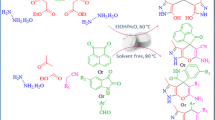
Abstract
We have studied the catalytic ability of copper(II) acetate monohydrate as a mild, environmentally benign, natural and economical catalyst for the multi-component efficient synthesis of biologically active spiro-4H-pyran derivatives and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives with excellent yields and short reaction times. The most important advantages of this procedure are its mild, non-toxic and inexpensive catalyst, one-pot synthesis, environmentally benign nature, solvent-free conditions, simple operational procedures, and highly efficient conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
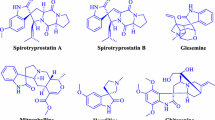
Nowadays, most research has been focused on the study of synthesising heterocyclic compounds. Organic compounds containing nitrogen heterocyclic rings are important compounds in medicinal chemistry. The synthesis of biologically active spiropyran derivatives (spirooxindole) and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives have been given much attention because of their advantages in various biological (Figs. 1, 2) and pharmaceutical fields, for example, as anti-HIV [1], anti-tubercular [2], fungicidal [3], anticonvulsant [4] and anticancer [5, 6] agents; in addition, these spirocycles are MDM2 inhibitors [7] and progesterone receptor modulators [8].
In recent decades, a number of methodologies for preparation of these compounds have been reported that have included various catalysts such as [BMIm] BF4 [9], urea–choline chloride [10], sulfated choline-based heteropolyanion [11], β-cyclodextrin [12], lipase [13], CsF [14], carbon–SO3H [15], Et3N [16], [Bmim]OH [17], ultrasound-assisted [18], NiCl2·6H2O [19], PTSA/[Bmim]Br [20], CuI nanoparticles [21], InCl3 [22, 23], STA [24], SBA-Pr–SO3H [25], PTSA [26], Ce(SO4)2·4H2O [27] and Cu(OAc)2/sodium L-ascorbate [28]. Some of these methodologies have limitations such as a difficult work-up, toxic and expensive catalysts and solvents, long reaction times and low yields.
Recently, increasing interest has been paid to the multi-component domino reactions (MCRs) [29–40]. Because of their notable advantages such as atom economy, environmental friendliness, low-cost, one-pot nature, simple work-up they have become powerful tools in the synthesis of organic compounds possessing biological and pharmaceutical properties.
Our recent research has focused on the development of clean and simple methodologies with environmentally benign natures. Therefore, given that one of the factors that reduces environmental pollution in synthesis of organics is solvent-free conditions, we have developed multicomponent reactions under solvent-free conditions.
In this regard, we have reported copper(II) acetate monohydrate for the synthesis of spiro-4H-pyran derivatives and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones via MCRs under thermal and solvent-free conditions. The advantages of copper(II) acetate monohydrate as a catalyst in organic compound synthesis are that it is environmentally benign, economical, mild, inexpensive, non-toxic and highly efficient [41–43].
Results and discussion
In this protocol, we have reported copper(II) acetate monohydrate as an efficient catalyst under thermal and solvent-free conditions for simple, environmentally benign synthesis of biologically active spiro-4H-pyran derivatives 4 from reaction between isatin 1, malononitrile 2 and 1,3-dicarbonyl compounds/4-hydroxycumarin/naphthlol 3 (Scheme 1).
In order to optimize the reaction conditions, the synthesis of compound 4a (Table 3, entry 1) was used as a model reaction. The effect of different amounts of catalyst on the reaction has been studied in this protocol. No product could be detected in the absence of the catalyst even after 10 h (Table 1, entry 1). Good yields were obtained in the presence of the catalyst. The best amount of catalyst was 15 mol% (0.03 g; Table 1, entry 4). The higher amount of catalyst did not increase the yields products (Table 1, entry 5).
Also, the effect of temperature on the reaction has been investigated. At room temperature, the product was not detected (Table 2, entry 1). The reaction was investigated at various temperatures and a high yield of product was obtained at 80 °C (Table 2, entry 4).
In order to study this procedure, we synthesized a one-pot, three-component condensation reaction of isatin (1.0 mmol), malononitrile (1.0 mmol) and compounds 3 (1.0 mmol) in the presence of copper(II) acetate monohydrate (15 mol%) as a mild catalyst under thermal and solvent-free conditions, and the results are shown in Table 3.
After the successful synthesis of spiro-4H-pyran derivatives 4, we turned our attention to the synthesis of spiro-4H-pyran derivatives from reaction between acenaphthoquinone 5, malononitrile 2 and compounds 6 in the present of copper(II) acetate monohydrate (Scheme 2) and these compounds were synthesized under similar conditions in good yields. The results are shown in Table 4.
Then, we turned our attention toward the synthesis of pyrazolo[1,2-b]phthalazine-5,10-dione derivatives. In order to discern the optimal conditions, we investigated the four components of phthalic anhydride 8, hydrazine monohydrate 9, aromatic aldehydes derivatives 10 and malononitrile 2 in the presence of copper(II) acetate monohydrate as an efficient, environmentally benign and natural catalyst (Scheme 3).
In order to optimize the reaction conditions, the synthesis of compound 11a (Table 7, entry 1) was used as a model reaction. The effect of different amounts of catalyst on the reaction has been studied in this protocol. No product could be detected in the absence of the catalyst even after 10 h (Table 5, entry 1). Good yields were obtained in the presence of catalyst. The best amount of catalyst was 20 mol% (0.04 g; Table 5, entry 5). The higher amount of catalyst did not increase the yields products (Table 5, entry 6).
Also, the effect of temperature on the reaction has been investigated. At room temperature, the product was not detected (Table 6, entry 1). The reaction was investigated at various temperatures and a high yield of product was obtained at 80 °C (Table 6, entry 4).
In order to study this procedure, we synthesized a series of compounds with various types of electron-donating and electron-withdrawing aldehyde derivatives such as Cl, Br, NO2, OH, OMe,….substituted banzaldehydes which gave excellent yields. Also, the generality of this four-component condensation reaction was studied by using copper(II) acetate monohydrate (20 mol%) via phthalic anhydride (1.0 mmol), hydrazine monohydrate (1.0 mmol), the various aldehyde derivatives (1.0 mmol) and malononitrile (1.0 mmol) under thermal and solvent-free conditions, and the results are shown in Table 7.
Comparison of the catalytic ability of some catalysts reported in the literature for the synthesis of spiro-4H-pyran and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives are shown in Tables 8 and 9. This study reveals that copper(II) acetate monohydrate has shown its extraordinary potential to be an alternative cheap, cost-effective, eco-friendly, efficient catalyst for the synthesis of these compounds. In addition, the use of solvent-free conditions with excellent yields and short reaction times are notable advantages of this presented methodology.
Experimental
General
Melting points of all compounds were determined using an Electrothermal 9100 apparatus. Also, proton nuclear magnetic resonance (1H NMR) analyses were recorded on a Bruker DRX-400 Avance instrument with deuterated dimethyl sulfoxide (DMSO-d6 ) as a solvent. In this article, all reagents and solvents were purchased from Merck, Fluka and Acros chemical companies and were used without further purification.
General procedure for preparation of spiro-4H-pyran derivatives (4a–f) and (8a–f)
A mixture of isatin/acenaphthoquinone (1.0 mmol), malononitrile (1.0 mmol) and 1,3-dicarbonyl compounds/4-hydroxycumarin/naphthlol 3 (1.0 mmol) in the presence of copper(II) acetate monohydrate as a mild, environmentally benign and natural catalyst under thermal and solvent-free conditions was heated for the appropriate time. After completion of the reaction [as determined by thin layer chromatography (TLC)], the mixture was cooled to room temperature (rt), the solid products were filtered and then were recrystallized from ethanol to give pure compounds (4a–f) and (7a–f). All products were characterized by comparison of spectroscopic data (1HNMR). Spectra data of selected and known products are represented below:
7-Amino-1,3-dimethyl-5-nitro-2,2,4-trioxo-1,2,3,4-tetra-hydrospiro[indoline-3,5-pyrano[2,3-d]pyrimidine]-6-carbonitrile (4a)
mp: 288–290 °C; 1H NMR (400 MHz, DMSO-d6): 1.00 (3H, s, CH3), 1.03 (3H, s, CH3), 2.07–2.19 (2H, m, CH2), 2.50–2.57 (2H, m, CH2), 6.79 (1H, d, J = 7.2 Hz, ArH), 6.89 (1H, t, J = 7.2 Hz, ArH), 6.98 (1H, d, J = 6.8 Hz, ArH), 7.13 (1H, t, J = 6.4 Hz, ArH), 7.22 (2H, s, NH2), 10.38 (1H, s, NH).
2-Amino-7,7-dimethyl-2,5,6,7,8-tetrahydro-2H-spiro[acenaphthylene-1,4-chromene]-3-carbonitrile (7a)
mp: 270–272 °C; 1H NMR (400 MHz, DMSO-d6): 1.02 (3H, s, CH3), 1.04 (3H, s, CH3), 2.04–2.13 (1H, m, CH2), 2.50–2.51 (1H, m, CH2), 2.63 (2H, s, CH2), 7.32 (2H, s, NH2), 7.37–7.85 (6H, m, ArH).
General procedure for preparation of pyrazolo[1,2-b]phthalazine-5,10-dione derivatives (11a–o)
A mixture of phthalic anhydride (8, 1.0 mmol), hydrazine monohydrate (9, 1.0 mmol) and copper(II) acetate monohydrate was heated for 2 h at 80 °C. Then, aromatic aldehyde (10, 1.0 mmol) and malononitrile (12, 1.0 mmol) were added and the mixture was heated for the appropriate time. After completion of the reaction (as per TLC), the mixture was cooled to rt, the solid products were filtered and then were recrystallized from ethanol to give pure compounds (11a–o). All products were characterized by comparison of spectroscopic data (1HNMR). Spectra data of selected and known products are represented below:
3-Amino-1-(2-thenaldehyde)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (11g)
mp: 244–246 °C; 1H NMR (400 MHz, DMSO-d6): 6.09 (1H, s, CHAr), 6.88–7.30 (4H, m, ArH), 7.96–8.28 (6H, m, NH2 and ArH).
3-Amino-1-(3,4,5-trimethoxyphenyl)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (11h)
mp: 254–256 °C; 1H NMR (400 MHz, DMSO-d6): 3.66 (3H, s, OCH3), 3.76 (6H, s, 2 OCH3), 6.07 (1H, s, CHAr), 6.78 (2H, s, ArH), 7.89–8.29 (6H, m, NH2 and ArH).
3-Amino-1-(4-fluorophenyl)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (11i)
mp: 265–267 °C; 1H NMR (400 MHz, DMSO-d6): 6.17 (1H, s, CHAr), 7.20 (2H, t, J = 8.8 Hz, ArH), 7.53–7.57 (2H, m, ArH), 7.96–8.26 (6H, m, NH2 and ArH).
3-Amino-1-(3-methoxyphenyl)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (11l)
mp: 247–249 °C; 1H NMR (400 MHz, DMSO-d6): 3.34 (3H, s, OCH3), 6.09 (1H, s, CHAr), 6.88–7.30 (4H, m, ArH), 7.83–8.26 (6H, m, NH2 and ArH).
3-Amino-1-(4-methylphenyl)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[1, 2-b]phthalazine-2-carbonitrile (11m)
mp: 251–253 °C; 1H NMR (400 MHz, DMSO-d6): 2.30 (3H, s, CH3), 6.10 (1H, s, CHAr), 7.18 (2H, d, J = 8.0 Hz, ArH), 7.34 (2H, d, J = 8.0 Hz, ArH), 7.97–8.28 (6H, m, NH2 and ArH).
Conclusion
In summary, we have studied an efficient, environmentally benignm, natural and economical catalyst for the one-pot, multi-component synthesis of spiro-4H-pyran derivatives and pyrazolo[1,2-b]phthalazine-5,10-dione derivatives. Copper(II) acetate monohydrate catalyzed synthesis of these bioactive compounds under solvent-free and thermal conditions. The notable advantages of this methodology are its use of a mild, non-toxic and inexpensive catalyst, its excellent yields and short reaction times, its highly efficient yields, environmentally benign nature and one-pot and solvent-free conditions.
References
M.M. Garima Kumari, Eur. J. Med. Chem. 46, 1181 (2011)
V.V. Vintonyak, K. Warburg, H. Kruse, S. Grimme, K. Hübel, D. Rauh, H. Waldmann, Angew. Chem. Int. Ed. Engl. 49, 5902 (2010)
J.S. Kim, H.K. Rhee, H.J. Park, S.K. Lee, C.O. Lee, H.Y. Park Choo, Bioorg. Med. Chem. 16, 4545 (2008)
S. Grasso, G. Desarro, N. Micale, M. Zappala, G. Puia, M. Baraldi, C. Demicheli, J. Med. Chem. 43, 2851 (2000)
M.M.-C. Lo, C.S. Newmann, S. Nagayams, E.O. Perlstein, S.L. Schreiber, J. Am. Chem. Soc. 127, 10130 (2005)
J. Li, Y.F. Zhau, X.Y. Yuan, J.X. Xu, I. Gong, Molecules 11, 574 (2006)
S. Yu, D. Qin, S. Shangary, J. Chen, G. Wang, K. Ding, D. McEachern, S. Qiu, Z. Nikolovska-Coleska, R. Miller, S. Kang, D. Yang, S. Wang, J. Med. Chem. 52, 7970 (2009)
A. Fensome, W.R. Adams, A.L. Adams, T.J. Berrodin, J. Cohen, C. Huselton, A. Illenberger, J.C. Kern, V.A. Hudak, M.A. Marella, E.G. Melenski, C.C. McComas, C.A. Mugford, O.D. Slayden, M. Yudt, Z. Zhang, P. Zhang, Y. Zhu, R.C. Winneker, J.E. Wrobel, J. Med. Chem. 51, 1861 (2008)
K. Rad-Moghadam, L. Youseftabar-Miri, Tetrahedron 67, 5693 (2011)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62 (2014)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
R. Sridhar, B. Srinivas, B. Madhav, V.P. Reddy, Y.V.D. Nageswar, K.R. Rao, Can. J. Chem. 87, 1704 (2009)
S.J. Chai, Y.F. Lai, J.C. Xu, H. Zheng, Q. Zhu, P.F. Zhang, Adv. Synth. Catal. 353, 371 (2011)
Y.B. Wagh, Y.A. Tayade, S.A. Padvi, B.S. Patil, N.B. Patil, D.S. Dalal, Chin. Chem. Lett. 26, 1273 (2015)
B. Maheshwar Rao, G. Niranjan Reddy, T. Vijaikumar Reddy, B.L.A. Prasad, R.B.N. Prabhavathi Devi, J.S. Yadav, B.V. Subba Reddy, Tetrahedron Lett. 54, 2466 (2013)
M. Saeedi, M.M. Heravi, Y.S. Beheshtiha, A. Oskooie, Tetrahedron 66, 5345 (2010)
D.S. Raghuvanshi, K.N. Singh, Tetrahedron Lett. 52, 5702 (2011)
M.R. Nabid, S.J.T. Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrason. Sonochem. 17, 159 (2010)
S.H. Song, J. Zhong, Y.H. He, Z. Guan, Tetrahedron Lett. 53, 7075 (2012)
R. Ghahremanzadeh, G. Imani Shakibaei, A. Bazgir, Synlett 8, 1129 (2008)
J. Safaei Ghomi, H. Shahbazi Alavi, A. Ziarati, R. Teymuri, M.R. Saberi, Chin. Chem. Lett. 25, 401 (2014)
Y.D. Reddy, B. Suryanarayana, C.V.R. Reddy, P.K. Dubey, Heterocycl. Lett. 4, 341 (2014)
G. Shanthi, G. Subbulakshmi, P.T. Perumal, Tetrahedron 63, 2057 (2007)
M. Veeranarayana Reddy, P. Chenna Rohini Kumar, G. Chandra Sekhar Reddy, C. Suresh Reddy, C. R. Chim. 17, 1250 (2014)
G. Mohammadi Ziarani, N. Hosseini Mohtasham, A. Badiei, N. Lashkari, J. Chin. Chem. Soc. (2014). doi:10.1002/jccs.201300538
M. Sayyafi, M. Seyyedhamze, H.R. Khavasi, A. Bazgir, Tetrahedron 64, 2375 (2008)
E. Mosaddegh, A. Hassankhani, Tetrahedron Lett. 52, 488 (2011)
L. Torkian, M. Dabiri, P. Salehi, M. Bararjanian, Helv. Chim. Acta 94, 1416 (2011)
Z. Liqin, Z. Bo, L. Yiqun, Chin. J. Org. Chem. 31, 553 (2011)
A. Strecker, Leibigs. Ann. Chem. 7, 27 (1850)
Z. Madanifar, M.T. Maghsoodlou, M. Kangani, N. Hazeri, Res. Chem. Intermed. 41, 9863 (2015)
P. Iniyavan, S. Sarveswari, V. Vijayakumar, Res. Chem. Intermed. 41, 7413 (2015)
F. Mohamad Pour, M.T. Maghsoodlou, R. Heydari, M. Lashkari, Iran. J. Catal. 6, 127 (2016)
N. Xiao, S.H. Wang, A.Y. Zhang, H.Y. Li, P. Wang, W. Li, B.H. Chen, G.F. Chen, N. Li, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-1961-1
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, Res. Chem. Intermed. 41, 2503 (2015)
S. Salahi, M.T. Maghsoodlou, N. Hazeri, F. Movahedifar, R. Doostmohammadi, M. Lashkari, Res. Chem. Intermed. 41, 6477 (2015)
Xu Xiaowen, Yiqun Li, Res. Chem. Intermed. 41, 4169 (2015)
M.T. Maghsoodlou, N. Khorshidi, M. Mousavi, N. Hazeri, S.M. Habibi-Khorassani, Res. Chem. Intermed. 41, 7497 (2015)
B. Mirhosseini-Eshkevari, M.A. Ghasemzadeh, J. Safaei-ghomi, Res. Chem. Intermed. (2015). doi:10.1007/s11164-014-1854-8
G. Mohammadi Ziarani, N. Hosseini Mohtasham, N. Lashgari, A. Badiei, Res. Chem. Intermed. 41, 7581 (2015)
L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu, S. Liu, J. Acs. Comb. Sci. 15, 183 (2013)
X. Xin, D. Xiang, J. Yang, Q. Zhang, F. Zhou, D. Dong, J. Org. Chem. 78, 11956 (2013)
W. Zhang, X. He, B. Ren, Y. Jiang, Z. Hu, Tetrahedron Lett. 56, 2472 (2015)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamadpour, F., Maghsoodlou, M.T., Heydari, R. et al. Copper(II) acetate monohydrate: an efficient and eco-friendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under solvent-free conditions. Res Chem Intermed 42, 7841–7853 (2016). https://doi.org/10.1007/s11164-016-2565-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2565-0