Abstract
The authors describe a molecularly imprinted polymer (MIP) deposited on multiwalled carbon nanotubes (MIP/MWCNTs) for separation and preconcentration of L-cysteine (L-Cys). The MIP was characterized by scanning electron microscopy, X-ray diffraction and FT-IR and via adsorption kinetics and adsorption isotherms. The MIP is shown to be a viable sorbent for L-Cys which subsequently is quantified by spectrophotometry through formation of a charge transfer complex with the DDQ reagent. The experimental parameters affecting separation efficiency and spectrophotometric determination were optimized. Under optimum conditions and at an analytical wavelength of 478 nm, the calibration plot is linear in the 4.0 to 180 ng mL−1 concentration range, and the limit of detection (at an S/N ratio of 3) is 2.3 ng mL−1. The intra-day and inter-day precision are in the range from 2.4 to 3.6%. The method was successfully applied to determination of L-Cys in spiked human serum and water samples where it gave recoveries ranging from 96.6 to 102.4%.

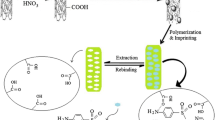
Schematic of the preparation of a molecularly imprinted polymer coated on the multiwalled carbon nanotube (MIP/MWCNT) by functionalization of MCNTs with methacrylic acid and subsequent polymerization. The MIP/MWCNTs were successfully applied for extraction and spectrophotometric determination of L-Cys by charge transfer (CT) complexation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
L-Cys participates in a variety of important cellular functions, among others protein synthesis, detoxification and metabolism [1]. L-Cys has several pharmaceutical applications; namely, it is used in some antibiotics for the treatment of skin damage, and as a radio protective agent. Also, it is widely used in the food industry as an antioxidant and in the pharmaceutical industry in drug formulation and as a biomarker [2]. Therefore, the detection of L-Cys is important, and considerable efforts have been made to develop high efficient methods for its determination. A number of analytical methods have been reported for determination of L-Cys, such as high performance liquid chromatography (HPLC) [3], capillary electrophoresis [4], gas chromatography [5], spectroflourimetry [6], electrochemical [7], spectrophotometry [8] and chemiluminescence [9]. These methods provide high sensitivity and low detection limit, but they suffer from some disadvantages, such as, multi-step operation, extend clean up steps and expensive instrumentation. Also, they need chemical derivatization to overcome interference effects and to increase sensitivity. Compared with the expensive methods, the cost of conventional spectrophotometric method and its simplicity makes it a widely accepted detection method. However the application of this method is mainly limited by its poor selectivity and sensitivity [10]. Therefore, Hyphenation of advanced sample preconcentration and cleanup methods with ordinary spectrophotometric method can be worthy. Solid phase extraction (SPE) has been recognized a solvent less and efficient sample preparation procedure for a wide range of samples [11]. The simplicity of the operation, selectivity and efficiency of SPE procedure are mainly dependent on the proper choice of the sorbent material [12]. In the past few years, molecularly imprinted polymers (MIPs) has been considered as a selective adsorbent in SPE, and found wide applications in analysis of real samples with complex matrices, as highlighted by several reviews [13–15]. MIPs are synthetic polymers possessing specific cavities designed for a target molecule and are synthesized by the polymerization of different components [16]. MIPs have attracted increasing attentions due to their advantages involve in predetermined recognition ability, chemical stability, relative ease and simple preparation. Aswni et al. used methacrylic acid (MAA) based MIPs for modification of carbon paste electrode (CPE) to determine L-Cys [17]. Also, Cai et al. used a MIPs for extraction and subsequent spectroflorimetric determination of L-Cys [18]. The conventional bulk MIP has intrinsic limitations, such as low adsorption capacity, poor site accessibility and irregular shape [19]. For avoiding of these defects, surface molecular imprinting technique has been proposed [20, 21]. Usefulness of active cavities, number of active cavities, binding kinetic and structure regularity of MIPs can be significantly improved by grafting of MIPs on supported materials such as carbon nanotubes (CNTs) [22, 23], mesoporous silica [24], nano particles [25] and nano capsule [26]. Coupling of spectrophotometric method with an advance sample preparation methods such as SPE with MIPs grafted with MWCNTs (MIP/MWCNTs) can be worthy. The aim of this study was to report an approach for preparation of MIP/MWCNTs based on the functionalization of MWCNTs by MAA and subsequent polymerization for preparation of a selective MIP/MWCNTs composite for separation and enrichment of L-Cys prior to its spectrophotometric determination. Spectrophotometric determination was carried out using charge transfer complex formation between removed L-Cys and 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) as a π-acceptor reagent. Extraction with MIP increases the selectivity and derivatization of extracted L-Cys improves the sensitivity of the spectrophotometric detection of L-Cys. The main factors affecting extraction efficiency and spectrophotometric reaction were investigated and optimized.
Experimental
Materials and reagents
MWCNTs (mean diameter of 30 nm) were obtained from Iranian Nanomaterials Pioneers Co. (Mashhad, Iran, https://sites.google.com/site/nanosuny/home). Methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), L-Cys, Tionilchloride and 2,3-dichloro 5,6-dicyano-p-benzoquinone (DDQ) were purchased from Sigma-Alderich (St. Louis, USA, http://www.sigmaaldrich.com). Acetonitrile, acetone, dimethyl formamide, sodium hydroxide (NaOH), hydrochloric acid (HCl), ethanol and methanol were obtained from Merck (Darmstadt, Germany, http://www.merckgroup.com).
Apparatus
A UV-Vis spectrophotometer model SPECORD 210 (Analytikjena, Germany) with a 300 μL uartz microcell (Fisher Co., Germany) was used for spectrophotometric measurements. The synthesized MIPs were characterized by a scanning electron microscopy (SEM), (Philips, CM120, 100 KV), APD 2000 X-ray diffractometer (XRD) (Italstructures, Italy) and FT-IR spectrometer (Perkin-Elmer, Spectrum version 10.01.00, USA). A Metrohm 713 pH-meter equipped with a combined glass calomel electrode was used for the pH adjustments at 25 ± 1 °C temperature. A centrifuge Model 16,105 (Farayand Co., Iran) was used for separation of adsorbents. An ultrasonic water bath model Parsonic 2600 s (Parsnahand Co., Iran) was used for dissolution of reagent.
Pretreatment of MWCNTs
The MWCNTs was preconditioned in two steps. At first, the surface of MWCNTs was oxidized by acid treatment [13]. Briefly, 500 mg of MWCNTs was added to 50 mL of H2SO4–HNO3 (3:1, v/v) solution and the suspension was refluxed at 60 °C for 4 h. The oxidized MWCNTs were filtered and washed with deionized water (200 mL, three times) and dried in a vacuum oven in 100 °C for 5 h. Then, the functionalized MWCNTs were suspended in 1 mL of DMF and 10 mL tionilcholoride, was added into the dispersion and stirred for 1 h. After the mixture was maintained at 80 °C for 24 h under nitrogen atmosphere, the product was washed with acetone. The product was dried under a vacuum oven at room temperature for 24 h.
Covalent functionalization of MWCNTs with MAA
For this step 500 mg of pretreated MWCNTs dispersed in 20 mL acetonitrile for 10 min, then 1 mL of MAA and 50 mg sodium bicarbonate were added, the mixture was maintained at 80 °C under nitrogen atmosphere for 2 h for covalent functionalization of MWCNTs with MAA. Then, the MAA-functionalized MWCNTs (MAA/MWCNTs) were filtrated, washed repeatedly (40 mL, five times) with deionized water and dried in vacuum.
Synthesis of L-Cys MIP/MWCNTs
1 mmol of L-Cys, 300 mg of MAA/MWCNTs and 4.0 mmol of MAA were added into a 100 mL round flask containing 20 mL of acetonitrile. The mixture was incubated for 1 h at room temperature for pre-polymerization. Then, 20 mmol of EGDMA was added and the mixture was purged with nitrogen for 10 min to remove oxygen. Afterwards, 30 mg of AIBN was added for initiation of polymerization. The reaction was allowed to proceed at 60 °C for 24 h. After the polymerization, the polymers were washed with ethanol/acetic acid (9:1, v/v) mixture for several times until the template was no longer detectable by spectrophotometry. Finally, the MIP/MWCNTs were dried in vacuum at 60 °C for 30 min. For comparison, non-molecularly imprinted polymers multiwalled carbon nanotubes (NIPs/MWCNTs) were prepared by the same procedure, only without addition of L-Cys in the polymerization process. The preparation process of MIP/MWCNTs is schematically depicted in Scheme 1.
Preconcentration procedure
An aliquot of sample solution containing 50 ng mL−1 of L-Cys was transferred into a 250 mL beaker. The pH of the solution was adjusted to 6.5 by the drop wise addition of aqueous HCl (0.1 M). Then 50 mg of the MIP/MWCNTs was added to solution and the mixtures were shaken for 20 min. After removal of the supernatant by centrifugation (3500 rpm, 3 min), the adsorbents was eluted with 1.5 mL ethanol/acetic acid (9:1, v/v) mixture. Then, the obtained eluent was pipetted into a test tube for charge transfer complex formation. Thus, 75 μL of DDQ 5 × 10−4 M was added to the solution for 20 min at 25 °C, and the resultant solution was subjected to the final spectrophotometric determination at 478 nm.
Adsorption studies
Adsorption study was performed to examine the adsorption kinetic and adsorption isotherms at 25 °C. The adsorption kinetic of the MIP/MWCNTs toward L-Cys was investigated by following procedure: In a centrifuge tube, 20.0 mg of the MIP/MWCNTs and/or NIPs/MWCNTs were suspended in 10 mL of 80.0 mg L−1 L-Cys. These tubes were incubated at room temperature under shaking. Ten samples were taken out at time range of 5–50 min with a five min interval. Then, L-Cys concentration in supernatant was measured spectrophotometrically and the adsorption capacity Q (mg g−1) of the polymers for L-Cys was calculated according to the following formula:
where Ci (mg L−1) and Ct (mg L−1) represent the initial and final L-Cys concentration, respectively. V (L) is the sample volume and m (g) is the MIP mass.
For study of adsorption isotherms, suitable aliquots of MIP/MWCNTs (20 mg) were introduced into different centrifuge tubes. Then, 10 mL of L-Cys with initial concentration ranging from 10.0 to 100.0 mg L−1 was added into each centrifuge tube. The centrifuge tubes were stirred at 25 °C for 90 min. The adsorbent was centrifuged immediately at 3500 rpm (RCF = 1369) for 3 min and the supernatant solution was analyzed to determine the remaining concentration (Ce, mg L−1) using spectrophotometry. Finally, the equilibrium adsorption capacity Qe (mg g−1) was calculated according to the following equation:
Real sample analysis
To evaluate the applicability of the method for real samples analysis, tap water and human serum samples were selected for investigated of application of the presented method. The samples were pretreated by the relevant procedure as follows:
Human serum
The human serum sample was stored in a refrigerator after collection. A 1 mL of the human serum was directly diluted to the 100 mL by deionized water. Due to high sensitivity of the presented method, the concentration of the L-Cys in final sample solution should controlled in the linear rang of determination. An aliquot of the prepared sample was analyzed by analytical procedure.
Tap water
Tap water sample were filtered through a filter paper (45 μm) and then used directly for analysis.
Results and discussion
The MIP/MWCNTs was synthesized in three steps: pretreatment of MWCNTs surface, grafting of MAA on surface of nanotubes and subsequent polymerization. The Spectrophotometric detection was carried out by L-Cys reaction with DDQ, as a π acceptor, to form a highly colored charge transfer complex. Due to poor sensitivity of direct spectrophotometric determination of L-Cys, the reaction of L-Cys with DDQ, to form of a highly colored charge transfer complex, was used for improving of detection sensitivity. Regarding to absorption spectra of the sample and blank after SPE (Fig. S1), the corrected absorbance at 478 nm was selected as the extraction efficiency under different experimental conditions. The reagent blank at this wavelength show a low absorption. The effects of experimental parameters for both micro extraction procedure and spectrophotometric reaction have been investigated and optimized. The corrected absorbance at 487 nm was used to assess the extraction efficiency under different experimental conditions. A unit variety approach was employed for optimization and all results were average of five replicate measurements.
Characterization
The FTIR spectra of the products in each step of the MIP/MWCNTs synthesis were recorded to verify the formation of the expected products. The FT-IR spectrum for oxidized MWCNTs is shown in Fig. 1a. The absorption bands at 3435 cm−1 (which is attributed to the stretching vibration of OH groups) and 1733 cm−1 (corresponding to the C = O vibrations) are indicating the presence of functional groups (COOH) on the surface of the MWCNTs. Furthermore, as shown in Fig. 1b, the characteristic absorption band at 732 cm−1 which corresponds to the C–Cl absorbance, confirmed the surface choloration. The FT-IR spectrum for covalent functionalization of MWCNTs with MAA is shown in Fig. 1c. The peak of C = O stretching vibration was shifted down to 1726 cm−1, which was characteristically assigned to carbonyl stretching of carboxylic acid. Also, the characteristic peaks at 3439 cm−1 (O–H stretching peak), and 732 cm−1 are related to COOH group of MAA stretching vibration. The obvious stretching vibration of C– O group at 1732 cm−1 and the increasing of C–O–C band intensity at 1157 cm−1 revealed the existence of EGDM. Moreover, new absorption peaks at 2989 and 2958 cm−1 are related to the stretching modes of aliphatic C–H bonds in the final product (Fig. 1d). Based on the above results, it can be concluded that the fabrication procedure has been successfully performed. Fig. 2a and b show the SEM images of MWCNTs and MIP/MWCNTs which demonstrate that the MWCNTs have a rod structure. The comparison of SEM images of the MCNTs and MIP/MWCNTs indicates that the MWCNTs are enwrapped rather homogeneously by MWCNT amount layer. Fig. 3 shows the XRD of MIP/MWCNTs and MWCNTs. The typical diffraction peaks of MWCNTs can be observed at 2θ = 26.0°. The average crystallite size of the MIP/MWCNTs nanoparticles, based on the Scherer equation, was estimated to be approximately 25 nm according to Scherer equation.
Point of zero charge of the MIP/MWCNTs (pHpzc)
Surface charge of MWCNT amount and therefore their recognition properties can be changed at different pHs when ionizable functional groups such as methacrylic acid, vinyl benzoic acid and etc. are introduced into the polymer matrix [13]. In this study, the pHpzc of the MIP/MWCNTs was determined in degassed 0.01 mol L−1 NaNO3 solution at 20 °C. Aliquots of 30 mL of 0.01 mol L−1 NaNO3 were mixed with 50 mg MIP/MWCNTs in several beakers. The pH of the solutions was adjusted in the range 2.0–10.0 using HNO3 and/or NaOH solution. The initial pH values of the solutions were recorded, and the beakers were covered with par film and shaken for 24 h. Then, difference of final pH and initial pH (∆pH) for each solutions was plotted against their initial pH values (Fig. S2). According to the results of this study, the pHpzc was determined to be o be 6.3.
Optimization of experimental parameters
Effect of pH
The pH of the sample solution plays an important role in the adsorption process of the L-Cys by affecting both the forms of analyte in solution and the surface charge of adsorbent. Therefore, the effect of sample pH on the adsorption of L-Cys was investigated in the range of 2.0–11.0 (Fig. 4). Depending on the sample pH, L-Cys can exist in various forms, such as, H3A+ at pH values <4.0 due to protonated amino group, H2A (uncharged) at the pH range 4.0–9.0, HA− (carrying a COO− group) at pH range 9.0–12.0 and A2− (carrying COO− and S−) at pH ≥12. As Fig. 4 shows, the adsorbent provided the highest affinity to L-Cys at pH range of 6.0–7.0. Regarding to the pHpzc of MIP/MWCNTs (6.3), uncharged form of L-Cys is preferentially extracted by MIP/MWCNTs due to non-electrostatic interactions. The electrostatic repulsion forces are responsible for lower extraction efficiencies in other pHs. Therefore pH 6.5 was selected for the further experiments.
Effect of pH on the adsorption efficiency of L-Cys. Conditions: Sample volume: 50 mL, L-Cys: 50.0 ng mL−1, sample volume: 50 mL, Adsorbent amount: 50 mg, adsorption time: 20 min, desorption time: 10 min, eluent volume: 2 mL, analytical wavelength: 487 nm. Error bars represent the standard deviation for three experiments
Effect of adsorption time
To obtain satisfactory extraction with a short analysis time, it is necessary to select an adsorption time that provides the equilibrium between the sample solution and the adsorbent. The effect of adsorption time on recovery of L-Cys was investigated in the range of 5–50 min. The results showed that an adsorption time of 20 min was sufficient to attain adsorption equilibrium. So an adsorption time of 20 min was selected for subsequent investigations.
Effect of sample volume
In order to obtain a higher enrichment factor in SPE, a larger volume of sample solution is required. The effect of sample volume on the L-Cys extraction was investigated using different sample volumes in the range of 5–150 mL, which were spiked with 50 ng mL−1 of L-Cys. It was found that the quantitative recoveries were obtained up to 110 mL. The extraction efficiency was decreased at higher sample volumes due to the analyte loss from the adsorbent surface. Thus, the volume of 100 mL was selected for subsequent experiments to obtain a higher preconcentration factor.
The MIP/MWCNTs amount
Compared to conventional micro-size sorbents, MIPs sorbents have higher surface areas and satisfactory results can be achieved with fewer amounts of MIPs. Thus, to study the effect of adsorbent amount on the extraction efficiency, different amounts of adsorbent in the range of 10–70 mg were added to the 50 mL of 50 ng mL−1 of analyte solution. The results showed that the extraction efficiency increased with increasing amounts of adsorbent up to 60 mg and then leveled off. Therefore, 50 mg of adsorbent was found to be the optimum.
Desorption conditions
In most of SPE producers, desorption process is a rather critical step and selection of desorption solvent is very important. A suitable desorption solvent should effectively remove the template with the minimum volume. It also should not damage the favorable structure of decorated MIP/MWCNTs. On the basis of the above considerations, the usefulness of several types of desorption solvents, including methanol, ethanol, methanol/acetic acid (9:1 v/v), ethanol/acetic acid (9:1 v/v), ethanol/HCl 0.01 mol L−1 (2:1 v/v), methanol/ HCl 0.01 mol L−1 (2:1 v/v) and acetic acid 0.01 mol L−1was examined. The results (Fig. 5) showed that the mixture of ethanol/acetic acid (9:1 v/v) is the most effective eluent and can be used for the quantitative recovery of the L-Cys. Also the effect of desorption time was investigated in the range of 1–25 min. The obtained results showed that a duration time of 10 min appeared to be sufficient for complete desorption and no significant effect was observed when the time of desorption was greater than 10 min. The effect of eluent volume on L-Cys recovery was further investigated in the range of 1–6 mL. A smaller volume of the eluent is preferred for efficient elution efficiency (> 95%) and higher enrichment factor. The experimental results showed that a volume of 1.5 mL is enough to obtain the best enrichment of L-Cys.
Effect of the complexation conditions
The effect of DDQ concentration on the measured absorbance was investigated by adding different amounts of DDQ (5 × 10−4 mol L−1) to desorbed L-Cys in the range of 10–100 μL. The results revealed that the highest absorbance was obtained over the 75 μL. Therefore a volume of 75 μL was selected for subsequent experiments. The effect of complexation time on the absorbance signal was also investigated in the range of 1–30 min at room temperature (25 ± 2 °C). The results showed that reaction was complete within 20 min. Thus the time of 20 min was selected as optimal complexation time.
Reusability and stability
Reusability of adsorbent is very important in solid phase extraction procedures. In order to examine the potential of the fabricated MIP/MWCNTs for recycling, the used adsorbent was rinsed with deionized water (20 mL, once) and subjected to loading and elution procedure under optimum conditions. The results showed that the adsorbent can be used at least for seven times without any significant loss in recoveries of L-Cys (less than 5%). Over seven cycle of sorption/desorption, the performance of adsorbent decreased gradually due to shrinking–swelling of MIP during desorption process and partial destruction of imprinted cavity [27]. This study indicates that the prepared adsorbent shows good reusability and stability with no analyte carryover up to seven successive extraction procedures.
Adsorption study of MIP/MWCNTs
Adsorption kinetics
The rate of adsorption is important for evaluation of the adsorption efficiency. Therefore, the adsorption kinetics of templates on the MIP/MWCNTs and NIPs/MWCNTs were investigated. The results (Fig. S3) showed that the adsorption amounts of L-Cys increased with time and leveled off gradually in the end. At the beginning, as MIP/MWCNTs provides large quantities of unoccupied imprinted binding sites, L-Cys molecules are adsorbed preferentially to react with the binding sites of the MIP/MWCNTs, displaying rapid adsorption for L-Cys. The adsorption rates of L-Cys decrease after 20 min because the internal diffusion of template molecules into binding sites has to overcome the mass transfer resistance. NIPs/MWCNTs and MIP/MWCNTs are similar in the adsorption tendency for L-Cys, but MIP/MWCNTs exhibits higher adsorption capacity. The reason should be attributed to the presence of enough imprinted cavities in MIP/MWCNTs [28]. Furthermore, the adsorption equilibrium of MIP/MWCNTs is achieved within 25 min and the adsorption capacity reaches 8.7 mg g−1 (L-Cys), respectively. In previous studies, the adsorption of some template onto MIP/MWCNTs was equilibrated after 3 h or even more [29]. The short adsorption equilibrium time is one of the MIP/MWCNTs advantages. It is also obvious that the adsorption amount of the MIP/MWCNTs is higher than those of the NIPs/MWCNTs. This suggests that the imprinted cavities of the MIP/MWCNTs provide high binding affinity of the templates to the polymer. Therefore, the short equilibrium time and the high adsorption capacity suggest the potential applications of MIP/MWCNTs for the removal of L-Cys.
Adsorption isotherms
Adsorption isotherms were used to estimate binding capacity and to evaluate binding behavior of the prepared MIP/MWCNTs. The result of this study (Fig. S4) showed that, adsorption capacity of polymers towards L-Cys increased with the increasing of initial concentration of L-Cys up to 8.6 mg L−1and then leveled off, indicating saturation of the active binding sites of the polymers. The saturated adsorption values were found to be 9.07 mg g−1 and 2.53 mg g−1 for MIPs/MWNTs and NIPs/MWCNTs, respectively. Also it can be seen that the amounts of L-Cys bound to MIP/MWCNTs are remarkably higher than those of bound to NIP/MWCNTs under same experimental conditions. This can be attributed to the generation of cavity recognition sites in MIP/MWCNTs during imprinting process. The equilibrium binding data were further used to evaluate the binding properties of MIP/MWCNTs and NIPs/MWCNTs by Langmuir and Freundlich models. Langmuir model assumes that each active binding site can only hold one template, however the Freundlich model is not restricted to the formation of a mono layer [30]. The equilibrium data were fitted to Langmuir and Freundlich isotherm models by linear regression. The results of this study (Table S1) showed that Langmuir model give the better R2 coefficient (0.99) and therefore can better describe the binding processes. In addition, due to imprinting effect, the maximum adsorption and the Langmuir adsorption equilibrium constant of the MIP/MWNTs were higher than those of the NIPs/MWCNTs.
Selectivity of the MIP/MWCNTs
Selectivity of prepared MIP/MWCNTs toward L-Cys was investigated by recovery studies of L-Cys in the percent of potential interferences with similar structures (glycine (Gly), homocysteine (Hcy), gluthatione (GSH), histidine (His), arginine (Arg)) in concentrations up to 100 times higher than that of L-Cys. The obtained results (Table S2) showed that recoveries of L-Cys were not significantly changed in the presence of interferences. All the experiments were performed in triplicate. This high selectivity of this MIP for L-Cys is attributed to the presence of tailor- made binding sites for L-Cys in terms of interaction and size.
Analytical parameters
Analytical characteristics of the presented method were evaluated under optimized conditions (Table S3). Calibration curve was obtained by least-squares linear regression analysis of the corrected absorbance (n = 3) versus concentration of analyte using ten concentration levels. The calibration curve was linear over the range 4.0–180.0 ng mL−1 with linear regression eq. A = 0.009C + 0.044 (A, corrected absorbance at 487 nm and C ng mL−1of L-Cys) and correlation coefficient R2 = 0.998. The limit of detection (LOD), defined as LOD = 3Sb /m (where, Sb and m are standard deviation of the blank and the slope of the calibration graph, respectively) was found to be 2.3 ng mL−1. The precision of the method was evaluated through of the investigation intra-day precision and inter-day precision as relative standard deviation (RSD %). The intra-day precision was evaluated over five replicates spiked concentration (50.0 ng mL−1of L-Cys) within one day (n = 5). The inter-day precision was evaluated over five daily replicates, spiked at same level per work day, over a period of three days (n = 15). The intra-day and inter-day precisions were found to be 2.4 and 3.6, respectively. Furthermore, enrichment factor (EF) was calculated by EF = Vs/Ve × R% definition (where Vs is the sample volume, Ve is the elution solvent volume, and R% is extraction yield). By considering the optimum elution volume of 1.5 mL and sample volume of 100 mL (R = 98.9%), an enrichment factor of 72.52 was achieved for L-Cys determination. A comparison of the analytical characteristics of the presented method and other methods is presented in Table 1. The presented method has distinct advantages in term of simplicity, low detection limit and ease of operation.
Real sample analyses
The applicability of the presented method to the real samples was investigated by determination of L-Cys in human serum and tap water. Recovery studies were carried out by adding known concentrations of L-Cys at three levels to dilute solutions of samples. For each concentration level, six replicate experiments were made, and the results obtained were compared with the added concentrations. Also the recovery tests were carried out by spiking the human serum and tap water with different amounts of L-Cys (30, 50 and 100 ng mL−1). The obtained relative recoveries (Table 2) varied from 96.6% to 102.4%, showing the absence of a matrix effect on the performance of the presented method. The accuracy of the method was checked with the reference method [35]. The results are presented in Table 3. The statistical analysis of the results using Student’s t-test showed that there are no significant differences between the results obtained by two methods at a 95% confidence level. These good recoveries demonstrate that the purposed method is sufficiently applicable to determine L-Cys in real samples.
Conclusion
In this study, a fast and sensitive method based on the SPE with MIP/MWCNTs followed by spectrophotometric detection through charge transfer complexation has been developed for separation and determination of L-Cys. The MIP/MWCNTs composite was prepared by functionalization of MWCNTs by MAA and subsequent polymerization. The prepared MIPs proved to be an efficient and selective adsorbent for separation and enrichment of trace level of L-Cys from real samples. Coupling of SPE by MIP/MWCNTs and subsequent charge transfer complex formation significantly improved selectivity and sensitivity of the ordinary spectrophotometric determination of L-Cys. The presented method successfully discriminated L-Cys from Hcy, GSH and other amino acids in real samples. Also, this method provides excellent analytical performance toward L-Cys, such as high sensitivity and wide linear range. Moreover, the combination of MIP/MWCNTs and spectrophotometry will greatly enrich the research contents as well as broaden the applications of molecular imprinting techniques. The comparison of presented method and other reported methods shows that the analytical performance of presented method is comparable with some sensitive instrumental methods.
References
Ensafi AA, Rezaei B, Nouroozi S (2009) Flow injection Spectrofluorimetric determination of Cystine and cysteine. J Braz Chem Soc 20(2):288–293
Ahmad M, Pan C, Zhu J (2010) Electrochemical determination of L-cysteine by an elbow shaped, Sb-doped ZnO nano wire-modified electrode. J Mater Chem 20(34):7169–7174
Kaniowska E, Chwatko G, Głowacki R, Kubalczyk P, Bald E (1998) Urinary excretion measurement of cysteine and homocysteine in the form of their S-pyridinium derivatives by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A 798(1–2):27–35
Jin W, Chen H (2000) A new method of determination of diffusion coefficients using capillary zone electrophoresis (peak-height method). Chromatographia 52(1):17–21
Kubec R, Svobodova M, Valisec J (2001) Gas-chromatographic determination of Smethylcysteine sulfoxide in cruciferous vegetables. Eur Food Res Technol 213(4–5):386–388
Huang S, Wang L, Huang C, Hu B, Su W, Xiao Q (2016) Graphene quantum dot coupled with gold nanoparticle based “off-on” fluorescent probe for sensitive and selective detection of L-cysteine. Microchim Acta 183(6):1855–1864
Devasenathipathy R, Mani SM, Chen SM, Kohilarani K, Ramaraj S (2015) Determination of L- cysteine at iron tetrasulfonated phthalocyanine decorated multiwalled carbon nanotubes film modified electrode. Int J Electrochem Sci 10:682–690
Blasco F, Medina-Hernandez MJ, Sagrado S (1997) Use of pH gradients incontinuous-flow systems and multivariate regression techniques applied to the determination of methionine and cysteine in pharmaceuticals. Anal Chim Acta 348(1–3):151–159
Dong Y, Su YM, Chen P, Sun H (2015) Chemiluminescence of carbon dots induced by diperiodato-nicklate (IV) in alkaline solution and its application to a quenchometric flow-injection assays of paracetamol, L-cysteine and glutathione. Microchim Acta 182(5-6):1071–1077
Wen X, Yang Q, Yan Z, Deng Q (2011) Chemical analysis of food: techniques and applications. Microchem J 97(2):249–254
Pohl P, Stecka H, Jamroz P (2012) Solid phase extraction with flame atomic absorption spectrometry for determination of traces of Ca, K, mg and Na in quality control of white sugar. Food Chem 130(2):441–446
Ozdemi N, Soylak M, Elci L, Dogan M (2004) Speciation analysis of inorganic Sb(III) and Sb(V) ions by using mini column filled with Amberlite XAD-8 resin. Anal Chim Acta 505(1):37–41
Martín-Esteban A (2001) Molecularly imprinted polymers: new molecular recognition materials for selective solid-phase extraction of organic compounds. Fresenius J Anal Chem 370(7):795–802
Lasakova M, Jandera P (2009) Molecularly imprinted polymers and their application in solid phase extraction. J Sep Sci 32(5–6):799–812
Cheong WJ, Yang SH, Ali F (2013) Molecular imprinted polymers for separation science: a review of reviews. J Sep Sci 36(3):609–628
Lin Z, Cheng W, Li Y, Liu Z, Chen X, Huang C (2012) Novel superparamagnetic surface molecularly imprinted nanoparticle adopting dummy template: an efficient solid-phase extraction adsorbent for bisphenol. Anal Chim Acta 720:71–76
Aswini KK, Vinu Mohan AM, Biju VM (2014) Molecularly imprinted polymer based electrochemical detection of L-cysteine at carbon paste electrode. Mater Sci Eng C 37:321–326
Cai X, Li J, Zhang Z, Wang G, Song X, You J, Chen L (2014) Chemodosimeter-based fluorescent detection of L-cysteine after extracted by molecularly imprinted polymers. Talanta 120:297–303
Xie CG, Zhang ZP, Wang DP, Guan GJ, Gao DM, Liu JH (2006) Surface molecular self-assembly strategy for TNT imprinting of polymer nanowire/nanotube arrays. Anal Chem 78(83):39–46
Yang H-H, Zhang S-Q, Tan F, Zhuang Z-X, Wang X-R (2005) Surface molecularly imprinted nanowires for biorecognition. J Am Chem Soc 127(5):1378–1379
Tan CJ, Chua HG, Ker KH, Tong YW (2008) Preparation of bovine serum albumin surface-imprinted submicrometer particles with magnetic susceptibility through core shell miniemulsion polymerization. Anal Chem 80(3):683–692
Hu TP, Zhang YM, Zheng LH, Fan GZ (2010) Molecular recognition and adsorption performance of benzothiophene imprinted polymer on silica gel surface. J Fuel Chem Technol 38(6):722–729
Dai H, Xiao DL, He H, Li H, Yuan DH, Zhang C (2015) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta 182(5–6):893–908
Xu Z, Zhou W, Xu PP, Pan JM, Wu XY, Yan YS (2011) A molecularly imprinted polymer based on TiO2 as sacrificial support for selective recognition of dibenzothiophene. J Chem Eng 172(1):191–198
Niu M, Pham-Huy C, He H (2016) Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183(10):2677–2695
Ki C, Chang J (2006) Preparation of a molecularly imprinted polymeric nanocapsule with potential use in delivery applications. Macromolecules 39(9):3415–3419
Janiak DS, Ayyub OB, Kofinas P (2009) Effects of charge density on the recognition properties of molecularly imprinted polymeric hydrogels. Macromolecules 42(5):1703–1709
Hua Z, Chen Z, Li Y, Zhao M (2008) Thermo sensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin. Langmuir 24(12):5773–5780
Yang YZ, Liu XG, Guo MC, Li S, Liu WF, Xu BS (2011) Molecularly imprinted polymer on carbon microsphere surfaces for adsorbing dibenzothiophene. Colloids Surf A Physicochem Eng Asp 377(1–3):379–385
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I Solids J Amer Chem Soc 38(11):2221–2295
Negi D, Chanu T (2008) Surface-modified CdS nanoparticles as a fluorescent probe for the selective detection of cysteine. Nanotechnology 19(46):465503–465508
Wang Y, Wang J, Yang F, Yang X (2010) Spectrophotometric determination of cysteine with gold nanoparticles stabilized with single-stranded oligo nucleotides. Anal Sci 26(5):545–549
Wei X, Qi L, Tan J, Liu R, Wang F (2010) A colorimetric sensor for determination of cysteine by carboxymethyl cellulose-functionalized gold nanoparticles. Anal Chim Acta 671(1):80–84
Pan N, Li-Ying W, Wu LL, Peng CF, Xie ZJ (2016) Colorimetric determination of cysteine by exploiting its inhibitory action on the peroxidase-like activity of au@Pt core-shell nanohybrids. Microchim Acta 184(1):65–72
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104(2):627–633
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Hashemi, M., Nazari, Z. & Bigdelifam, D. A molecularly imprinted polymer based on multiwalled carbon nanotubes for separation and spectrophotometric determination of L-cysteine. Microchim Acta 184, 2523–2532 (2017). https://doi.org/10.1007/s00604-017-2236-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2236-x