Abstract
A novel and efficient sulfadiazine imprinted polymer was synthesized via co-precipitation method and successfully grafted on magnetic multi-walled carbon nanotubes. The synthesized magnetic imprinted polymer was characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, X-ray powder diffraction analysis, thermal analysis and applied as a sorbent for selective magnetic solid-phase extraction of sulfadiazine. The retained sulfadiazine was eluted by 150.0 µL methanol/acetic acid (6:4) solution and quantified by fiber optic linear array spectrophotometry via formation of a detectable azo dye. All parameters affecting the extraction of sulfadiazine were investigated and optimized. Under the optimized conditions, the method exhibited a linear dynamic range of 2.0–50.0 µg L−1 with a detection limit of 0.56 µg L−1 and enrichment factor of 300.0. The relative standard deviation at 30.0 µg L−1 of sulfadiazine (N = 6) was 2.8 and 4.6% for intra-day and inter-day, respectively. The method was successfully applied to determine sulfadiazine in human urine, honey, milk and environmental water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfadiazine (4-amino-N-2-pyrimidinyl-benzene sulfonamide, SD) is an effective, inexpensive and broad-spectrum synthetic antibiotic agent which is widely used for veterinary purposes to control bacterial infection. Thus, it may enter the food chain via consumption of animal products, water contaminated by surface runoff or transport through soil and uptake by plants [1]. On the other hand, it is well known that sulfadiazine has relatively long half-life and potential carcinogenic effect. Furthermore, the spreading of antibiotics into the environments may lead to the generation of resistant pathogens [2]. Accordingly, there is an urgent need to develop a selective, simple, sensitive and reliable analytical method for monitoring its concentration in different matrices.
Several analytical methods have been reported for the determination of sulfadiazine using different modes of chromatography (HPLC, LC/MS, GC and TLC) [3,4,5], capillary zone electrophoresis [6], enzyme-linked immunosorbent assay (ELISA) [7], different electrochemical techniques [8, 9], chemiluminescence [10], spectrophotometric methods [11,12,13] and flame atomic absorption spectrometry [14]. Among them, spectrophotometric techniques are the most commonly utilized technique for the analysis of different analytes due to the wide availability of the instrument, inherent simplicity, low cost, adequate precision and accuracy. Accordingly, various spectrophotometric methods have been developed for the determination of sulfadiazine in different matrices which the majority of them are based on a derivative reaction of sulfadiazine with different coupling agents such as iminodibenzyl [11], phloroglucinol [12] and α-naphthylamine [13] to produce a detectable azo dye. However, due to the lack of selectivity of spectrophotometry beside the low concentration of sulfadiazine in real samples with the complex matrix, an efficient sample pretreatment is indispensable prior to instrumental quantitative analysis. Several sample preparation methods including cloud point extraction [14], dispersive liquid–liquid microextraction [15] and solid-phase extraction [16] have been used for the separation and preconcentration of sulfadiazine.
Molecular imprinting technique is a promising and powerful method to design and construct tailor-made biomimetic receptors with specific molecular recognition ability. This technique is based on the synthesis of a polymeric matrix in the presence of template molecule/ion. Removal of the template leaves behind specific recognition cavities complementary in size, shape and chemical functionality to the template. Due to the high selectivity, high stability, relative ease of production, reusability and low cost, these polymer-based materials have been applied for separation and solid-phase extraction of a wide variety of target analytes [17]. Despite widespread application, traditionally prepared imprinted polymers suffer some drawbacks including high rigid structures, incomplete template removal, poor site accessibility, low binding capacity and slow mass transfer. However, surface imprinting through grafting imprinted polymer on the surface of a support material has been proved to be a promising technique to overcome these drawbacks via controlling the template molecules to locate at the surface of imprinted materials [18]. Different materials such as Fe3O4 nanoparticles [19], titanium dioxide [20], carbon nanotubes [21] and graphene [22] have been used as a support material in the surface imprinting process. Among them, multi-walled carbon nanotubes (MWCNTs) have attracted great attention due to their extraordinarily high specific surface area, unique atomic structures, high mechanical strength and ease of surface modification [23]. Several kinds of imprinted polymers have been synthesized and applied for the recognition of sulfadiazine, but the synthesis of MIP based on MWCNTs is not reported yet [10, 24,25,26,27,28].
In our previous study, a simple, sensitive and fast spectrophotometric method was developed for quantification of sulfadiazine based on the formation of an azo dye with thenoyltrifluoroacetone as a new coupling agent [29]. In this study, we intended to synthesize a novel molecularly imprinted polymer based on magnetic multi-walled carbon nanotubes (MMWCNTs-MIP) and develop a novel magnetic micro-solid-phase extraction method for selective separation and preconcentration of sulfadiazine. Furthermore, the developed microextraction method was combined with our previously reported spectrophotometric technique to provide a more reliable method for determination of sulfadiazine in complex matrices. The developed method was thoroughly investigated and optimized and successfully applied to determine sulfadiazine in human urine, honey, milk and environmental water samples.
Experimental
Apparatus
An Avantes (Netherlands) photodiode array spectrophotometer model Ava-Spec-2048 with a source model of Ava Light-DH-S-BAL was used for all absorbance measurements. The spectral measurements were done against the blank solution as a reference. The pH measurements were performed with a Metrohm pH meter (model 827, Switzerland) equipped with a combined glass calomel electrode. A model MR 3200 heater-stirrer (Heidolph, Germany) was used for the synthesis of the polymer. A strong magnet (1.2 T, 10 cm × 5 cm × 2 cm) was applied for the magnetic separation.
Standard solutions and reagents
All reagents were of analytical reagent grade and used without any further purification. Multi-walled carbon nanotubes (diameter: 30–50 nm, length: ~20 μm) were obtained from Research Institute of the Petroleum Industry (Tehran, Iran). Sulfadiazine was purchased from Alfa Aesar (Karlsruhe, Germany). 2,2′-Azobisisobutyronitrile (AIBN) was purchased from the ACROS Company (New Jersey, USA). Thenoyltrifluoroacetone was purchased from Sigma-Aldrich (Missouri, USA). All the other chemicals including, methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), ammonium iron (II) sulfate hexahydrate and cetyltrimethylammonium bromide (CTAB) were purchased from the Merck Company (Darmstadt, Germany). Double distilled water was used during the study. A 1000.0 mg L−1 stock standard solution of sulfadiazine was prepared by dissolving an accurately weighed amount of powdered standard in diluted alkaline solution. Working solutions were prepared daily through serial dilutions of the standard stock solution with distilled water. A 1% (w/v) thenoyltrifluoroacetone solution was prepared by dissolving an appropriate amount of the reagent in ethanol.
Real sample preparation
Milk and honey samples were purchased from a local supermarket. Twenty mL of an ethanol/acetic acid (97:3, v/v) solution was added to 200.0 mL of the milk sample and homogenized under sonication for 30 s. The mixture was centrifuged for 5 min at 5000 rpm [30], and 150.0 mL of the supernatant was treated according to the developed procedure. Ten mL double distilled water was added to 20.0 mL of honey sample. The solution was mixed and filtered using a filter paper. The filtrate was then diluted to 150.0 mL with double distilled water, mixed thoroughly and centrifuged at 5000 rpm for 15 min [31]. The supernatant was treated according to the developed method. The urine sample provided by a healthy volunteer was stored at −4 °C until analysis. The sample was centrifuged at 5000 rpm for 15 min. Then, 50.0 mL of the supernatant was diluted to 150.0 mL and treated according to the developed procedures [32]. The Well water sample was filtered using a 0.45 Millipore filter. Then 150.0 mL of it was treated according to the given procedure.
Synthesis of MMWCNTs-MIP
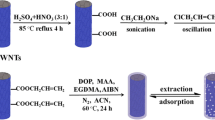
As shown in Fig. 1, the MMWCNTs-MIP was synthesized in three steps. The first step was to introduce the carboxylic groups onto MWCNTs surface. For this purpose, 500.0 mg of MWCNTs was dispersed in 100.0 mL 3:1 (v/v) mixture of concentrated sulfuric/nitric acid under sonication for 2 h and refluxed under magnetic stirring at 80 °C for 3 h. The mixture was then filtered through a 0.45 µm polycarbonate membrane and washed thoroughly until the filtrate was neutral. The resultant carboxylic acid functionalized MWCNTs were dried under vacuum at 60 °C overnight.
The second step was to introduce magnetic property into the carboxyl-functionalized MWCNTs (MWCNTs-COOH) which was achieved through a simple solution method [33]. Briefly, 0.6 g of ammonium iron (II) sulfate hexahydrate was dispersed into 20.0 mL of double distilled water and hydrazine hydrate solution (volume ratio 3:1) which resulted in the instant formation of a grass-green solution. Then 250.0 mg of carboxyl-functionalized MWCNTs was added to the solution, and the mixture was stirred vigorously. Subsequently, the pH of the mixture was adjusted in the range of 11.0–13.0 by the addition of ammonia solution and it was then refluxed at boiling point for 2 h. Finally, the magnetic product was separated using an external magnet and washed several times with double distilled water and ethanol, and dried at 60 °C under vacuum for 24 h.
In the third step, the MMWCNTs-MIP sorbent was prepared. To synthesize MMWCNTs-MIP, 0.5 mmol of sulfadiazine powder was dissolved in 100.0 mL of DMSO followed by the addition of 2.0 mmol of MAA. After 60 min stirring, 500.0 mg of MMWCNTs along with 200.0 mg CTAB (as dispersant [21, 34]) was added and mixed for another 60 min. Subsequently, 10.0 mmol of EGDMA and 50.0 mg of AIBN were added and mixed thoroughly. The mixture was cooled to 0 °C and degassed under a nitrogen atmosphere for 15 min. Finally, the flask of the mixture was sealed and transferred to a water bath. The temperature was slowly raised to 60 °C, and the reaction was allowed to proceed at 60 °C for 24 h. After completion of polymerization, the synthesized magnetic sulfadiazine imprinted polymer was collected using external magnet, washed with double distilled water and ethanol several times and dried under vacuum at 60 °C. To remove sulfadiazine from the MMWCNTs-MIP, the obtained polymer was treated successively with 6:4 mixture of methanol/acetic acid solution until no SD was detected by spectrophotometer in the eluent. The control polymer was prepared by the same procedure, but without the presence of sulfadiazine.
General procedure
20.0 mg of the MMWCNTs-MIP was added to the standard or sample solution of sulfadiazine with pH of ~4. The mixture was stirred by means of a mechanical stirrer for 10 min. The sorbent was then separated using an external magnet followed the decantation of the supernatant. To desorb retained sulfadiazine, 150.0 µL of 6:4 mixture of methanol/acetic acid solution was added to the sorbent and sonicated for 4 min. Subsequently, the sorbent was separated with a magnet and the supernatant was transferred to a spectrophotometric cell for quantification.
Sulfadiazine was spectrophotometrically quantified based on the derivative reaction between diazotized sulfadiazine and thenoyltrifluoroacetone as a coupling agent in the alkaline medium [29]. For this purpose, 70.0 µL of sodium nitrite (5.0 × 10−3 mol L−1) and 90.0 µL of hydrochloric acid (3.0 mol L−1) were added to the extract. The contents were mixed well for a few seconds the sulfadiazine diazotization was completed in acidic medium. Then, 120.0 µL of sodium hydroxide (6.0 mol L−1) along with 90.0 µL of thenoyltrifluoroacetone (1.0%) was added to the solution which resulted in the instant formation of an orange azo dye. The content was mixed thoroughly and the resultant orange azo dye was detected for quantification of sulfadiazine using fiber optic linear array spectrophotometry at 510 nm against the reagent blank.
Results and discussion
Sorbent characterization
The stepwise surface modification of MWCNTs and successful synthesis of the MMWCNTs-MIP were ascertained by the FT-IR spectroscopy (Fig. 2). Figure 2a, b represents the FT˗IR spectra of MWCNTs and MWCNTs-COOH. As it is demonstrated in Fig. 2b, the appearance of the characteristic absorption band of C=O stretching vibration at 1684 cm−1, COO− asymmetric stretching band at 1621 cm−1 and O–H stretching band at 3405 cm−1 confirms the successful introduction of carboxylic groups on the MWCNTs surfaces. The spectrum of magnetic multi-walled carbon nanotubes (MMWCNTs-COOH) (Fig. 2c) showed the same characteristic bands with decreased intensity or a slight shift in some peaks which is due to further modification of MWCNTs–COOH with the magnetic nanoparticle. The observed characteristic band at 559 cm−1, corresponding to Fe–O in Fe3O4, is indicative of successful synthesis of MMWCNTs. Furthermore, the spectrum of MMWCNTs-MIP (Fig. 2d) is quite different. The appearance of absorptions peaks corresponded to O–H stretching vibration at 3441 cm−1, C–H stretching vibration in CH2 and CH3 at 2853 and 2956 cm−1 and the related bending vibrations at 1390 and 1458 cm−1, C=O stretching vibration of carboxyl group (in MAA) at 1731 cm−1, C=C stretching vibration in the benzene ring of sulfadiazine at 1559 cm−1, asymmetric and symmetric C–O stretching of ester (in EGDMA) at 1265 and 1152 cm−1 and the Fe–O stretching vibration at 470 cm−1 indicates that the sulfadiazine imprinted polymer has been grafted successfully on the MMWCNTs–COOH.
The morphological structure of MWCNTs, MMWCNTs and MMWCNTs-MIPs was characterized using SEM (Fig. 3). Comparison of these three images revealed the homogeneous distribution of iron oxide nanoparticles on the MWCNTs (Fig. 3b vs. a) and the successful grafting of MIP on the MMWCNTs surface (Fig. 3c).
The successful synthesis of the sorbent was further verified by investigating the X-ray diffraction pattern of prepared MMWCNTs-MIP. As indicated in Fig. 4a, the appearance of characteristic diffraction peak of MWCNTs (JCPGS Card No. 01-0640) at 2θ = 26.13° and also the characteristic diffraction peaks of Fe3O4 (JCPDS Card No. 19-0629) at 2θ = 30.29, 35.70, 43.28, 53.38, 57.31 and 62.92 confirms the presence of MWCNTs and magnetic phase in the final MMWCNTs-MIP. The TG/DTA analysis was also carried out to investigate the thermostability of the synthesized MWCNTs-MIP. Based on the thermogram represented in Fig. 4b, the sorbent was stable up to 250 °C and the weight loss due to decomposition of MIP layer occurred between 250 and 400 °C. Also, the weight fraction of MP layer was estimated to be ~21.4%. This evidence further verifies the successful synthesis of MMWCNTs-MIP.
Optimization of extraction condition
To assess the capability of synthesized MMWCNTs-MIP as a sorbent for selective magnetic micro-solid-phase extraction of sulfadiazine, different parameters affecting the extraction conditions were investigated and optimized. All optimization experiments were done in triplicate using univariable approach.
Effect of the pH
The sample solution pH may drastically affect the retention capability of the analyte by the sorbent and thereby improve the extraction efficiency. To investigate this effect, the extraction procedure was performed at various pH in the range of 2.0–7.0. As it is shown in Fig. 5a, the extraction recovery increased with increasing the sample pH, reached its maximum amount in the range of 3.5–4.5 and then decreased gradually. These observations can be explained by considering the chemistry of sulfadiazine and the surface binding sites of the sorbent in the aqueous phase. According to the pKa value of MAA (pKa = 4.7), at pH lower than 4.7, the surface charge of MAA, the recognition site on the sorbent, is positive and in higher pHs it is negative. On the other side, sulfadiazine with the pKa values of pKa1 = 1.57 and pKa2 = 6.5 can exist in cationic, neutral or anionic forms depending on the environmental relevant pH [35]. Accordingly, at low pH range (<1.57), sulfadiazine exists mainly in the cationic form which is not favorable for interaction with the protonated recognition sites of the sorbent. Hence, negligible amounts of sulfadiazine are adsorbed to the sorbent. With the increase in pH, the ratio of neutral to the cationic form of sulfadiazine increases and in the pH range of 3.5–4.5 the interaction of analyte and sorbent reaches its maximum, resulting in higher extraction efficiency. At higher pH, the anionic form of sulfadiazine dominates and the extraction recovery decreases as a consequence of the repulsion with negatively charged MAA. Based on this evidence, the pH of ~4 was chosen as optimum pH for quantitative extraction of sulfadiazine.
Effect of a pH of sample solution b Time of extraction c Time of desorption d Ionic strength on the extraction efficiency of sulfadiazine. Conditions: sample volume, 50.0 mL; sulfadiazine concentration, 30.0 µg L−1; amount of sorbent, 20.0 mg; desorbing solution, 150.0 µL mixture (6:4) of methanol/acetic acid
Effects of desorption condition
Effective desorption of analyte from the sorbent is necessary to ensure its quantitative recovery. To optimize the desorption condition, type and volume of desorbing solution were investigated and optimized. For this purpose, different solvents including acetonitrile, methanol, acetic acid and two mixture of methanol/acetic acid with the volume ratio of (9:1) and (6:4) were examined. Among them, 6:4 mixture of methanol/acetic acid was found to be superior in comparison with other eluents. Therefore, it was used as a desorbing solution in subsequent experiments. Likewise, the effect of volume of the desorbing solution on the extraction recovery was examined by varying its volume in the range of 50.0–250.0 µL. The results showed that 150.0 µL of the desorbing solution is sufficient for quantitative extraction of sulfadiazine.
Effect of the sorption and desorption time
To attain quantitative and satisfactory results in a proper time, the effect of sorption and desorption time was considered. To optimize this parameter, the extraction process was carried out at different times in the range of 1–40 min. As demonstrated in Fig. 5b, the extraction recoveries increased with increasing the extraction time from 1 to 10 min and then remained constant by a further increase in the extraction time. Thus, 10 min was sufficient to achieve quantitative extraction of sulfadiazine. The time of desorption of sulfadiazine was also investigated and optimized via varying the time from 1 to 10 min. It was found that the complete removal of sulfadiazine from the sorbent was achieved in 4 min (Fig. 5c). These results confirm the fast sorption/desorption kinetic of the sorbent.
Effect of the amount of sorbent
The effect of the amount of sorbent on the extraction efficiency of sulfadiazine was studied by using a different amount of MMWCNTs-MIP in the range of 2.0–50.0 mg. According to the obtained results, maximum extraction of sulfadiazine was achieved using 20.0 mg of sorbent and more amounts of the sorbent did not lead to a significant improvement. Thus, 20.0 mg of the sorbent was used in subsequent studies.
The effect of ionic strength
To investigate the effect of ionic strength on the extraction efficiency of the sulfadiazine, different amount of NaCl (0–10% (w/v)) was added to the sample solution. Based on the results of this investigation (Fig. 5d), the addition of salt up to 5% did not have a significant and beneficial effect on the analytical signals. However, further addition of salt resulted in a slightly negative effect on the extraction efficiency. This behavior can be related to the increase in viscosity due to the addition of salt, which hinders mass transfer and resulted in lower extraction efficiency. Furthermore, the reduction of interaction between analyte and sorbent surface in the presence of high amount of salt may be another reason for the reduction of extraction efficiency. Consequently, subsequent experiments were done without salt addition.
Effect of sample volume
The ability of the developed method for the enrichment of the trace amount of sulfadiazine from the large sample volume was investigated. For this purpose, the extraction of 1.5 µg of sulfadiazine from different sample volumes (25.0–250.0 mL) was carried out under optimum conditions. The results revealed that the quantitative recovery (≥95%) was achievable up to a sample volume of 150.0 mL. Accordingly, the developed method has the potency of achieving high enrichment factor for the extraction of sulfadiazine.
Capacity and reusability of the sorbent
The sorption capacity of the synthesized imprinted sorbent toward sulfadiazine was measured upon addition of 10.0 mg of MMWCNTs-MIP to the 50.0 mL of sulfadiazine solution with a concentration of 10.0 mg L−1. After 60 min mixing, the amount of sulfadiazine remained in the solution was determined and the sorption capacity of the sorbent was found to be 48.4 mg g−1.
Reusability is a key factor in the assessment of the performance and stability of the sorbent materials. The possibility of regeneration and reusability of the prepared MMWCNTs-MIP was evaluated through the accomplishment of five consecutive sorption–desorption cycles under the optimized conditions. According to the results, extraction recoveries were quantitative without any significant loss in the extraction capability of the sorbent. Therefore, the MMWCNTs-MIP was stable and reusable at least for five sorption–desorption processes.
Selectivity assessment
The selectivity of the synthesized MMWCNTs-MIP was assessed by applying the developed method to sulfadiazine, sulfamethoxazole and sulfathiazole as its structural analogues and metronidazole as the non-analogue. For this purpose, 20.0 mg of MMWCNTs-MIP or MMWCNTs-NIP was added to the solution containing each compound at a concentration level of 30.0 µg L−1. As it can be deduced from the extraction efficiencies demonstrated in Fig. 6, compared with MMWCNTs-NIP, the MMWCNT-MIP exhibited higher rebinding selectivity for sulfadiazine and its analogues. The specific recognition property of the synthesized MIP was evaluated using imprinting factor (α) and selectivity factor (β) defined as follows:
where Q A and Q B are the amounts of the examined compounds retained on the MMWCNT-MIP and MMWCNT-NIP, respectively. α 1 and α 2 represent the imprinting factor with respect to the template and other compounds, respectively. The imprinting factors of 2.58, 1.19, 1.59 and 0.50 for sulfadiazine, sulfamethoxazole, sulfathiazole and metronidazole and the selectivity factors of 2.16, 1.62 and 5.16 for sulfamethoxazole, sulfathiazole and metronidazole were obtained, respectively. Based on these results the yielded imprinting factors for the template is bigger than those of other compounds which indicate the good imprinting effect of the synthesized MIP toward sulfadiazine.
Analytical performance
Once the developed method was optimized, different analytical characteristics including dynamic range, correlation coefficient (R 2), limit of detection, relative standard deviation (RSD) and preconcentration factor were evaluated to assess the method performance. Calibration curve constructed with the sample volume of 150.0 mL was linear over the range of 2.0–50.0 µg L−1 of sulfadiazine with a correlation coefficient of 0.9996 and equation of A = 0.0248C + 0.0011 (where A is absorbance and C is the concentration of sulfadiazine in initial solution). Limit of detection (LOD) of 0.56 µg L−1 was obtained for sulfadiazine based on 3S b/m, where S b is the standard deviation of blank measurements and m is the slope of calibration graph. The preconcentration factor defined as the ratio of the maximum volume of the aqueous phase (150.0 mL) to the final volume of the extract (0.5 mL) was found to be 300.0. The relative standard deviation (RSD) for the six independent measurements of the 30.0 µg L−1 of sulfadiazine was 2.8 and 4.6% for intra-day and inter-day analysis, respectively.
The efficiency of the developed method was further investigated by comparing the analytical parameters of the method with those of recently reported methods for the extraction and determination of sulfadiazine. The results are presented in Table 1. As it can be concluded from Table 1, the developed method provides a lower detection limit and comparable precision. In comparison with our previously developed dispersive micro-solid-phase extraction (DMSPE) method for sulfadiazine which is superior in simplicity, ultra-fast extraction process and being cost-effective by using the very low amount of sorbent, this method provides higher preconcentration factor, lower detection limits as well as higher selectivity.
Application
To assess the feasibility and applicability of the developed method for determination of sulfadiazine in different matrices, the method was utilized for the determination of sulfadiazine in different real samples including milk, honey, environmental water samples and human urine. However, the examined samples were found to be free of sulfadiazine. Therefore, the reliability of the developed method was assessed through the recovery experiments by spiking the samples with three levels of sulfadiazine. As it can be seen from the results given in Table 2, the quantitative recoveries were obtained for all samples type examined (from 94.8 to 101.2%). Thus, the developed procedure is promising and reliable for the determination of sulfadiazine in the wide variety of real samples.
Conclusion
In this study, an efficient molecularly imprinted polymer for sulfadiazine was synthesized via co-precipitation method and successfully grafted on magnetic multi-walled carbon nanotubes (MMWCNTs-MIP) which provide the enhancement in the surface area and facilitate its separation. Furthermore, the synthesized MMWCNTs-MIP was characterized and applied as a novel sorbent for selective magnetic micro-solid-phase extraction of sulfadiazine. The experimental results indicated that the imprinted sorbent possesses fast kinetics and good selectivity for sulfadiazine. Quantification of sulfadiazine was performed using a simple and fast spectrophotometric method based on the formation of an azo dye. Ease of separation of magnetic sorbent, excellent selectivity and fast sorption/desorption kinetic toward sulfadiazine in combination with simplicity, rapidity, sensitivity and specificity of the spectrophotometric method provide a simple, cost-effective, selective and reliable procedure for the determination of sulfadiazine in a wide variety of real samples with complex matrices. Furthermore, the developed method provided good accuracy and precision, low detection limit and high enhancement factor.
References
I. Braschi, S. Blasioli, L. Gigli, C.E. Gessa, A. Alberti, A. Martucci, J. Hazard. Mater. 178, 218–225 (2010)
L. Wang, J. Wu, Q. Wang, C. He, L. Zhou, J. Wang, Q. Pu, J. Agric. Food Chem. 60, 1613–1618 (2012)
Y. Li, J. Han, Y. Yan, B. Chen, G. Zhang, Y. Liu, C. Sheng, J. Iran. Chem. Soc. 10, 339–346 (2013)
J.F. Huertas-Pérez, N. Arroyo-Manzanares, L. Havlíková, L. Gámiz-Gracia, P. Solich, A.M. García-Campaña, J. Pharm. Biomed. Anal. 124, 261–266 (2016)
I.S. Ibarra, J.M. Miranda, J.A. Rodriguez, C. Nebot, A. Cepeda, Food Chem. 157, 511–517 (2014)
F. Tong, Y. Zhang, F. Chen, Y. Li, G. Ma, Y. Chen, K. Liu, J. Dong, J. Ye, Q. Chu, J. Chromatogr. B 942–943, 134–140 (2013)
C. Cháfer-Pericás, Á. Maquieira, R. Puchades, J. Miralles, A. Moreno, Anal. Bioanal. Chem. 396, 911–921 (2010)
S.A.A. Almeida, A.M. Heitor, M.C.B.S.M. Montenegro, M.G.F. Sales, Talanta 85, 1508–1516 (2011)
I. Campestrini, O.C. de Braga, I.C. Vieira, A. Spinelli, Electrochim. Acta 55, 4970–4975 (2010)
F. Lu, H. Li, M. Sun, L. Fan, H. Qiu, X. Li, C. Luo, Anal. Chim. Acta 718, 84–91 (2012)
P. Nagaraja, K.R. Sunitha, R.A. Vasantha, H.S. Yathirajan, Eur. J. Pharm. Biopharm. 53, 187–192 (2002)
K. Upadhyay, A. Asthana, N. Tiwari, Asian J. Pharm. Clin. Res. 5, 222–226 (2012)
J. Fan, Y. Chen, S. Feng, C. Ye, J. Wang, Anal. Sci. 19, 419–422 (2003)
S. Dadfarnia, A.M. Hajishabani, H. Fazeli Rad, J. Chin. Chem. Soc. 58, 503–508 (2011)
W. Yu, Z. Liu, S. Cui, S. Zhang, X. Yang, L. Lei, H. Zhang, A. Yu, Anal. Methods 6, 2545–2552 (2014)
H. Liu, J. Ren, Y. Hao, P. He, Y. Fang, Talanta 72, 1036–1041 (2007)
B. Rezaei, S. Mallakpour, O. Rahmanian, J. Iran. Chem. Soc. 7, 1004–1011 (2010)
Y. Mao, Y. Bao, S. Gan, F. Li, L. Niu, Biosens. Bioelectron. 28, 291–297 (2011)
E. Kazemi, A.M. Haji Shabani, S. Dadfarnia, Microchim. Acta 182, 1025–1033 (2015)
W. Xu, W. Zhou, P. Xu, J. Pan, X. Wu, Y. Yan, Chem. Eng. J. 172, 191–198 (2011)
L. Gao, L. Chen, X. Li, Microchim. Acta 182, 781–787 (2014)
H. Qiu, C. Luo, M. Sun, F. Lu, L. Fan, X. Li, Anal. Chim. Acta 744, 75–81 (2012)
D. Xiao, P. Dramou, N. Xiong, H. He, D. Yuan, H. Dai, H. Li, X. He, J. Peng, N. Li, Analyst 138, 3287–3296 (2013)
P. Ma, Z. Zhou, W. Yang, B. Tang, H. Liu, W. Xu, W. Huang, J. Appl. Polym. Sci. (2015). doi:10.1002/APP.41769
S. Sadeghi, A. Motaharian, Mat. Sci. Eng. C 33, 4884–4891 (2013)
Y.H. Huang, Y. Xu, Q.H. He, Y.S. Cao, B.B. Du, Chin. J. Anal. Chem. 40, 1011–1018 (2012)
L. Xu, J. Pan, Q. Xia, F. Shi, J. Dai, X. Wei, Y. Yan, J. Phys. Chem. C 116, 25309–25318 (2012)
Z. Zhang, M. Li, F. Shen, X. Ren, Anal. Methods 7, 5794–5800 (2015)
E. Kazemi, S. Dadfarnia, A.H.M. Shabani, A. Abbasi, M.R.R. Vaziri, A. Behjat, Talanta 147, 561–568 (2016)
X. Huang, D. Yuan, B. Huang, Talanta 72, 1298–1301 (2007)
F. Yu, C. Liu, Y. Guo, Y. Yang, Anal. Methods 5, 3920–3926 (2013)
N. Unceta, A. Gómez-Caballero, A. Sánchez, S. Millán, M.C. Sampedro, M.A. Goicolea, J. Sallés, R.J. Barrio, J. Pharm. Biomed. Anal. 46, 763–770 (2008)
T. Madrakian, A. Afkhami, M. Ahmadi, H. Bagheri, J. Hazard. Mater. 196, 109–114 (2011)
D. Xiao, P. Dramou, N. Xiong, H. He, H. Li, D. Yuan, H. Dai, J. Chromatogr. A 1274, 44–53 (2013)
C.Y. Lin, S.D. Huang, Anal. Chim. Acta 612, 37–43 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazemi, E., Dadfarnia, S. & Haji Shabani, A.M. Synthesis of a novel molecularly imprinted polymer based on functionalized multi-walled carbon nanotubes for selective extraction of sulfadiazine prior to spectrophotometric determination. J IRAN CHEM SOC 14, 1935–1944 (2017). https://doi.org/10.1007/s13738-017-1132-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1132-y