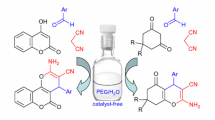
Abstract
A facile, eco-friendly, and efficient approach for the multicomponent synthesis of 2-amino-pyran analogues (4a–j) is described that involves the reaction of substituted aldehydes, methyl cyanoacetate, and 1,3-cyclohexadione in a one-pot method using ruthenia-doped alumina (RuO2/Al2O3) as heterogeneous catalyst in a green solvent system. A simple wet-impregnation approach was used to prepare the catalyst material and was well-characterized using several analytical techniques like PXRD, TEM, SEM, SEM–EDX, and BET analysis. The key benefits of the current protocol are operational simplicity, economy, green reaction conditions, easy workup, short reaction time (10 min), higher product yields (94–98%), and no need for column chromatographic purification. The additional key advantage of this method is the recyclability and reusability of catalyst material up to eight runs through simple filtration without any significant loss of its catalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) are significant protocols in synthetic and medicinal chemistry [1,2,3]. MCRs involve a reaction between more than two substrates to form a single complex product having new carbon–carbon, carbon–nitrogen, and other carbon–heteroatom bonds via a one-pot process without isolation of any intermediate [4]. MCRs are utilized in various fields such as medicinal, computational, agrochemical, petroleum, and pharmaceutical [2,3,4]. Further, MCRs offer advantages like minimizing waste production, simple handling, reducing reaction time, using non-toxic solvents/catalysts, increasing product yields, and mild reaction conditions [5,6,7,8]. Environmental-friendly, cost-effectiveness, and non-requirement of purification procedures are additional viable benefits of MCRs.
Oxygen-containing heterocyclic molecules revealed influential research owing to their remarkable biological properties and extensive applications to agrochemicals, fine chemicals, pharmaceuticals, computational, and biological systems [9]. Pyran is a six-membered oxygen-containing heterocyclic ring [9,10,11]. Many natural products such as benzopyrans, flavonoids, coumarins, xanthones, and sugars contain pyran scaffold as an essential structural subunit. Various pyran analogues show various pharmaceutical activities like antitumor, anti-plasmodial, Alzheimer’s, antihistamine, anti-malarial, antidepressant, anti-inflammatory, antioxidant, analgesic, and antimicrobial [12,13,14,15,16,17,18,19,20]. Recently, a few protocols have been described for the synthesis of these analogues by using different catalysts such as (NH4)2HPO4, trichloroisocyanuric acid, glutamic acid, Zr@IL-Fe3O4, cellulose biocomposite, triethanolamine, WEMFSA, Zn(L-proline)2, and Fe3O4@D-NH2-HPA [21,22,23,24,25,26,27,28,29]. On the other hand, many of these protocols need harsh reaction conditions, prolonged reaction time, expensive, extremely corrosive, lessened product yields, toxic solvents and catalysts, and a complex separation procedure. Thus, improving a facile, suitable, and environmental-friendly protocol using economic and non-toxic reagents/solvents would enhance the scope of preparing 2-aminopyrans.
Nowadays, heterogeneous catalysts have swiftly developed in various chemical, pharmaceutical, petroleum, and materials sciences [30,31,32,33]. It has distinctive chemical and physical properties like highly stable, long reaction life, and huge surface area to perform as heterogeneous promoters for different catalytic reactions [34]. Heterogeneous catalysts usually comprise metal oxides such as titania, alumina, ruthenia, silica, zirconia, and ceria [35]. Amongst them, alumina has fascinated much attention due to its vital properties like economy, environmental friendliness, operational simplicity, non-corrosive nature, high surface area, greater reactivity, higher selectivity, non-toxicity, and moisture insensitivity [35,36,37]. Further, it tends to aggregate and show insignificant dispersion in organic and aqueous solvents systems because of their vast surface area and surface energy. Ruthenium and its oxides are currently gaining much attention due to their excellent thermal, mechanical, and chemical properties. These catalysts are extensively utilized as Lewis acid and/or strong bases in significant catalytic reactions like Fischer–Tropsch synthesis, hydrogenation ammonia fabrication, water-splitting, olefin metathesis, organic synthesis, CO-methanation, steam reforming, and fine chemicals manufacture [35, 38,39,40,41,42,43]. These catalysts' expedience depends on the nature of support, particle size, and reaction limits. Further, these catalysts show simple handling, regular cost, non-hazardous nature, and excellent activity. Therefore, Ru-based composites as heterogeneous catalysts evolve superior in green organic synthesis. Currently, immense efforts are underway to study combined units for their synergetic behaviour. Ruthenia and alumina materials have exquisite catalytic efficacy and vital importance in catalysis applications. Hence, combining these complex catalytic materials will aid in potentially advancing the catalytic properties.
Recently, our research team focussed on various green approaches like ultrasonication, microwave irradiation, mechanochemical and heterogeneous catalysis for preparing novel organic compounds having biological activities including anti-cancer, antimicrobial, anti-malarial, antiviral, etc. [44,45,46,47,48]. With a desire to emerge with the most efficient synthetic protocol, we wish to report an effective approach for synthesizing 2-amino pyrans via multicomponent single-step reaction of substituted aldehydes, methyl cyanoacetate, and 1,3-cyclohexadione in the presence of 2.5% RuO2-doped Al2O3 catalyst under EtOH solvent media and mild reaction conditions. There are no reactions reported utilizing this heterogeneous catalyst to prepare 2-amino pyran derivatives to the best of our knowledge.
Experimental section
Synthesis of RuO2-doped Al2O3 catalysts
Ruthenia-doped alumina (RuO2/Al2O3) catalysts were synthesized by a simple wet-impregnation protocol. An appropriate amount of the ruthenium (III) chloride [RuCl3.XH2O (Sigma Aldrich, 99.98%) was dissolved in deionized water (40 mL). Then, the suitable amount of γ-Alumina [γ-Al2O3, Sigma-Aldrich] was added to the above solution and stirred for 3 h at RT. Later, it was continued overnight. Further, the above mixture was dried for 10 h at 150 °C in a hot air oven. Later, the solid material was calcined in the presence of air, at 450 °C for 3 h to afford appropriate amounts of target catalysts (1%, 2.5% & 5%w/w). All the instrumental information is incorporated into the supporting information (SI-I).
General procedure for the preparation of 2-amino-pyran derivatives (4a–j) in the presence of catalysts
A mixture comprising substituted aldehyde (1 mmol), methyl cyanoacetate (1.1 mmol), 1,3-diketone (1,3-cyclohexadione, 1 mmol), nanocatalyst (RuO2/Al2O3, 30 mg), and 10 mL of EtOH was agitated and stirred for 10 min at RT (Scheme 1). The reaction progress was noticed by thin-layer chromatography, and the reaction was allowed to cool and then concentrated under reduced pressure. Next, the resulting solid was washed with ethyl acetate, filtered, evaporated, and dried to obtain the crude product. Further, the crude product was purified from EtOH solvent to afford pure targets (4a–j) in higher yields. Moreover, the insoluble separated catalyst was washed with acetone, dried in an oven at 200 °C and reused for the next cycle. The structural data of final molecules were evidenced by 1H, 13C, 15 N, and HRMS spectroscopic analysis in supplementary information (SI-II).
Results and discussion
Catalyst characterization
Powdered X-ray diffraction (P-XRD) analysis
Figure 1 represents the P-XRD patterns for the prepared catalyst of 2.5% RuO2-doped Al2O3 material. The peaks sited at the 2θ angles of 28.1°, 35.3°, 40.1° and 54.5° were perceived matching to planes (1 1 0), (1 0 1), (2 0 0) and (2 1 1), respectively, which agreed with the (JCPDS No. 21-1172). Notably, Al2O3 nanoparticle peaks at 46.3°, 58.1°, and 67.2° correspond to (4 0 0), (4 2 2), (4 4 0) which match with the (JCPDS No. 79-1558). The P-XRD signals identified in the figure ascertain the nano-crystalline nature of the nanocatalyst. The average crystallite size in 2.5% RuO2/Al2O3 was figured to be 15.2 nm, which agreed with the Scherrer formula.
SEM–EDX analysis
The SEM image of 2.5%RuO2/Al2O3 catalyst shown in Fig. 2a reveals the nanocomposite material's morphology and particle size. The morphology of the catalyst obtained displayed agglomerated well-defined tiny needle ruthenium-like particles 12–23 nm in size. The EDX spectrum of the RuO2/Al2O3 catalyst verifies that all elements in the nano-complex containing Ru, Al, O are detected in the spectrum, and there are no additional elements related to impurities in Fig. 2b. Further, EDX analysis was also used to map the nanocatalyst composite elements. In view of the energy dispersive analysis on the catalyst, as shown in Fig. 2c, no impurity was identified in the prepared nanocomposite and it comprised of pure Ru, Al, and O, respectively. Moreover, Fig. 2d displays the elements' homogenous distribution within RuO2/Al2O3 catalyst structure.
TEM analysis
TEM analysis was utilized to establish the comprehensive morphology and shape of the constituting 2.5%RuO2/Al2O3 catalyst. The image reveals the presence of RuO2/Al2O3 nanoparticles in the catalyst. It seems that the bar shape ruthenia particles are covered in a layer of alumina. Further, particle size was in the range 10–21 nm (Fig. 3a). The TEM image of pure RuO2 shows only bar-shaped particles having dimensions 18–29 nm (Fig. 3b).
BET analysis
The BET approach was employed to assess the N2 adsorption/desorption construction of 2.5% RuO2-doped Al2O3 catalyst (Fig. 4). The BET performance on catalyst material was used to note the surface area, pore volume, and pore size of the structure. In the BET analysis, the catalyst material was characterized by the type-IV adsorption–desorption isotherm and H1 type hysteresis loop with P/P0 value lying between 0.61 and 0.96. The RuO2/Al2O3 catalyst measured a specific surface area of 85 m2 g−1 with a pore volume of 1.42 cm3 g−1 and an average pore size of 47 nm, revealing a mesoporous structure.
Thermo-gravimetric analysis
The thermal stability of the catalyst was analysed by thermo-gravimetric analysis (Fig. 5) under air up to 600 °C. The TGA curve of the catalyst material exhibited two-step mass change between 160–246 °C and 246–336 °C. The first loss of mass at a lower temperature is ascribed to sample dehydration. The successive second weight loss is due to the thermal decomposition of the organic functional groups.
Optimization conditions
The model reaction containing equimolar amounts of 2-methoxy benzaldehyde, 1,3-cyclohexadione, and methyl cyanoacetate in slight excess as reactants was investigated in diverse reaction conditions, including: with/without different catalytic systems, solvents, and catalyst loading for optimization. Firstly, the reaction was led without any catalyst in EtOH solvent. However, there were no signs of progress even after a long reaction time of 6 h, both at room temperature and under reflux conditions (Table 1). The identical reaction was then tested using different acidic catalysts like para toluene sulphonic acid, trifluoroacetic acid, formic acid, and acetic acid in ethanol solvent. The product was formed at RT after 3 to 4.5 h, but the yields were relatively marginal (Table 1). Then, the same reaction was brought about with different basic catalysts such as sodium bicarbonate, sodium carbonate, triethylamine, and pyridine in ethanol. However, these catalysts generated no product yields even after prolonged reaction time (Table 1).
The pilot reaction was considered again using catalysts like ferric chloride, metal, and non-metal oxides like alumina, ceria, ruthenia, and silica, in EtOH at RT. The desired product was obtained in around 50% yields with FeCl3 and ceria after 3 h. The reaction using silica gave a moderate yield of 64% in a 2 h reaction time. On the other hand, the reaction mediated using alumina and ruthenia as catalytic systems resulted in good yields of 82% and 84% in less time, corresponding to 1 h and 0.5 h, respectively. Encouraged by these results, and in an endeavour to enhance the efficiency of the model reaction, a combination of the two solid catalysts, namely ruthenia doped in alumina, was organized in weight percentages of 1% RuO2/Al2O3, 2.5% RuO2/Al2O3, and 5% RuO2/Al2O3 was employed as a mixed catalyst. The multicomponent reaction gave a notable product yield (90%) of the target compound at RT in 15 min with 1% RuO2/Al2O3. However, with 2.5% RuO2/Al2O3 and 5% RuO2/Al2O3 catalyst, an excellent reaction yield of 98% was witnessed in mere 9 min (Table 1). Hence, 2.5% RuO2/Al2O3 was designated as optimized catalyst per cent, as an excess per cent did not change either the product yields or the reaction time.
To enhance reaction conditions for the effect of catalyst loading, the typical reaction was studied by various catalytic loadings of RuO2-doped alumina in mild reaction conditions in the presence of a green solvent system (Table 2). Initially, no product was observed when the model reaction was investigated without a catalyst for 2 h at RT. Next, the reaction was conducted by using 10 to 30 mg of catalyst; then, product yields increased. But, no significant improvement in the yield or reaction time was noticed on using more than 30 mg catalyst material. Moreover, 20 mg and 10 mg of catalyst material influenced the target yield by decreasing it to 70 and 63%, respectively. Hence, 2.5% RuO2/Al2O3 (30 mg) optimum catalyst material was utilized to effectively synthesize the target products in excellent yields (98%) in a short reaction time.
The solvent shows a significant role in chemical conversions in terms of reaction time and product yield. Initially, no reaction was proceeded even after an elongated reaction time in the presence of catalyst under solvent-free conditions. Solvents such as n-hexane and toluene did not give any product (Table 3). While lower yields were obtained by using various solvents (polar aprotic) like DMSO, acetonitrile, and DMF under same reaction conditions. Remarkably, the similar reaction displayed a prominent result with (polar protic) solvents MeOH, and EtOH owing to improved activity of the catalyst surface. Based on the above investigations, and considering the incorporation of green solvent, green protocols, economy, shorter reaction times, and excellent yields, EtOH was found to be the better solvent over others.
The green multicomponent synthesis of 2-amino pyrans by nano-ruthenia loaded on alumina proved to catalyse the reaction in an efficient, and facile manner producing excellent yields via single-pot procedure. The scope of the approach was studied by using a range of aromatic aldehydes in place of 2-methoxy benzaldehyde. The reactions when extended to differently functionalized aromatic aldehydes confirmed that aldehydes with both electron-withdrawing or donating groups contributed comparable yields of the product. Hence, this indicates that substitutions on aromatic aldehydes play nearly an insignificant role both in the ease of getting the products or on their corresponding product yields (Table 4).
A possible reaction mechanism for the one-pot, multicomponent reaction of aldehyde, methyl cyanoacetate, and 1,3-cyclohexadione to prepare 2-amino-pyran derivatives is depicted in Scheme 2. In conformity with the reaction mechanism, 2.5% RuO2/Al2O3 is a very active nanocatalyst for the formation of cyanophenylacrylate as intermediate (III) via the condensation reaction of aromatic aldehyde (I) and methyl cyanoacetate (II). The catalyst activates the aromatic aldehyde and also helps in the deprotonation of the active methylene compound due to its Lewis acidic sites. In the subsequent stage, the above Knoevenagel intermediate (III) goes through a Michael addition reaction with 1,3-cyclohexadione (IV) underneath the catalytic acceleration to obtain the adduct (V). Finally, enolization of (VI) occurs to produce the intermediate (VII), which undergoes intramolecular cyclization to obtain the target molecule (VIII).
Recyclability
The recyclability and reusability of the catalyst material were investigated for the one-pot, multicomponent reaction of aldehyde, methyl cyanoacetate and 1,3-cyclohexadione as a typical reaction in EtOH solvent using 30 mg of 2.5%RuO2/Al2O3 as heterogeneous catalyst. Upon accomplishment of the reaction, the solid material was separated successfully by using a simple filtration approach from the product. The recovered solid catalyst material was washed with EtOH and dried under reduced pressure. Then, the recovered catalyst was reused in further reactions for eight successive cycles, as there was no significant loss of activity (Fig. 6).
Additionally, an investigation of heterogeneity of RuO2/Al2O3 solid catalytic activity was accomplished by the hot-filtration technique on a pilot reaction for the final compound production. While the reaction (10 min) progressed, the catalyst composite detached from the reaction mixture and the left-over filtrate was constant stirred until the reaction time reached 60 min under similar optimized conditions. No perceptible increase in the product yield of the outcome was observed and also the reaction time increased after 8th cycle. Based on the results, the catalyst material (RuO2/Al2O3) was detected to be stable, and apparently, no leaching of metal substance from synthesized catalyst witnessed under the optimized reaction conditions. Moreover, the recycled catalyst (after the 8th cycle) was analysed by TEM, XRD, and SEM analysis. The catalyst did not lose any weight. The obtained results revealed no considerable variation in their structural morphology as compared to the fresh catalyst composite (Fig. S1–S3). Based on the outcomes, no erosion of the active material from the support material, and the catalyst composite demonstrated it as an exceptionally vigorous heterogeneous class that retains constant crystal composition following repeated usage.
The efficiency of the as-synthesized catalyst material (RuO2/Al2O3) for the preparation of amino pyran analogues was compared with the previous literature-described approaches with various catalysts, in terms of the time, yield and reaction conditions (Table 5). An examination of the data exposes that the RuO2/Al2O3 proves to be a superior catalyst composite with greater efficiency and scope for use in the one-pot synthesis of aminopyran analogues, as compared to the earlier reported protocols in all standards.
Conclusion
To sum up, a facile, novel, and environmentally benign protocol for preparing 2-amino pyran analogues using an efficient 2.5%RuO2/Al2O3 as heterogeneous catalyst under green solvent media has been developed. The ready availability of the reagents, simple synthesis, effortless handling, eco-friendly, mild reaction conditions, use of non-toxic solvent/catalysts, short reaction times, higher product yields, reduction of waste, and no use of column chromatography technique are essential features of this protocol. Further, the prepared catalyst offered major benefits like environmental friendliness, non-corrosive nature, economy, high surface area, greater reactivity, non-toxicity, moisture insensitivity, low catalytic amount, recyclability, and reusability.
References
Ch. Madhavi, H. Ganja, N. Kerru, S. Maddila, S.B. Jonnalagadda, Appl. Organomet. Chem. 34, e6442 (2021). https://doi.org/10.1002/aoc.6442
N. Kerru, S. Maddila, S.B. Jonnalagadda, Front. Chem. 9, 638832 (2021). https://doi.org/10.3389/fchem.2021.638832
K.K. Gangu, J.V.S.K.V. Kalyani, T.S. Guru, S. Maddila, S.B. Jonnalagadda, Mater. Today Commun. 28, 102206 (2021)
N. Kerru, S. Maddila, S.B. Jonnalagadda, Curr. Org. Chem. 23, 3156 (2019). https://doi.org/10.2174/1385272823666191202105820
N. Kerru, L. Gummidi, S. Maddila, S.B. Jonnalagadda, Curr. Org. Chem. 25, 1 (2021). https://doi.org/10.2174/1385272824999201020204620
D.J. Rao, N. Kerru, S. Maddila, Chem. Data Collect. 32, 100669 (2021). https://doi.org/10.1016/j.cdc.2021.100704
N. Kerru, L. Gummidi, S. Maddila, S.B. Jonnalagadda, Inorg. Chem. Commun. 123, 108321 (2021). https://doi.org/10.1016/j.inoche.2020.108321
S. Harikrishna, A.R. Robert, H. Ganja, S. Maddila, S.B. Jonnalagadda, Sustain. Chem. Pharm. 16, 100265 (2020). https://doi.org/10.1016/j.scp.2020.100265
M. Costa, T.A. Dias, A. Brito, F. Proenca, Eur. J. Med. Chem. 123, 487 (2016). https://doi.org/10.1016/j.ejmech.2016.07.057
S. Maddila, S. Gorle, S.B. Jonnalagadda, Expert Opin. Drug Discov. 15, 203 (2020). https://doi.org/10.1080/17460441.2020.1696768
N. Kerru, V.H.S.S. Bhaskaruni, R. Kishore, S. Maddila, S.B. Jonnalagadda, Lett. Drug Des. Discov. 15, 118 (2018). https://doi.org/10.2174/1570180814666170710161844
Y.D. Duan, Y.Y. Jiang, F.X. Guo, L.X. Chen, L.L. Xu, W. Zhang, B. Liu, Fitoterapia 135, 114 (2019). https://doi.org/10.1016/j.fitote.2019.04.012
F.M. Wunsch, B. Wünsch, F.A. Bernal, T.J. Schmidt, Molecules 26, 5249 (2021). https://doi.org/10.3390/molecules26175249
C. Hu, L. Jiang, L. Tang, M. Zhang, R. Sheng, Bioorg. Med. Chem. (2021). https://doi.org/10.1021/jm800869t
N.A. Farag, S.R. Mohamed, G.A. Soliman, Bioorg. Med. Chem. 16, 9009 (2008). https://doi.org/10.1016/j.bmc.2008.08.039
P. Lerdsirisuk, C. Maicheen, J. Ungwitayatorn, Bioorg. Chem. 57, 142 (2014). https://doi.org/10.1016/j.bioorg.2014.10.006
B. Gopishetty, S. Hazeldine, S. Santra, M. Johnson, G. Modi, S. Ali, J. Zhen, M. Reith, A. Dutta, F J. Med. Chem. 54, 2924 (2011). https://doi.org/10.1021/jm200020a
L.Y. Zeng, B. Xi, K. Huang, J. Bi, L. Wei, C. Cai, S. Liu, ACS Comb Sci. 21, 656 (2019). https://doi.org/10.1021/acscombsci.9b00050
A.R. Saundane, K. Vijaykumar, A.V. Vaijinath, Bioorg. Med. Chem. Lett. 23, 1978 (2013). https://doi.org/10.1016/j.bmcl.2013.02.036
K. Nicole, C. Gianluca, M. Domenico, L. Erik, P. Sabrina, V.C. Carlo, H. Hans-Ulrich, A. Carmen, R.N. Francisco, S. Dirk, W. Bernhard, Eur. J. Med. Chem. 219, 113443 (2021). https://doi.org/10.1016/j.ejmech.2021.113443
B. Saeed, B. Morteza, S.A. Masoumeh, Syn. Commun. 37, 1097 (2007)
S.F. Hojati, N.M. Eghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018). https://doi.org/10.1080/00304948.2018.1468982
K. Khandan-Barani, M. Kangani, M. Mirbaluchzehi, Z. Siroos, Inorg. Nano-Met. Chem. 47, 751 (2017). https://doi.org/10.1080/15533174.2016.1212233
M. Aghaei-Hashjin, A. Yahyazadeh, E. Abbaspour-Gilandeh, RSC Adv. 11, 23491 (2021). https://doi.org/10.1039/D1RA04381A
S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F.F. Bamoharram, M.M. Heravi, Sci. Rep. 10, 1 (2020). https://doi.org/10.1038/s41598-020-70738-z
R. Rahnamafa, L. Moradi, M. Khoobi, Res. Chem. Intermed. 46, 2109 (2020). https://doi.org/10.1007/s11164-020-04081-3
P.B. Hiremath, K. Kantharaju, ChemistrySelect 5, 1896 (2020). https://doi.org/10.1002/slct.201904336
D. Tahmassebi, J.E. Blevins, J.S. Gerardot. Appl. Organomet. Chem. 33, e4807 (2019). https://doi.org/10.1002/aoc.4807
A. Jamshidi, B. Maleki, F.M. Zonoz, R. Tayebee, Mater. Chem. Phys. 209, 46 (2018). https://doi.org/10.1016/j.matchemphys.2018.01.070
S.V.H.S. Bhaskaruni, K.K. Gangu, S. Maddila, S.B. Jonnalagadda, Chem. Rec. 19, 1793 (2019). https://doi.org/10.1002/tcr.201800077
H. Ganja, A.R. Robert, P. Lavanya, S. Chinnam, S. Maddila, S.B. Jonnalagadda, Inorg. Chem. Commun. 114, 107 (2020). https://doi.org/10.1016/j.inoche.2020.107807
S.V.H.S. Bhaskaruni, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Arab. J. Chem. 13, 1142 (2020). https://doi.org/10.1016/j.arabjc.2017.09.016
S. Harikrishna, A.R. Robert, H. Ganja, S. Maddila, S.B. Jonnalagadda. Appl. Organomet. Chem. 34, e5796 (2020). https://doi.org/10.1002/aoc.5796
S.N. Maddila, S. Maddila, N. Kerru, S.V.H.S. Bhaskaruni, S.B. Jonnalagadda, ChemistrySelect 5, 1786 (2020). https://doi.org/10.1002/slct.201901867
S.V.H.S. Bhaskaruni, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, Catal. Commun. 100, 24 (2017). https://doi.org/10.1016/j.catcom.2017.06.023
S. Shabalala, S. Maddila, W.E. Van Zyl, S.B. Jonnalagadda, ACS-Ind. Eng. Chem. Res. 56, 11372 (2017). https://doi.org/10.1016/j.psep.2022.01.054
K.K. Gangu, S. Maddila, S.N. Maddila, S.B. Jonnalagadda, RSC Adv. 7, 423 (2017). https://doi.org/10.1039/C6RA25372E
X. Pan, F. Jiao, D. Miao, X. Bao, Chem Rev. 121, 6588 (2021). https://doi.org/10.1021/acs.chemrev.0c01012
Q. Song, W.D. Wang, X. Hu, Z. Dong, Nanoscale 11, 21513 (2019). https://doi.org/10.1039/C9NR08483E
W. Wang, M. Xu, X. Xu, W. Zhou, Z. Shao, Angew Chem. Int. Ed. Engl. 59, 136 (2020). https://doi.org/10.1002/anie.201900292
C. Theunissen, M.A. Ashley, T. Rovis, J. Am. Chem. Soc. 141, 6791 (2019). https://doi.org/10.1021/jacs.8b13663
S. Chen, A.M. Abdel-Mageed, D. Li, J. Bansmann, S. Cisneros, J. Biskupek, W. Huang, R.J. Behm, Angew Chem. Int. Ed. Engl. 58, 10732 (2019). https://doi.org/10.1002/anie.201903882
R.L. Arevalo, S.M. Aspera, M.C. SisonEscano, H. Nakanishi, H. Kasai, ACS Omega 2, 1295 (2017). https://doi.org/10.1021/acsomega.6b00462
S. Maddila, S. Gorle, S. Shabalala, O. Oyetade, S.N. Maddila, P. Lavanya, S.B. Jonnalagadda, Arab. J. Chem. 12, 671 (2019). https://doi.org/10.1016/j.arabjc.2016.04.016
M.R. Khumalo, S.N. Maddila, S. Maddila, S.B. Jonnalagadda, ChemistrySelect 4, 12503 (2019). https://doi.org/10.1002/slct.201903222
M.R. Khumalo, S.N. Maddila, S. Maddila, S.B. Jonnalagadda, RSC Adv. 9, 30768 (2019). https://doi.org/10.1039/C9RA04604F
N. Kerru, L. Gummidi, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Molecules 25, 1909 (2020). https://doi.org/10.3390/molecules25081909
S. Gorle, K.K. Gangu, S. Maddila, S.B. Jonnalagadda, Chem. Data Collect. 28, 100 (2020). https://doi.org/10.1016/j.cdc.2020.100471
D. Zhenhua, L. Xiaohua, F. Juhua, W. Min, L. Lili, F. Xiaoming, Eur. J. Org. Chem. 1, 137 (2011). https://doi.org/10.1002/ejoc.201001151
B. Saeed, B. Morteza, M. Sheikh-Ahmadi, S. Hekmat, P. Salehi, Syn. Commun. 37(7), 1097 (2007). https://doi.org/10.1080/00397910701196579
L. Rong, X. Li, H. Wang, D. Shi, S. Tu, Q. Zhuang, Synth. Commun. 36, 2363 (2006). https://doi.org/10.1080/003979106006402302363
W. Xiang-Shan, S. Da-Qing, T. Shu-Jiang, Y. Chang-Sheng, Synth. Commun. 33(1), 119 (2003). https://doi.org/10.1081/SCC-120015567
L. Ji-Tai, X. Wen-Zhi, Y. Li-Chao, L. Tong-Shuang, Synth. Commun. 34(24), 4565 (2004). https://doi.org/10.1081/SCC-200043233
R. Naresh, A. Santhi, D. Derong, A. Hadi, Z. John, C.-G.J. Heterocyc, Chemistry 54(1), 677 (2017). https://doi.org/10.1002/jhet.2641
H. Alireza, S. Mohsen, G. Nooshin, Z. Abdolkarim, D.M. Mohammad, Appl. Catal. A Gen. 402(1–2), 11 (2011). https://doi.org/10.1016/j.apcata.2011.04.012
Acknowledgements
The authors are very thankful to the Andhra University, and GITAM Deemed to be University, Visakhapatnam, India, for instrumentation, research, and financial support.
Funding
This work received no fund from any source.
Author information
Authors and Affiliations
Contributions
BA performed experimental studies and conceptualization; ARR performed writing—original draft and conceptualization; MMKK provided facilities for the spectral characterization; RM provided facilities for the catalyst characterization; PM performed validation, data curation, and formal analysis; SM: performed conceptualization, project administration, writing—original draft, supervision and funding acquisition; SBJ performed review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apparao, B., Robert, A.R., Kumar, M.M.K. et al. Design of novel 2-amino-pyrans via a green and facile one-pot multicomponent protocol using RuO2/Al2O3 as reusable catalyst. Res Chem Intermed 49, 1043–1058 (2023). https://doi.org/10.1007/s11164-022-04949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04949-6