Abstract
New cationic surfactants, bis-quaternary ammonium salts, were prepared from N,N-dimethylaminoalkyl esters of saturated fatty acids and products of the reactions of epichlorohydrin with primary amines: pentyl-, hexyl- and octylamine. The bis (ester–ammonium) salts obtained were examined in respect to their surface-active properties: critical micelle concentration (CMC), effectiveness of surface tension reduction (γCMC), and adsorption efficiency (pC20). All these surfactants showed good water solubility and low critical micelle concentrations of more than two orders of magnitude lower than these of corresponding mono-alkylammonium salts. They also showed good wetting capability, but worse foaming properties. All the surfactants tested were nontoxic to gram-negative bacteria, but some of them inhibited the growth of gram-positive bacteria and yeast.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ammonium salts are an important group of cationic surfactants. Conventional ammonium salts (made up of one amphiphilic moiety) are widely used in many applications such as: fabric softeners, antielectrostatics, adhesion promoters in asphalt, corrosion inhibitors, dispersants, hair conditioners, and, of course, are used as germicides and biocides.
Bis-quaternary ammonium salts (made up of two amphiphilic moieties connected by a spacer) also belong to the group of cationic surfactants. These salts are more surface-active by some orders of magnitude than the comparable conventional ammonium salts containing one hydrophilic and one hydrophobic group. These salts have shown interesting physical properties such as very low critical micelle concentration, high viscoelasticity, detergency, high solubilization property, good wetting and foaming ability [1–3]. They are biologically active toward bacteria, yeasts and moulds [4–6]. In addition, these groups of cationic surfactants have excellent antistatic effects [7]. Consequently, bis-ammonium salts are potential compounds for the next generation of surfactants and they are attracting a lot of interest.
In the present paper, the synthesis and surface-active properties of bis (ester–ammonium) salts are described. Bis-ammonium salts, which contain cleavable ester groups show good surface-activity, are hydrolyzable and easily biodegradable [8]. Also, it is known that mono-ammonium salts, with an ester group in their molecule (esterquats) are widely used as softeners [9]. Therefore, in this paper, the antimicrobial activity and wetting ability of bis (ester–ammonium) salts are presented.
Experimental Procedures
Materials
All reagents used were commercially available. Amines: pentyl-, hexyl-, octylamine, (purity > 98%), N,N-dimethylethanolamine, N,N-dimethylpropanolamine (purity > 98%) were purchased from Fluka; decanoyl, lauroyl, mirystoyl chloride (purity 98%) were purchased from Aldrich. Epichlorohydrin (purity > 98%) was purchased from VEB Laborchemie Apolda and freshly distilled before synthesis. N,N-dimethylaminoisopropanol was prepared in our lab according to the method described in [10] from propylene oxide and N,N-dimethylamine.
Series of 2-dimethylaminoethyl esters of saturated fatty acids were synthesized by esterification of N,N-dimethylaminoethanol with alkanoyl chlorides [11]. Hydrochlorides of dimethylaminoethylesters were separated and purified by crystallization from a chloroform-hexane system; the purity of the crystallized hydrochlorides was over 99%. A free dimethylaminoester EA-m-n (see Scheme 1) was liberated from the hydrochlorides of dimethylaminoethylesters using a 10% NaHCO3 solution; the ethereal extract was dried over anhydrous MgSO4 and the solvent was evaporated to dryness. Analogously, series of 3-dimethylaminopropyl and 3-dimethylaminoisopropyl esters were prepared.
Surface tension versus the logarithm of the aqueous molar concentration (log C) of bis (ester–ammonium) salts at 25 °C. See Scheme 1 for compounds
Synthesis of bis(2-hydroxy-3-chloropropyl)alkylamine. General procedure. A mixture of epichlorohydrin (0.50 mol), alkylamine (0.20 mol) and methanol (50 cm3) was stirred at room temperature for 12 h. The solvent and unreacted epichlorohydrin were then evaporated under reduced pressure (12–15 mm Hg) at room temperature until a constant mass was achieved. The yields of the reaction products were almost quantitative (90–96%). A transparent and very viscous liquid was obtained and was used in subsequent synthesis without further purification. 1H NMR for selected compound bis(2-hydroxy-3-chloropropyl)hexylamine. 1H NMR (CDCl3) δ 0.88 (t, J = 6.9 Hz, 3H, CH 3CH2), 1.20–1.26 [m, 6H, CH3(CH 2)3], 1.50 [m, 2H, CH3(CH2)3CH 2], 2.55–2.79 [m, 6H, CH 2N(CH 2)CH 2], 3.56–3.70 [m, 4H, (CH 2CH)2], 3.98–4.07 [m, 2H, (CH)2], 4.46–4.58 [m, 2H, (OH)2].
The preparation of bis (ester–ammonium) salts bis (EA-m-n) R followed the typical procedure described in [7].
Bis{2-hydroxy-3-[2-(dodecanoyloxy)ethyldimethylammonio]propyl}pentylamine dichloride- [Bis (EA-11-2) C 5 ] was obtained as a pale-yellow, waxy material, yield = 83%. 1H NMR (CDCl3) δ 0.86 [t, J = 6.6 Hz, 9H, (CH 3CH2)3], 1.23–1.32 {m, 38H, [CH3(CH 2)8]2, CH3(CH 2)3}, 1.48 (m, 4H, (CH 2CH2C=O)2], 2.20 [m, 4H, (CH2CH 2C=O)2], 2.48–2.65 [m, 6H, CH 2N(CH 2)CH 2], 3.38–3.41 {s, 12H, [N+(CH 3)2]2}, 3.52 [t, 4H, (N+CH 2)2], 3.57–3.70 [m, 4H, (N+CH 2CH)2], 4.09–4.20 [m, 2H, (CH)2], 4.25–4.47 [m, 6H, (OH)2, (OCH 2CH2N+)2].
Bis{2-hydroxy-3-[2-(dodecanoyloxy)ethyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-11-2) C 6 ] was obtained as a pale-yellow, waxy material, yield = 85%. 1H NMR (CDCl3) δ 0.86 [t, J = 6.6 Hz, 9H, (CH 3CH2)3], 1.23–1.32 {m, 40H, [CH3(CH 2)8]2, CH3(CH 2)4}, 1.49 (m, 4H, (CH 2CH2C=O)2], 2.22 [m, 4H, (CH2CH 2C=O)2], 2.48–2.67 [m, 6H, CH 2N(CH 2)CH 2], 3.38–3.41 {s, 12H, [N+(CH 3)2]2}, 3.52 [t, 4H, (N+CH 2)2], 3.57–3.70 [m, 4H, (N+CH 2CH)2], 4.11–4.20 [m, 2H, (CH)2], 4.23–4.44 [m, 6H, (OH)2, (OCH 2CH2N+)2].
Bis{2-hydroxy-3-[2-(dodecanoyloxy)ethyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-11-2) C 8 ] was obtained as a pale-yellow, waxy material, yield = 80%. 1H NMR (CDCl3) δ 0.86 [t, J = 6.6 Hz, 9H, (CH 3CH2)3], 1.23–1.32 {m, 44H, [CH3(CH 2)8]2, CH3(CH 2)6}, 1.50 (m, 4H, (CH 2CH2C=O)2], 2.22 [m, 4H, (CH2CH 2C=O)2], 2.50–2.71 [m, 6H, CH 2N(CH 2)CH 2], 3.38–3.41 {s, 12H, [N+(CH 3)2]2}, 3.52 [t, 4H, (N+CH 2)2], 3.57–3.70 [m, 4H, (N+CH 2CH)2], 4.08–4.20 [m, 2H, (CH)2], 4.25–4.40 [m, 6H, (OH)2, (OCH 2CH2N+)2].
Bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}pentylamine dichloride- [Bis (EA-9-3) C 5 ] was obtained as a pale-yellow, waxy material, yield = 75%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.5 Hz, (CH 3CH2)3], 1.26 {m, 30H, [CH3(CH 2)6]2, CH3(CH 2)3}, 1.59 [m, 4H, (CH 2CH2C=O)2], 2.20 [m, 4H, (OCH2CH 2CH +2 N)2], 2.32 [m, 4H, (CH2CH 2C=O)2], 2.65 [m, 6H, CH 2N(CH 2)CH 2], 3.41 {s, 12H, [+N(CH 3)2]2}, 3.72 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.19 [m, 2H, (OH)2].
Bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-9-3) C 6 ] was obtained as a pale-yellow, waxy material, yield = 72%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.5 Hz, (CH 3CH2)3], 1.26 {m, 32H, [CH3(CH 2)6]2, CH3(CH 2)4}, 1.59 [m, 4H, (CH 2CH2C=O)2], 2.20 [m, 4H, (OCH2CH 2CH +2 N)2], 2.32 [m, 4H, (CH2CH 2C=O)2], 2.65 [m, 6H, CH 2N(CH 2)CH 2], 3.41 {s, 12H, [+N(CH 3)2]2}, 3.72 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.19 [m, 2H, (OH)2].
Bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-9-3) C 8 ] was obtained as a pale-yellow, waxy material, yield = 70%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.5 Hz, (CH 3CH2)3], 1.26 {m, 36H, [CH3(CH 2)6]2, CH3(CH 2)6}, 1.59 [m, 4H, (CH 2CH2C=O)2], 2.20 [m, 4H, (OCH2CH 2CH +2 N)2], 2.32 [m, 4H, (CH2CH 2C=O)2], 2.65 [m, 6H, CH 2N(CH 2)CH 2], 3.41 {s, 12H, [+N(CH 3)2]2}, 3.72 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.19 [m, 2H, (OH)2]. Anal. Calcd. for C44H91N3Cl2O6·2 H2O: C, 61.08; H, 10.96; N, 4.86; Cl, 8.19%. Found: C, 61.02; H, 10.96; N, 4.84; Cl, 8.11%.
Bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}pentylamine dichloride- [Bis (EA-11-3) C 5 ] was obtained as a pale-yellow, waxy material, yield = 85%. 1H NMR (CDCl3) δ 0.88 {m, 9H, J = 6.8 Hz, [CH 3(CH2)8]2, CH 3(CH2)4}, 1.26 {m, 38H, [CH3(CH 2)8]2, CH3(CH 2)3}, 1.57 [m, 4H, (CH 2CH2C=O)2], 2.15 [m, 4H, (OCH2CH 2CH +2 N)2], 2.33 [m, 4H, (CH2CH 2C=O)2], 2.67 [m, 6H, (CH 2N(CH 2)CH 2], 3.40 {s, 12H, [+N(CH 3)2]2}, 3.71 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.19 [m, 2H, (OH)2].
Bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-11-3) C 6 ] was obtained as a pale-yellow, waxy material, yield = 83%. 1H NMR (CDCl3) δ 0.87 {m, 9H, J = 6.7 Hz, [CH 3(CH2)8]2, CH 3(CH2)4}, 1.25 {m, 40H, [CH3(CH 2)8]2, CH3(CH 2)4}, 1.58 [m, 4H, (CH 2CH2C=O)2], 2.16 [m, 4H, (OCH2CH 2CH +2 N)2], 2.33 [m, 4H, (CH2CH 2C=O)2], 2.66 [m, 6H, CH 2N(CH 2)CH 2], 3.40 {s, 12H, [+N(CH 3)2]2}, 3.69 [m, 8H, (+NCH 2CH2CH2O)2, (CHCH +2 N)2], 4.14 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.40 [m, 2H, (OH)2]. Anal. Calcd. for C46H95N3Cl2O6·2.2 H2O: C, 61.61; H, 11.17; N, 4.68; Cl, 7.90%. Found: C, 61.66; H, 11.11; N, 4.60; Cl, 8.00%.
Bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-11-3) C 8 ] was obtained as a pale-yellow, waxy material, yield = 78%. 1H NMR (CDCl3) δ 0.87 {m, 9H, J = 6.6 Hz, [CH 3(CH2)8]2, CH 3(CH2)4}, 1.25 {m, 44H, [CH3(CH 2)8]2, CH3(CH 2)6}, 1.55 [m, 4H, (CH 2CH2C=O)2], 2.14 [m, 4H, (OCH2CH 2CH +2 N)2], 2.33 [m, 4H, (CH2CH 2C=O)2], 2.66 [m, 6H, CH 2N(CH 2)CH 2], 3.42 {s, 12H, [+N(CH 3)2]2}, 3.73 [m, 8H, (+NCH 2CH2CH2O)2, (CHCH +2 N)2], 4.15 [m, 6H, (OCH 2CH2CH +2 N)2, (CH)2], 4.41 [m, 2H, (OH)2]. Anal. Calcd. for C48H99N3Cl2O6·2 H2O: C, 62.57; H, 11.27; N, 4.56; Cl, 7.70%. Found: C, 62.50; H, 11.17; N, 4.50; Cl, 7.66%.
Bis{2-hydroxy-3-[3-(tetradecanoyloxy)propyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-13-3) C 8 ] was obtained as a pale-yellow, waxy material, yield = 73%. 1H NMR (CDCl3) δ 0.89 {m, 9H, J = 6.3 Hz, [CH 3(CH2)10]2, CH 3(CH2)6}, 1.20–1.27 {m, 52H, [CH3(CH 2)10]2, CH3(CH 2)6}, 1.61 {m, 4H, [O=CCH2CH 2(CH2)10]2}, 2.33–2.45 [m, 4H, (O=CCH 2CH2)2], 2.63–2.81 [m, 6H, CH 2N(CH 2)CH 2], 3.50 {s, 12H, [N+(CH 3)2]2}, 3.72 {m, 8H, (N+CH 2CH2O)2, (N+CH 2CH)2], 3.94–4.27 [m, 6H, (N+CH2CH 2O)2, (CH)2], 4.59 [m, 2H, (OH)2].
Bis{2-hydroxy-3-[3-(decanoyloxy)isopropyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-9-3 iso ) C 6 ] was obtained as a pale-yellow, waxy material, yield = 74%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.5 Hz, (CH 3CH2)3], 1.23–1.29 {m, 32H, [CH3(CH 2)6]2, CH3(CH 2)4}, 1.59–1.61 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.31–2.37 [m, 4H, (CH2CH 2C=O)2], 2.50 [m, 6H, CH 2N(CH 2)CH 2], 3.35 {s, 12H, [+N(CH 3)2]2}, 3.67 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.10–4.14 [m, 4H, (CH)2, (OH)2], 5.15–5.19 {m, 4H, [OCH(CH3)CH2]2}.
Bis{2-hydroxy-3-[3-(decanoyloxy)isopropyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-9-3 iso ) C 8 ] was obtained as a pale-yellow, waxy material, yield = 78%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.6 Hz, (CH 3CH2)3], 1.24–1.28 {m, 38H, [CH3(CH 2)6]2, CH3(CH 2)6}, 1.59–1.61 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.28–2.36 [m, 4H, (CH2CH 2C=O)2], 2.45–2.59 [m, 6H, CH 2N(CH 2)CH 2], 3.35 {s, 12H, [+N(CH 3)2]2}, 3.70 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.08–4.13 [m, 4H, (CH)2, (OH)2], 5.12-5.18 {m, 4H, [OCH(CH3)CH2]2}. Anal. Calcd. for C44H91N3Cl2O6·0.8 H2O: C, 62.66; H, 11.06; N, 5.00; Cl, 8.41%. Found: C, 62.70; H, 11.09; N, 4.94; Cl, 8.56%.
Bis{2-hydroxy-3-[3-(dodecanoyloxy)isopropyldimethylammonio]propyl}pentylamine dichloride- [Bis (EA-11-3 iso ) C 5 ] was obtained as a pale-yellow, waxy material, yield = 83%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.6 Hz, (CH 3CH2)3], 1.24–1.29 {m, 38H, [CH3(CH 2)6]2, CH3(CH 2)3}, 1.59–1.63 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.28–2.36 [m, 4H, (CH2CH 2C=O)2], 2.45–2.61 [m, 6H, CH 2N(CH 2)CH 2], 3.35 {s, 12H, [+N(CH 3)2]2}, 3.70 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.04–4.10 [m, 4H, (CH)2, (OH)2], 5.11–5.16 {m, 4H, [OCH(CH3)CH2]2}.
Bis{2-hydroxy-3-[3-(dodecanoyloxy)isopropyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-11-3 iso ) C 6 ] was obtained as a pale-yellow, waxy material, yield = 80%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.7 Hz, (CH 3CH2)3], 1.22–1.26 {m, 40H, [CH3(CH 2)8]2, CH3(CH 2)4}, 1.58–1.61 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.30–2.34 [m, 4H, (CH2CH 2C=O)2], 2.40–2.51 [m, 6H, CH 2N(CH 2)CH 2], 3.41 {s, 12H, [+N(CH 3)2)]2}, 3.70 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11–4.29 [m, 4H, (CH)2, (OH)2], 5.12–5.17 {m, 4H, [OCH(CH3)CH2]2}.
Bis{2-hydroxy-3-[3-(dodecanoyloxy)isopropyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-11-3 iso ) C 8 ] was obtained as a pale-yellow, waxy material, yield = 82%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.7 Hz, (CH 3CH2)3], 1.22–1.26 {m, 44H, [CH3(CH 2)8]2, CH3(CH 2)6}, 1.58–1.61 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.29–2.35 [m, 4H, (CH2CH 2C=O)2], 2.58–2.66 [m, 6H, CH 2N(CH 2)CH 2], 3.43 {s, 12H, [+N(CH 3)2]2}, 3.72 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11–4.13 [m, 4H, (CH)2, (OH)2], 5.07–5.13 {m, 4H, [OCH(CH3)CH2]2}. Anal. Calcd. for C48H99N3Cl2O6·1.5 H2O: C, 63.20; H, 11.27; N, 4.61; Cl, 7.77%. Found: C, 63.23; H, 11.21; N, 4.57; Cl, 7.69%.
Bis{2-hydroxy-3-[3-(tetradecanoyloxy)isopropyldimethylammonio]propyl}hexylamine dichloride- [Bis (EA-13-3 iso ) C 6 ] was obtained as a pale-yellow, waxy material, yield = 56%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.7 Hz, (CH 3CH2)3], 1.22–1.26 {m, 48H, [CH3(CH 2)10]2, CH3(CH 2)4}, 1.59–1.61 {m, 10H, (CH 2CH2C=O)2, [OCH(CH 3)CH2]2}, 2.29–2.38 [m, 4H, (CH2CH 2C=O)2], 2.67 [m, 6H, CH 2N(CH 2)CH 2], 3.45 {s, 12H, [+N(CH 3)2]2}, 3.67 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11–4.13 [m, 4H, (CH)2, (OH)2], 5.10–5.15 {m, 4H, [OCH(CH3)CH2]2}.
Bis{2-hydroxy-3-[3-(tetradecanoyloxy)isopropyldimethylammonio]propyl}octylamine dichloride- [Bis (EA-13-3 iso ) C 8 ] was obtained as a pale-yellow, waxy material, yield = 61%. 1H NMR (CDCl3) δ 0.88 [m, 9H, J = 6.7 Hz, (CH 3CH2)3], 1.22–1.26 {m, 50H, [CH3(CH 2)10]2, CH3(CH 2)6}, 1.59–1.61 {m, 10H, (CH 2CH2C=O)2, ([OCH(CH 3)CH2]2}, 2.29–2.35 [m, 4H, (CH2CH 2C=O)2], 2.59–2.67 [m, 6H, CH 2N(CH 2)CH 2], 3.41 {s, 12H, [+N(CH 3)2]2}, 3.56–3.67 [m, 8H, (+NCH 2CH2CH2O)2, (CH2CH +2 N)2], 4.11–4.13 [m, 4H, (CH)2, (OH)2], 5.08–5.12 {m, 4H, [OCH(CH3)CH2]2}. Anal. Calcd. for C52H107N3Cl2O6·2 H2O: C, 63.90; H, 11.24; N, 4.30; Cl, 7.26%. Found: C, 63.83; H, 11.22; N, 3.97; Cl, 7.19%.
Analytical Method
The structures of the prepared compounds were confirmed by their spectral data. 1H-NMR (300 MHz) spectra were recorded on a Bruker DRX 300 spectrometer using CDCl3 solutions and TMS (tetramethylsilane) as the internal reference. Elemental analyses were carried out using a VarioEl III CHNS analyzer. The surface tension of the aqueous solutions of the bis (ester–ammonium) salts was measured by the Wilhelmy plate method at 25 °C. Foaming abilities were determined at 2 g/dm3 and 25 °C according to the Ross–Miles method. Foam stabilities were determined by comparing height at 10 min to the initial foam height in the Ross-Miles device. Wetting ability was determined at 2 g/dm3 and 25 °C according to Polish standard, PN-74/C 04800, and is defined as the time needed to sink a standard cotton fabric circle.
Antimicrobial Activity
Antimicrobial activity of bis (ester–ammonium) salts against various prokaryotic and eukaryotic microorganisms was evaluated by a dilution method. Nutrient agar and mycological agar were used for bacteria and yeast, respectively.
The compounds studied were dissolved in distilled water and after sterilization (filtration, 0.45 μm, Millipore), a half millilitre of each concentration of the compound was added to the warm medium to give a final concentration range 512–8 μg/mL. The liquid media were then poured into Petri dishes and after solidification were individually inoculated with 20 μL of cell suspensions each containing separately tested microorganisms.
Inocula of the bacteria and yeasts were prepared by growing the microorganisms overnight in the nutrient broth or mycological broth and diluting cultures to approximately 106 cfu (colony forming units/mL). Gram-positive bacteria species: Staphylococcus aureus PCM 1944, Bacillus subtilis PCM 1949, and gram-negative rods: Escherichia coli PCM 2057, Serratia marcescens PCM 549 and wild yeast: Rhodotorula rubra was used for the test.
The incubation was carried out for 48 h at 37 °C for gram-positive bacteria and for 48 h at 28 °C for other species. The antimicrobial activity of the bis (ester–ammonium) salts tested was determined on the basis of their MIC. Minimal inhibitory concentrations (MIC) are defined as the lowest concentration of compounds at which the microorganisms tested do not show visible growth.
Results and Discussion
Synthesis
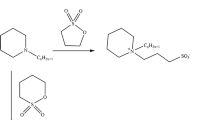
Bis (ester–ammonium) salts were prepared in two-step reactions as shown in Scheme 1, according to the procedure described in [4, 7].
The first step is the preparation of dichloroamines as the intermediate. These compounds were almost quantitatively obtained at room temperature due to the high reactivity of the primary amine with two molecules of epichlorohydrin. Pentyl-, hexyl- and octylamine were used as the primary amine. The second step is the quaternization reaction of N, N-dimethylaminoalkyl esters of saturated fatty acids [ω-(alkanoyloxy)alkyldimethylamine, EA-m-n] with the prepared dichlorides. 2-(decanoyloxy)-, 2-(dodecanoyloxy)-, 2-(tetradecanoyloxy)ethyldimethylamine, 3-(decanoyloxy)-, 3-(dodecanoyloxy)-, 3-(tetradecanoyloxy)propyldimethylamine and 3-(decanoyloxy)-, 3-(dodecanoyloxy)-, 3-(tetradecanoyloxy)isopropyldimethylamine were used as ω-(alkanoyloxy)alkyldimethylamine. The bis (ester–ammonium) dichlorides can be purified by washing them with hexane or diethyl ether, and recrystallization from a mixture of 2-propanol and acetone. However, after the evaporation of the solvents, the final products are highly hygroscopic, so even after drying in the desiccator over P2O5 and CaCl2, the salts are amorphous solids.
Surface-Active Properties
The surface-active properties of the prepared bis (ester–ammonium) salts are summarized in Table 1. Also, plots of the surface tension against the molar concentration are shown in Fig. 1.
It can be seen from Table 1 that the values of the critical micelle concentration of the salts obtained are lower by two to three orders of magnitude than that of DTAC (dodecyltrimethylammonium chloride, CMC = 12 mM), and sometimes even lower than that of gemini salts [3, 7, 12]. In a previous work [7], bis- and tris-ammonium salts were investigated with this same spacer, but with dodecyl chains at the ammonium groups. The CMC values of those salts were about 10−4 mol/dm3, and those values hardly depended on the length of alkyl chain at the central nitrogen atom. For bis (ester–ammonium) salts, there is a significant influence of the length of oxycarbonyl groups on the CMC.
A relationship between the number of carbon atoms in the hydrophobic chain and the CMC for a homologues series of the quaternary ammonium salts is described by the following equation: log CMC = A − Bn, where A and B are constant [13]. Figure 2 shows the relationship between the carbon number in the hydrophobic group and CMC for multiple ammonium salts obtained . The constant B for bis (EA-11-2) R salts is 0.068 (the correlation coefficient is 0.988), for bis (EA-11-3) R salts it is 0.025 (the correlation coefficient is 0.971) and for bis (EA-11-3 iso ) R salts it is 0.021 (the correlation coefficient is 0.993). The correlation coefficients obtained show good linearity between them. The decrease in the CMC with an increase in total carbon number is smaller for salts bis (EA-11-3) R and bis (EA-11-3 iso ) R than for salts bis (EA-11-2) R (about three times). Also the constant B for compounds bis (EA-11-2) R is smaller than the value B for bis-ammonium salts [7], which was equal to 0.085. This slight relationship between the CMC and the total carbon number for the bis (ester–ammonium) salts studied is probably caused by the weakly hydrophilic properties of oxycarbonyl groups.
Plots of log CMC versus the total carbon number in alkyl chains for bis (ester-ammonium) salts. Series 1 (open circles), bis (EA-11-2) C5; (filled circles), bis (EA-11-2) C6; (open squares), bis (EA-11-2) C8. Series 2 (open circles), bis (EA-11-3) C5; (filled circles), bis (EA-11-3) C6; (open squares), bis (EA-11-3) C8. Series 3 (open circles), bis (EA-11-3iso) C5; (filled circles), bis (EA-11-3iso) C6; (open squares), bis (EA-11-3iso) C8. See Scheme 1 for compounds
The length of oxycarbonyl groups also affected the values of γCMC and the effectiveness of surface tension reduction—pC20. From the data in Table 1, the highest efficiency in lowering the surface tension of water is observed for the compounds bis (EA-11-2) R. This efficiency is similar to the effect of salts with dodecyl chains at the quaternary center [7]. The highest values of γCMC were for the series of bis (EA-m-3 iso ) R, which is probably caused by the difficulties in adsorption on interface air/water because of dimethylaminoisopropanol groups and their steric hindrance in packing at the interface.
The pC20 is defined as the negative logarithm of the surfactant concentration in the bulk phase required to reduce the surface tension of the water by 20 mN/m and it represents the efficiency of surfactant adsorption on an air–water interface. The pC20 values of all investigated bis (ester–ammonium) salts are rather high and comparable with surface-active properties of gemini ammonium salts.
Bis-ammonium salts have been also reported to have better solubilizing, wetting, and foaming properties than conventional surfactants [14]. Also bis (ester–ammonium) salts described earlier [12], had good foaming ability and high foaming stability. In the present case, the foam ability of the salts obtained was poor. The worst foaming ability was shown for the group of bis (EA-m-3 iso ) R. No relationship was observed between the foaming ability and the length of the oxycarbonyl groups.
The wetting ability was measured as the time needed to wet and subsequently sink a special cotton material. Wetting times of the salts studied were short, meaning that all the salts obtained had a good wetting ability. Wetting time decreased with increasing alkyl chain length at the central nitrogen atom in the molecule (Table 1). Surprisingly, the shortest times were shown by bis (EA-m-3 iso ) R. Despite having the same molecular weight bis (EA-m-3 iso ) R and bis (EA-m-3) R had different wetting times.
Antimicrobial Activity
The antimicrobial activity of bis (ester–ammonium) salts on various prokaryotic and eukaryotic microorganisms was evaluated by determining the minimal inhibitory concentration (μg/mL), the values for which are given in Table 2.
All the compounds studied were completely inactive toward gram-negative bacteria (E. coli, S. marcescens) up to the concentration of 512 μg/mL. However, the ammonium salts investigated inhibited the growth of gram-positive bacteria slightly. The most sensitive was the B. subtilis strain. The MIC values for the majority of salts investigated, in this case, were 256 μg/mL. A relationship between antimicrobial activity and chemical structure of bis (ester–ammonium) salts was observed. All the salts bis (EA-m-3 iso ) R are more active toward B. subtilis and S. aureus, but when m = 13, these compounds are less toxic (MIC ≥ 512 μg/mL). Also, salt bis (EA-13-3) C 8 is inactive toward gram-positive bacteria. So, it can be inferred that the increase in acyl chain length caused the decrease in antimicrobial activity toward gram-positive bacteria. This relationship is well known for cationic gemini ammonium salts with alkyl chains [6]. Devinsky observed that ammonium salts with alkyl chains above C12 had higher MIC values than salts with alkyl chain C10–C12.
In the case of the effect of the alkylene length between the oxycarbonyl group and the ammonium group, salts of bis (EA-m-3 iso ) R are more active than bis (EA-m-2) R and bis (EA-m-3) R.
It is also noteworthy that among the salts studied the most active were bis (EA-9-3 iso ) C 8 , bis (EA-11-3 iso ) C 5 , bis (EA-11-3 iso ) C 6 and bis (EA-11-3 iso ) C 8 . These compounds inhibited the growth of cocci S. aureus and the endospore-forming rod B. subtilis at a concentration of 256 μg/mL.
It is significant that all salts bis (EA-m-n) R studied are less active toward gram-positive bacteria than the corresponding bis-ammonium salts with dodecyl chains at the ammonium groups [5]. The MIC values of those salts were equal to 128 μg/mL for S. aureus and B. subtilis. It supports the thesis that esterquats have low ecotoxicity. Also, indirect products EA-m-2, after quaternization by CH3Br, were investigated in previous studies [15]. For example MIC values of (CH3)3N+CH2CH2OCOC11H23•Br− toward S. aureus was 150 mM, B. subtilis MIC > 1,000 mM and E. coli MIC < 30 mM, which means that the spacer between the ammonium groups also has an effect on antimicrobial activity. The ammonium salts investigated did not have an antifungal activity.
References
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203
Cationic surfactants: Physical chemistry (1991) Marcel Dekker, New York
Tatsumi T, Zhang W, Nakatsuji Y, Miyake K, Matsushima K, Tanaka M, Furuta T, Ikeda I (2001) Preparation, surface-active properties and antimicrobial activities of bis(alkylammonium) dichloride having a butenylene or a butynylene spacer. J Surfact Deterg 4:271
Chlebicki J, Węgrzyńska J, Oświęcimska M, Maliszewska I (2005) Preparation, surface-active properties, and antimicrobial activities of bis-quaternary ammonium salts from amines and epichlorohydrin. J Surfact Deterg 8:227
Węgrzyńska J, Chlebicki J, Maliszewska I (2006) Synthesis and antimicrobial activity of multiple quaternary ammonium salts. Pol J Chem Technol 8:59
Devinsky F, Lacko I, Mlynarcik D, Racansky V, Krasnec L (1985) Relationship between critical micelle concentrations and minimum inhibitory concentrations for some non-aromatic quaternary ammonium salts and amine oxide. Tenside Deterg 22:10
Węgrzyńska J, Chlebicki J (2006) Surface-active and antielectrostatic properties of multiple quaternary ammonium salts. J Surfact Deterg 9:221
Tatsumi T, Zhang W, Kida T, Nakatsuji Y, Ono D, Takeda T, Ikeda I (2000) Novel hydrolyzable and biodegradable cationic gemini surfactants: 1,3-bis [(acyloxyalkyl)-dimethylammonio]-2-hydroxypropane dichloride. J Surfact Deterg 3:167
Puchta R, Krings P, Sandkühler P (1993) A new generation of softeners. Tenside Surf Deterg 30:186
Ślipko K, Chlebicki J (1979) Reactions of propylene oxide with amines. Pol J Chem 11:2231
Obłak E, Lachowicz TM, Sauter E, Łuczyński J, Witek S (2001) Comparative studies of the biological activities of lysosomotropic aminoesters and quaternary ammonium salts on the yeast Saccharomyces cerevisiae. Cell Mol Biol Lett 6:871
Tatsumi T, Zhang W, Kida T, Nakatsuji Y, Ono D, Takeda T, Ikeda I (2001) Novel hydrolizable and biodegradable cationic gemini surfactants: bis(ester–ammonium) dichloride having a butenylene or a butynylene spacer. J Surfact Deterg 4:279
Klevens HB (1948) Critical micelle concentration as determined by refraction. J Phys Colloid Chem 52:130
Rosen MJ (1993) Chem Technol 30:23
Łuczyński J, Czarny A Witek S (2003) Antimicrobial activity of selected cationic surfactants, Scientific conference May 20–23, 2003. In: Wilk KA (ed) Polanica Zdrój, surfactants and dispersed systems in theory and practice. Science Printing House Wrocław University of Technology, Wrocław, pp 357–361
Acknowledgments
Support of this work by the MNiI, Grant no 3 T09B 065 28 is gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Węgrzyńska, J., Chlebicki, J. & Maliszewska, I. Preparation, Surface-Active Properties and Antimicrobial Activities of Bis(Ester Quaternary Ammonium) Salts. J Surfact Deterg 10, 109–116 (2007). https://doi.org/10.1007/s11743-007-1020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-007-1020-z