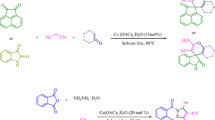
Abstract
This article aimed to present two facile and environmental friendly routes for the rapid assembly of biologically active compounds including pyrazol core using aspirin as a novel and green catalyst. The synthesis of bis(pyrazol-5-ol) derivatives was developed via one-pot, pseudo-five-component condensation, and the target dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles were prepared by one-pot, four-component reaction. These reactions can be performed in tandem from readily available starting materials. The main merits of the present methods are operational simplicity, no need for column chromatography, inexpensive materials, avoidance of harmless and corrosive acid catalysts, short reaction times, good yields of the products, and utilization of aspirin as a non-toxic, cheap, commercially available, and efficient catalyst.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, one of enduring challenges facing chemists is protection of our environment as an endowment of nature. Thus, shifting to the extension of methods that decrease the consumption of hazardous materials has an utmost priority. Solvent and type of catalyst along with notable parameters such as atom and step economy of process as well as nature of by-products are the main aspects in designing green synthetic routes for the synthesis of prominent heterocyclic architectures of medicinal connection. In this context, multicomponent reactions (MCRs) have served as pivotal synthetic procedures toward preparation of assemble libraries of various drug-like chemical entities [1,2,3,4,5,6].
Pyrazoles and pyranopyrazoles as N-fused heterocyclic compounds have exhibited a situation of prominence with a broad spectrum of biological and pharmacological activities such as anti-pyretic [7] analgesic [8], anti-microbial [9], anti-fungal [10], anti-inflammatory [11], anti-anxiety [12], anti-proliferative, and anti-tumor [13, 14]. Some compounds such as celecoxib a NSAIDs (anti-inflammatory and analgesic agent) [15], ENMD-2076 and R1530 (anti-angiogenic) [16], PNU-32945 (HIV-1 non-nucleoside reverse transcriptase inhibitor) [17], and sulfaphenazole (anti-bacterial) [18] have been manufactured as commercial drugs (Fig. 1). 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) as anti-viral agents restrain the peste des petits ruminants virus (PPRV) [19]. Recently, the α-glucosidase inhibitory activity of dihydropyrano[2,3-c]pyrazoles has been reported as a useful factor to reduce post-prandial hyperglycemia in diabetic individuals [20]. Also, these classes of compounds have served as valuable synthetic intermediates [21].
Not surprisingly, due to existence of these precious scaffolds in biologically functional molecules, considerable efforts have been documented toward the synthesis of these heterocyclic motifs using various methodologies. In the literature, synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) has occurred in the presence of catalysts namely pyridine trifluoroacetate [22], ZnAl2O4 NPs [23], sodium dodecyl sulfate [24], and N-methylimidazolium perchlorate [25]. On the other hand, some approaches for synthesis of dihydropyrano [2,3-c] pyrazoles involve the use of catalysts such as [MNP-PIm-SO3H]Cl [26], Fe3-xTixO4@SO3H MNPs [27], β-cyclodextrin [28], lemon juice [29], morpholine triflate [30], triphenylphosphine [31], γ-alumina [32], imidazole [33], [ChCl][ZnCl2]2 [34], and cerium ammonium nitrate (CAN) [35].
Over the last decade, organocatalytic methods have been extensively employed in medicinal chemistry for designing multicomponent and cascade reactions due to the need for convenience, generality, and robustness catalysts for the synthesis of complex molecular motifs without the presence of any metal atoms. Therefore, the development of new and versatile catalysts is beneficial for chemists [36]. Acetylsalicylic acid or aspirin is a simple chemical compound which was produced by Hoffmann. Pharmacological effects of aspirin include analgetic, anti-pyretic, anti-tumor, anti-inflammatory [37], anti-platelet [38], anti-thrombotic [39], and anti-oxidan [40]. Aspirin as a non-steroidal anti-inflammatory drug (NSAIDs) has earned remarkable position in the prevention of myocardial infarction, stroke, dementia, and schizophrenia [41].
In a continuation of our endeavors toward the development of green catalytic fashion for important organic conversions, [42,43,44,45,46] herein, we wish to report an efficient one-pot, pseudo-five-component strategy for the rapid synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) from reaction between ethyl acetoacetate 1, hydrazine monohydrate 2, and aldehydes 3 and also an efficient one-pot, four-component strategy for effective synthesis of dihydropyrano[2,3-c]pyrazole, and spiroindoline-pyranopyrazole derivatives by condensation of hydrazine monohydrate 1, ethyl acetoacetate 2, malononitrile derivatives 5, and arylaldehydes 3 or isatins in the presence of aspirin as a catalyst in an environmentally benign conditions (Scheme 1).
The combination of readily available substrates with a non-toxic, cheap, commercially available, and efficient catalyst features would offer an attractive gateway to assemble a library of heterocyclic with pyrazole motifs.
Experimental
General
Melting points and IR spectra of all compounds were determined using an Electro thermal 9100 apparatus and FT-IR-JASCO-460 plus spectrometer. The 1H and 13C NMR spectra of known compounds were recorded on a Bruker DRX-300 and 400 Avance instrument in DMSO at 300, 400, and 75 MHz. All chemicals were provided from the chemical producer Merck (Darmastadt, Germany) and Fluka (Buchs, Switzerland) and used without further purification. Aspirin was prepared by procedure reported by Palleros [51].
General procedure for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)derivatives
A mixture of ethyl acetoacetate (2.0 mmol), hydrazine hydrate (2.0 mmol), and aspirin (15 mol%) as catalyst was stirred in EtOH/H2O (3 mL). After 5 min, aromatic aldehyde (1.0 mmol) was added, and the mixture was stirred at 60 °C for the appropriate time. The completion of the reaction was monitored through thin layer chromatography (TLC). Finally, the reaction mixture was cooled to room temperature, and then ethanol (5 mL) was added to the mixture of reaction, and filtered to separate the product. Finally, the crude product was recrystallized from ethanol to afford the pure product.
General procedure for the synthesis of dihydropyrano[2,3-c]pyrazole and spiroindoline-pyranopyrazole derivatives
A mixture of hydrazine hydrate (1.0 mmol) and ethyl acetoacetate (1.0 mmol) was stirred for 5 min until 3-methyl-2-pyrazolin-5-one was precipitated. Aromatic aldehydes (1.0 mmol) or isains (1.0 mmol), malononitrile derivatives (1.0 mmol), and aspirin (20 mol%) were then added, and the mixture was heated to 80 °C under solvent-free conditions. The progress of the reaction was monitored by TLC. Then, the reaction mixture was cooled to room temperature. The mixture was washed with EtOH for separating the product. Finally, the crude product was recrystallized from ethanol to afford the pure pyranopyrazole derivatives.
Spectral data for the selected compounds
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4b )
Yield: (93%). White powder, mp: 269–271 °C; IR (KBr) (νmax, cm−1): 3428, 3145, 2928, 1597, 1518; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.10 (s, 6H, 2CH3), 3.35 (2OH exchanged with water of DMSO-d6), 4.99 (s, 1H, CH), 7.39 (d, J = 8.4 Hz, 2H, H-Ar), 8.13 (d, J = 8.8 Hz, 2H, H-Ar), 11.36 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(3-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4c )
Yield: (89%). White powder, mp: 254–257 °C; IR (KBr) (νmax, cm−1): 3405, 3095, 2979, 1600, 1528; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.11 (s, 6H, 2CH3), 3.39 (2OH exchanged with water of DMSO-d6), 4.99 (s, 1H, CH), 7.02–7.23 (m, 4H, H-Ar), 11.34 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-chlorophenyl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4e )
Yield: (92%). White powder, mp: 215–217 °C; IR (KBr) (νmax, cm−1): 3392, 3180, 2925, 1601, 1521, 1488; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.08 (s, 6H, 2CH3), 3.39 (2OH exchanged with water of DMSO-d6), 4.81 (s, 1H, CH), 7.12 (d, J = 8.4 Hz, 2H, H-Ar), 7.26 (d, J = 8.4 Hz, 2H, H-Ar), 9.07 (s, 1H, OH), 11.28 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(p-tolyl))methyl)-3-methyl-1H-pyrazol-5-ol ( 4h )
Yield: (89%). Pale orange powder, mp: 197–198 °C; IR (KBr) (νmax, cm−1): 3301, 3104.77, 2924, 1607, 1512; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.06 (s, 6H, 2CH3), 2.22 (s, 3H, CH3), 3.36 (2OH exchanged with water of DMSO-d6), 4.76 (s, 1H, CH), 7.00 (s, 4H, H-Ar), 11.30 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(o-tolyl))methyl)-3-methyl-1H-pyrazol-5-ol (4i)
Yield: (77%). Pale orange powder, mp: 281–283 °C; IR (KBr) (νmax, cm−1): 3432, 3092, 2926, 1604.58, 1525.05; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 1.81 (s, 6H, 2CH3), 2.11 (s, 3H, CH3), 3.38 (2OH exchanged with water of DMSO-d6), 4.92 (s, 1H, CH), 7.02–7.23 (m, 4H, H-Ar), 10.66 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-hydroxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4j )
Yield: (82%). White powder, mp: 255–257 °C; IR (KBr) (νmax, cm−1): 3415, 3268, 3107, 2928, 1600, 1514; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.05 (s, 6H, 2CH3), 3.37 (2OH exchanged with water of DMSO-d6), 4.70 (s, 1H, CH), 6.59 (d, J = 8.8 Hz, 2H, H-Ar), 6.90 (d, J = 8.4, 2H, H-Ar), 9.07 (s, 1H, OH), 11.27 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(thiophen-2-yl))methyl)-3-methyl-1H-pyrazol-5-ol ( 4l )
Yield: (78%). White powder, mp: 239–241 °C; IR (KBr) (νmax, cm−1): 3582, 3112, 2926, 1606, 1483; 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 2.09 (s, 6H, 2CH3), 3.38 (2OH exchanged with water of DMSO-d6), 4.97 (s, 1H, CH), 6.84-6.86 (m, 1H, H-Ar), 6.59–6.60 (m, 1H, H-Ar), 7.27 (d, J = 5.2 Hz, 2H, H-Ar), 11.36 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(pyridin-3-yl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4m )
Yield: (82%). White powder, mp: 295–298 °C; IR (KBr) (νmax, cm−1): 3193, 3055, 2926, 1596, 1530; 1H NMR (300 MHz, DMSO-d6): δ (ppm) = 2.12 (s, 6H, 2CH3), 4.92 (s, 1H, CH), 7.26 (dd, J = 7.8 Hz, 1H, H-Ar), 7.54 (d, J = 7.8 Hz, 1H, H-Ar), 8.34-8.35 (m, 2H, H-Ar), 11.10 (brs, 4H, 2NH, 2OH). 13C NMR (75 MHz, DMSO-d6): δ (ppm) = 10.7 (CH3), 31.1 (CH), 103.8, 123.3, 135.5, 139.1, 140.1, 147.0, 149.5, 161.4 (C-Ar).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-hydroxy-3-methoxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol ( 4n )
Yield: (87%). White powder, mp: 254–257 °C; IR (KBr) (νmax, cm−1): 3373, 3193, 2959, 1609, 1485; 1H NMR (300 MHz, DMSO-d6): δ (ppm) = 2.07 (s, 6H, 2CH3), 3.65 (s, 3H, OCH3), 4.75 (s, 1H, CH), 6.55 (dd, J = 8.1 Hz, J = 1.2 Hz, 1H, H-Ar), 6.63 (d, J = 8.1 Hz, 1H, H-Ar), 6.76 (d, J = 1.5 Hz, 1H, H-Ar), 11.30 (brs, 5H, 2NH, 3OH). 13C NMR (75 MHz, DMSO-d6): δ (ppm) = 10.9 (CH3), 32.8 (CH), 56.0 (OCH3), 105.0, 112.7, 115.3, 120.4, 134.8, 140.0, 144.9, 147.4, 161.4 (C-Ar).
6-amino-1,4-dihydro-3-methyl-4-(2-chlorophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile ( 6f )
Yield: (86%). White powder, mp: 231–233 °C; IR (KBr) (νmax, cm−1): 3391, 3357, 3314, 3169, 2190,1609, 1489, 1408, 1350, 1052, 763; 1H NMR (400 MHz, DMSO-d6): 1.77 (s, 3H, CH3), 5.08 (s, 1H, CH), 6.99 (s, 2H, NH2), 7.18–7.43 (m, 4H, Ar), 12.16 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(4-bromophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile ( 6h )
Yield: (92%). White powder, mp: 254–257 °C; IR (KBr) (νmax, cm−1): 3470, 3227, 3120, 2195, 1651, 1595, 1560, 1401, 1353, 1107, 883, 810, 744, 543; 1H NMR (400 MHz, DMSO-d6): 1.80 (s, 3H, CH3), 4.63 (s, 1H, CH), 6.96 (s, 2H, NH2), 7.15 (d, J = 8 Hz, 2H), 7.52 (d, J = 8 Hz, 2H), 12.16 (s, 1H, NH).
Results and discussion
At first, we centralized our attention to the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) and the reaction between ethyl acetoacetate (2.0 mmol), hydrazine hydrate (2.0 mmol), and 4-nitrobenzaldehyde (1.0 mmol) was chosen as a model reaction for preliminary experiments. The effect of catalyst loading was envisaged, and the results were summarized in Table 1. At a catalyst loading of 15 mol%, the highest yield of product was obtained in EtOH (Table 1, entry 3). Further increases to the catalyst loading did not considerably influence the reaction progress. Next, the effects of solvents, water, ethanol, and aqueous ethanol were evaluated and it was found that the rate 2:1 EtOH/Water is better than other rates. Finally, to optimize the reaction temperature, the model reaction was carried out using 15 mol% of the catalyst at different temperatures. It was found that 60 °C is an efficient temperature in terms of reaction time and yield obtained (Table 1, entry 6). As shown in (Table 1, entry 11), a test reaction was accomplished in the absence of catalyst at the optimum condition and offered only 30% yield of the expected product.

In another study for the synthesis of dihydropyrano[2,3-c], pyrazoles, we selected reaction of ethyl acetoacetate (1.0 mmol), hydrazine hydrate (1.0 mmol), malononitrile (1.0 mmol), and benzaldehyde (1.0 mmol) as model under solvent-free conditions and the effect of amount of catalyst and temperature was envisaged. As shown in (Table 2, entry 6), optimization of the reaction conditions demonstrated that the best results were gained when the reaction was performed at 80 °C in the presence of aspirin (15 mol%) under solvent-free conditions.

After optimizing the reaction conditions, we evaluated the range and feasibility of reactions using a various aryl aldehydes. As shown in Tables 3 and 4, it was shown that the two reactions tolerated both electron-withdrawing and electron-donating groups on the aldehyde aromatic rings including ortho-, meta-, and para-substituted with the corresponding products in satisfied yields. In synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols), substitution of NO2 group in the 4th position of aromatic aldehyde led to a relatively faster rate and higher yield than the substitution of other groups in various positions of aromatic ring.

Then, we investigated the utilization of acenaphthylene-1,2-dione or isatins as a substrate to react with the hydrazine hydrate, ethyl acetoacetate, and malononitrile under the optimized conditions. As expected, the reaction developed well to afford spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives (8a–c) and spiro[acenaphthylene-1,4′-pyrano[2,3-c]pyrazole (10) in good yields.
A suggested mechanism, illustrating the role of aspirin in the tandem synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol), dihydropyrano[2,3-c]pyrazole derivative, and spiroindoline-pyranopyrazole was suggested in Scheme 4. At first, pyrazolone A would be formed from the reaction between ethyl acetoacetate 1 and hydrazine hydrate 2. For the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol), the activated carbonyl group of the aldehydes 3 by aspirin via Knoevenagel condensation with pyrazolone to create the intermediated B where through Michael addition to another pyrazolone to give desirable products (4a–n).
For the synthesis of dihydropyrano[2,3-c]pyrazoles is proposed the arylidene malononitrile C to generate in situ via Knoevenagel condensation active aldehyes 3 and malononitrile 4. Michael addition of A and C gives the acyclic adduct products D, which undergoes intramolecular cyclization and tautomerization to afford the corresponding products (6a–o) (Scheme 2).
In order to appraise the privileged features of our procedure, we compared our results for the synthesis of bis(pyrazol-5-ols) and dihydropyrano[2,3-c]pyrazoles with other results reported in the literature, as shown in Table 5. Compounds including pyrazol core have extensively been used in the synthesis of drugs and pharmaceuticals. Thus, the elimination of residual metal species plays a pivotal role when a metal-containing catalyst was applied [12]. So, this method, compared to the existing ones, uses aspirin as an efficient, non-toxic, inexpensive, and commercially available organocatalyst along with merits including high yields and short reaction time.
The reusability of aspirin was examined in the synthesis of 4b as an example. It was observed that the yield of product reduced in the 3rd and 4th runs.
Conclusions
In compendium, we expanded an efficient strategy for one-pot synthesis of biologically 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol), dihydropyrano[2,3-c]pyrazole, and spiropyranopyrazole derivatives in the presence of aspirin as a commercially available, and eco-compatibility catalyst under environmental benign conditions. Good to excellent yields, simplicity of operation, comfortable purification, and high atom-economy are the noteworthy advantages of the present method.
References
S. Karamthulla, S. Pal, M.N. Khan, L.H. Choudhury, RSC Adv. 4, 37889–37899 (2014)
P.T. Anastas, T.C. Williamson (eds.), Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes (Oxford University Press, Oxford, 1998)
G.M. Ziarani, S. Faramarzi, N. Lashgari, A. Badiei, J. Iran. Chem. Soc. 11, 701–709 (2014)
P.T. Anastas, M.M. Kirchhoff, Acc. Chem. Res. 35, 686–694 (2002)
E. Ruijter, R. Scheffelaar, R.V.A. Orru, Angew. Chem. Int. Ed. 50, 6234–6246 (2011)
J.L. Tucker, Org. Process Res. Dev. 10, 315–319 (2006)
O.A. Attanasi, D. Spinelli, Soc. Chim. Italiana, Roma. 4, 105–137 (2000)
M.M.F. Ismail, Y.A. Ammar, H.S.A. EI-Zahaby, S.I. Eisa, S.E. Barakat, Arch. Pharm. Chem. Life Sci. 340, 476–479 (2007)
S.P. Prajapati, D.P. Patel, P.S. Patel, J. Chem. Pharm. Res. 4, 2652–2655 (2012)
Y. Liu, G. He, C. Kai, Y. Li, H. Zhu, J. Heterocycl. Chem. 19, 1370–1375 (2012)
G. Mariappan, B.P. Saha, L. Sutharson, A. Singh, S. Garg, L. Pandey, D. Kumar, Saudi Pharm. J. 19, 115–122 (2011)
C.E. Rosiere, M.I. Grossman, Science 131, 651 (1951)
M.G. LaPorte, Z. Wang, R. Colombo, A. Garzan, V.A. Peshkov, M. Liang, P.A. Johnston, M.E. Schurdak, M. Sen, D.P. Camarco, Y. Hua, Bioorg. Med. Chem. Lett. 26, 3581–3588 (2016)
J.L. Wang, D. Liu, Z.J. Zheng, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S., Z. Huang. Proc. Natl. Acad. Sci. 97, 7124–7129 (2009)
B.P. Bandgar, H.V. Chavan, L.K. Adsul, V.N. Thakare, S.N. Shringare, R. Shaikh, R.N. Gacche, Bioorg. Med. Chem. Lett. 23, 912–916 (2013)
K.M. Kasiotis, E.N. Tzanetou, S.A. Haroutounian, Front. Chem. 2, 1–7 (2014)
M.J. Genin, C. Biles, B.J. Keiser, S.M. Poppe, S.M. Swaney, W.G. Tarpley, Y. Yagi, D.L. Romero, J. Med. Chem. 43, 1034–1040 (2000)
F. Abrigach, R. Touzani, Med. Chem. (Los Angeles) 6, 292–298 (2016)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501–4503 (2009)
H. Kashtoh, M.T. Muhammad, J.J. Khan, S. Rasheed, A. Khan, S. Perveen, K. Javaid, K.M. Khan, M.I. Choudhary, Bioorg. Chem. 65, 61–72 (2016)
A.V. Stachulski, N.G. Berry, A.C.L. Low, S.L. Moores, E. Row, D.C. Warhurst, I.S. Adagu, J.F. Rossignol, J. Med. Chem. 49, 1450–1454 (2006)
E. Soleimani, S. Ghorbani, M. Taran, A. Sarvary, C. R. Chim. 15, 955–961 (2012)
J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, H. Shahbazi-Alavi, RSC Adv. 4, 46106–46113 (2014)
W. Wang, S.X. Wang, X.Y. Qin, J.T. Li, Synth. Commun. 35, 1263–1269 (2005)
N.G. Khaligh, S.B.A. Hamid, S.J. Titinchi, Chin. Chem. Lett. 27, 104–108 (2016)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh, S. Azizian, J. Mol. Catal. A-Chem. 418–419, 54–67 (2016)
D. Azarifar, Y. Abbasi, Synth. Commun. 46, 745–758 (2016)
Y.A. Tayade, S.A. Padvi, Y.B. Wagh, D.S. Dalal, Tetrahedron Lett. 56, 2441–2447 (2015)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559–7579 (2016)
C.F. Zhou, J.J. Li, W.K. Su, Chin. Chem. Lett. 27, 1686–1690 (2016)
A.K. Imene, F. Amina, L. Oumeima, B. Raouf, B. Boudjemaa, D. Abdelmadjid, Lett. Org. Chem. 13, 85–91 (2016)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523–2525 (2011)
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta A. 81, 431–440 (2011)
A.R. Hajipour, M. Karimzadeh, H. Tavallaei, J. Iran. Chem. Soc. 12, 987–991 (2015)
K. Ablajan, W. Liju, Y. Kelimu, F. Jun, Mol. Divers. 17, 693–700 (2013)
D.W.C. MacMillan, Nature 455, 304–308 (2008)
C.M. Ulrich, J. Bigler, J.D. Potter, Nat. Rev. Cancer 6, 130–140 (2006)
C. Cena, M.L. Lolli, L. Lazzarato, E. Guaita, G. Morini, G. Coruzzi, S.P. McElroy, I.L. Megson, R. Fruttero, A. Gasco, J. Med. Chem. 46, 747–754 (2003)
A. Undas, K.E. Brummel-Ziedins, K.G. Mann, Blood 109, 2285–2592 (2007)
X. Shi, M. Ding, Z. Dong, F. Chen, J. Ye, S. Wan, S.S. Leonard, V. Castronova, V. Vallyathan, Mol. Cell. Biochem. 199, 93–102 (1999)
T. Roberts, F. Shokraneh, S. Nur, Cochrane Libr (2016). doi:10.1002/14651858.CD012116
M. Fatahpour, F. Noori Sadeh, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, Res. Appl. Chem. 6, 1569–1572 (2016)
M. Kangani, N. Hazeri, M.T. Mghsoodlou, K. Khandan-Barani, M. Kheyrollahi, F. Nezhadshahrokhabadi, J. Iran. Chem. Soc. 12, 47–50 (2015)
M. Fatahpour, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, Iran. J. Sci. Technol. Trans. A Sci. (2016). doi:10.1007/s40995-016-0064-1
F. Mohamadpour, M.T. Maghsoodlou, R. Heydari, M. Lashkari, J. Iran. Chem. Soc. 13, 1549–1560 (2016)
M.T. Maghsoodlou, M. Karima, M. Lashkari, B. Adrom, J. Aboonajmi, J. Iran. Chem. Soc. 14, 329–335 (2017)
J. Xu-dong, D. Hai-feng, L. Ying-jie, C. Jun-gang, L. Da-peng, W. Mao-cheng, Chem. Res. Chin. Univ. 28, 999–1002 (2012)
M. Babaie, H. Sheibani, Arab. J. Chem. 11, 159–162 (2011)
Y. Zou, H. Wub, Y. Hua, H. Liu, X. Zhao, H. Ji, D. Shi, Ultrason. Sonochem. 18, 708–712 (2011)
B. Maleki, H. Eshghi, M. Barghamadi, N. Nasiri, A. Khojastehnezhad, S. Sedigh Ashrafi, O. Pourshiani, Res. Chem. Intermed. 42, 3071–3093 (2016)
D.R. Palleros, Experimental Organic Chemistry (Wiley, Hoboken, 2000), p. 494
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS comb. Sci. 14, 38–43 (2011)
S. Paul, K. Pradhan, S. Ghosh, S.K. De, A.R. Das, Tetrahedron 70, 6088–6099 (2014)
M.N. Elinson, A.I. Ilovaisky, V.M. Merkulova, P.A. Belyakov, F. Barba, B. Batanero, Tetrahedron 68, 5833–5837 (2012)
Acknowledgements
We gratefully appreciate the financial support from the Research Council of University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatahpour, M., Noori Sadeh, F., Hazeri, N. et al. Aspirin: an efficient catalyst for synthesis of bis (pyrazol-5-ols), dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles in an environmentally benign manner. J IRAN CHEM SOC 14, 1945–1956 (2017). https://doi.org/10.1007/s13738-017-1133-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1133-x