities of some quaternary morpholinium chlorides. Pol. J. Microbiol. 59(1), 49–53 (2010)
M.J. Rosen, Surfactants and Interfacial Phenomena (Wiley-Interscience, 1989).
A. Sokołowski, J. Chlebicki, K.A. Wilk, Adsorption of oligooxypropylenated amines at the aqueous solution—air interface: homologous series of piperidine and morpholine derivatives. Coll. Surf. A: Physicochem. Eng. Asp. 80(2–3), 243–249 (1993)
M.T. Garcia, O. Kaczerewska, I. Ribosa, B. Brycki, P. Materna, M. Drgas, Hydrophilicity and flexibility of the spacer as critical parameters on the aggregation behavior of long alkyl chain cationic Gemini surfactants in aqueous solution. J. Mol. Liq. 230, 453–460 (2017)
E.M. Lee, R.K. Thomas, J. Penfold, R.C. Ward, Structure of aqueous decyltrimethylammonium bromide solutions at the air water interface studied by the specular reflection of neutrons. J. Phys. Chem. 93(1), 381–388 (1989)
P.A. Pires, O.A. El Seoud, Surfactants with an amide group “spacer”: Synthesis of 3-(acylaminopropyl)trimethylammonium chlorides and their aggregation in aqueous solutions. J. Coll. Interface Sci. 304(2), 474–485 (2006)
S. Shapovalov, V. Ponomariov, Interaction of dyes with cationic surfactants in solutions: determination of critical micelle concentration. Int. Lett. Chem. Phys. Astron. 81, 27–34 (2019)
N.A. Al-Omair, Thermodynamic study of interaction between a cationic surfactant and an anionic azo dye in aqueous solution. J. Indian Chem. Soc 93, 1–7 (2016)
H. Akbaş, T. Taner, Spectroscopic studies of interactions between C. I. reactive orange with alkyltrimethylammonium bromide surfactants. Spectrochimica Acta Part A: Mole. Biomole. Spectrosc. 73(1), 150–153 (2009)
M. Sarkar, S. Poddar, Studies on the interaction of surfactants with cationic dye by absorption spectroscopy. J. Coll. Interface Sci. 221(2), 181–185 (2000)
A.M. Khan, S.S. Shah, A UV-visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J. Dispers. Sci. Technol. 29(10), 1401–1407 (2008)
E.T. Eter, R.E. Richard, A. David, Biodegradable surfactants derived from corn starch. J Am Oil Chem Soc 51, 486–494 (1974)
T. Balson, M.S.B. Felix, The Biodegradability of Non-Ionic Surfactants Biodegradability of Surfactants (Springer, The Netherlands, 1995), pp. 204–230
D.A. Jaeger, J. Wettstein, A. Zafar, Cleavable quaternary hydrazinium surfactants. Langmuir 14, 1940 (1998)
K. Kuperkar, J. Modi, K. Patel, Surface-active properties and antimicrobial study of conventional cationic and synthesized symmetrical Gemini surfactants. J. Surfactants Deterg. 15(1), 107–115 (2011)
D. Wieczorek, A. Dobrowolski, K. Staszak, D. Kwaśniewska, P. Dubyk, Synthesis, surface and antimicrobial activity of piperidine-based Sulfobetaines. J. Surfactants Deterg. 20(1), 151–158 (2016)
M. Diz, A. Manresa, A. Pinazo, P. Erra, M. Infante, Synthesis, surface active properties and antimicrobial activity of new bis Quaternary ammonium compounds. J Chem Soc, Perkin Trans 2(8), 1871 (1994)
G. Oros, T. Cserháti, E. Forgács, Separation of the strength and selectivity of the microbiological effect of synthetic dyes by spectral mapping technique. Chemosphere 52(1), 185–193 (2003)
J.W. Costerton, K. Cheng, The role of the bacterial cell envelope in antibiotic resistance. J. Antimicrob. Chemother. 1(4), 363–377 (1975)
L. Tavano, M.R. Infante, M.A. Riya, A. Pinazo, M.P. Vinardell, M. Mitjans, M.A. Manresa, L. Perez, Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 9(1), 306–319 (2013)
Acknowledgements
Our appreciation and deep gratitude to Prof. Dr. M.S. Taher (Chemistry Department, Faculty of Science, Al-Azhar University,Nasr City, Cairo , Egypt) for his involvement and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilal, N.M., Badr, E., Gomaa, E.H. et al. Synthesis, characterization, interaction with anionic dye, biodegradability, and antimicrobial activity of cationic surfactants: quaternary hydrazinium derivatives. J IRAN CHEM SOC 18, 3047–3060 (2021). https://doi.org/10.1007/s13738-021-02247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02247-3