Abstract
An ionic liquid aqueous two-phase system (ILATPS) of 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4)/ammonium citrate ((NH4)3C6H5O7) coupled with high-performance liquid chromatography was developed for the separation and determination of sulfadiazine (SD) and sulfamethoxazole (SMX) in water samples as well as aquaculture products. The effect of such parameters as the types and concentrations of salts, temperature, the concentrations of SD and SMX and the extraction time on the partitioning behavior expressed in terms of extraction efficiency has been evaluated. Under the optimal conditions, this extraction method has been successfully applied to the analysis of SD and SMX in water samples and aquaculture products with the recoveries of 98.29–99.55 % (SD) and 92.09–99.82 % (SMX). The detection limits for two analytes were 0.9 ng mL−1 (SD) and 1.8 ng mL−1 (SMX). In comparison with the traditional solvent extraction, ILATPS is much simpler and more environmentally friendly for the separation and enrichment of the sulfonamides antibiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are usually used in human and veterinary medicines for diseases treatment and prophylaxis. Over the last decades, large quantities of veterinary drugs have been extensively used in animal husbandry for prophylactic and therapeutic purposes. However, the improper use of chemotherapeutics in veterinary medicine and insufficient withdrawal time for treating animals may lead to the occurrence of drug residues in edible tissue, posing health hazard to the consumers.
Sulfonamides (SAs), a series of drugs containing the chemical structure of sulfanilic amide, are highly effective chemotherapeutic drugs as well known as antibacterial agents widely used in medicine and veterinary practice [1, 2]. Owing to their potential impact on human health, the European Union has adopted a maximum residue level (MRL) of 100 ng mL−1 in edible animal tissue [3]. Up to now, the methods of the determination of sulfonamides in water samples [4–6], milk [7–9], meat [10–12], soil [13] and egg [14] mainly include photometric [15, 16], spectrophotometric [17, 18], gas chromatography [19, 20], electroanalytical [21], capillary electrophoresis [22] and high-performance liquid chromatography (HPLC) with ultraviolet–visible (UV–Vis) detector [23–25], mass spectrometry [26–28] or fluorescence detector [11, 29]. Compared HPLC with other detection methods, some of these methods are time and cost consuming, low-sensitivity, low detection limit. Because of the relatively low concentrations of most SAs and their inherent complexity in environmental samples, the preconcentration and clean-up steps are necessary before analysis. Current preconcentration and clean-up techniques primarily focus on the solid-phase extraction [30, 31] (SPE). However, it requires a solvent desorption step which is time-consuming and demands traditional volatile organic solvents. Moreover, sample recovery is not always satisfactory. Therefore, the development of simple and environmental friendly pretreatment methods is of great interest.
Liquid–liquid extraction (LLE) is one of the efficient techniques to separate and concentrate various substrates. However, traditional LLE [32] usually requires some poisonous volatile organic solvents, while aqueous two-phase systems (ATPSs) with advantages of short processing time and a biocompatible environment set foot into the research field of vision. ATPSs are usually formed as a result of two different polymers or a polymer and a salt above a certain concentration. Recently, a new type of ATPSs based on ionic liquids (ILs) and salts has been investigated since Gutowski et al. [33] reported ionic liquid aqueous two-phase systems (ILATPSs) for the first time. These new ILATPSs combine the advantages of ILs and ATPSs, such as no emulsification, quick-phase separation, high extraction efficiency and gentle biocompatible environment [34]. ILATPSs have been successfully applied in the separation, concentration and purification of proteins [35], drugs [36] and antibiotics [37]. Because high concentration of inorganic salt is not desirable in the effluent streams due to the environmental problems, we employed organic salt as a substitute for inorganic salt.
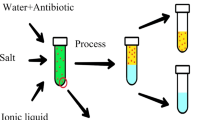
In this work, we explored a novel system based on 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4, Fig. 1)/ammonium citrate ((NH4)3C6H5O7) ILATPS coupled with HPLC for simultaneous the extraction together with the determination of sulfadiazine (SD, Fig. 1) and sulfamethoxazole (SMX, Fig. 1). After phase separation, these two SAs transferred into the [Bmim]BF4-rich phase, while the majority of concomitants remained in the bottom phase. The influence factors on extraction efficiency including the types of salt, the concentration of salt, the temperature, SD and SMX concentration, and extraction time were investigated. Under the optimal conditions, this method has been successfully applied to the analysis of SD and SMX in water samples and aquaculture products.
Experimental
Reagents and samples
The purity about mass fraction of 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim]BF4), 1-propyl-3-methylimidazolium tetrafluoroborate ([Pmim]BF4) and [Bmim]BF4 obtained from Chengjie Chemical Co., Ltd. (Shanghai, China) is greater than 0.99. Sulfadiazine (SD) and sulfamethoxazole (SMX) were bought from China Pharmaceutical Biological Products Analysis Institute (Shanghai, China). Sodium phosphate monobasic dihydrate (NaH2PO4·2H2O), ammonium citrate ((NH4)3C6H5O7) and sodium tartrate dibasic dihydrate (Na2C4H4O6·2H2O) were got from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The ILs and salts were used without further purification. All chemicals were of analytical grade, and all solutions were prepared from deionized water. The stock solutions of SD and SMX which should be replaced every 2 months were prepared by dissolving in methanol at the concentration of 100 μg mL−1 and stored at 4 °C [38, 39] in a refrigerator. The standard working solutions of SD and SMX were prepared by appropriate dilution of stock solutions with deionized water. All of the samples were collected in 2.5 × 103 mL amber glass bottles and stored at 4 °C until they were analyzed.
Preparation of actual samples
Water samples
Water samples were collected in 2.5 × 103 mL amber glass bottles from Yangzi River, fish farm of Jiangsu University which located in Zhenjiang (China). Both of the samples were centrifuged at 4,000 rpm for 30 min and the supernatant was collected, then different concentrations of SD and SMX working solutions (0, 10, 25, 50, 100 ng mL−1) were added. Finally, the mixture was filtered through 0.45-μm filter and stored at 4 °C for future use.
Aquaculture products
Crucian carp and shrimp purchased from local retail market were stored at −10 °C and thawed several hours at ambient temperature before using. 1.5 g of minced aquaculture products was placed into a 100-mL polypropylene tube, added in the SD and SMX working solutions (0, 10, 25, 50, 100 ng mL−1). Then, trichloroacetic acid (10 mL, 15 % in water) was added, and the mixture was thoroughly mixed using a homogenizer-disperser till it was in homogeneity. The solution was centrifuged at 4,000 rpm for 30 min; finally, the supernatant was filtered through microfilter with a pore size of 0.45 μm to remove the denatured proteins. The extracts were stored at 4 °C for future use.
Preparation of phase diagrams
The binodal curves were determined by titration method at T = 298.15 K. A few grams of pure ILs were weighed into a vessel, and a known mass of water was added and then mixed. The mixture was clear at first. Then, the known mass fraction of salt solution was added to the mixture until it became turbid. A few drops of water were added to make the mixture clear again, and then the above procedure were repeated to obtain sufficient data for the construction of a phase diagram. The vessel was immersed into a thermostatic waterbath (Gongyi Yuhua Instrument Co., Ltd. China) with an uncertainty of ±0.05 K. The composition of the mixture was determined by mass using an analytical balance (BS124S, Beijing Sartorius Instrument Co., Ltd. China) with an uncertainty of ±1.0 × 10−7 kg.
Extraction process
A special amount of (NH4)3C6H5O7 solution containing the standard working solution of SD and SMX was added into a 10.0-mL centrifuge tube, and then added 1 mL of [Bmim]BF4. The mixture was gently stirred for 5 min at ambient temperature, and then phase separation was achieved by centrifugation at 2,000 rpm for 20 min. After centrifugation, the tubes were placed into a thermostatic waterbath at 25 ± 0.05 °C for 30 min to equilibrate. The volume of top phase was recorded precisely. Most of SD and SMX were in the top phase where the [Bmim]BF4 was. A part of top phase was directly injected into the HPLC system for analysis.
HPLC–UV analysis
An Agilent 1200 HPLC system containing a quaternary pump and an ultraviolet–visible detector (Agilent, USA) was applied to the determination of SD and SMX. The Agilent ChemStation software was used for the instrument control and data processing. An Eclipse XDB-C18 reversed-phase column (250 mm × 4.6 mm, 5 μm, serial no. G1314B) was employed for chromatographic separation at the column temperature of 25 °C. The flow rate of mobile phase of methanol and water (the pH was adjusted to 3.00 with glacial acetic acid) was 1.0 mL min−1 with a ratio of 30:70. The injected volume was 20 μL and the column effluent was monitored at a wavelength of 265 nm.
Quantification
The partitions of SD and SMX in ILATPS were characterized by various parameters including the extraction efficiency (E) of SD and SMX calculated by
where C t represented mass equilibrium concentration of SD or SMX in the top phase, V t was the volume of the top phase, m s was the mass of SD or SMX initially added.
The enrichment factor (F) was calculated by
where V w stood for the volume of the water phase.
Results and discussion
Preparation of phase diagram for ILATPSs
Liquid–liquid equilibrium data are required for the design of aqueous two-phase extraction process, and for the comprehending of general factors that determine the partitioning behavior of solutes and particles. Our previous researches [40–42] have investigated the phase separation ability of [Bmim]BF4-inorganic/organic salt. The phase diagrams of [Bmim]BF4-(NH4)3C6H5O7/Na2C4H4O6/NaC2H3O2/NaH2PO4 are showed in Fig. 2 which indicated that these salts could form two-phase with [Bmim]BF4. The phase-forming ability followed the order: (NH4)3C6H5O7 > Na2C4H4O6 > NaH2PO4 > NaC2H3O2. These discussed salts were better salting-out agents. The anions with higher valence could hydrate more water molecules, resulting in the amount of water available to hydrate ILs decreasing. The Gibbs free energy of hydration of the ions could also have relation to the salting-out ability. The salt-out ability of (NH4)3C6H5O7 was slightly higher than that of Na2C4H4O6 with different anions and cations, though the ∆Ghyd of NH4 + ion (−285 kJ mol−1) is less than that of Na+ ion (−365 kJ mol−1) [43].
To see the efficacy of the imidazolium-based ILs in forming ATPSs with (NH4)3C6H5O7, the experimental binodal curves of the [Bmim]BF4/[Emim]BF4/[Pmim]BF4 + (NH4)3C6H5O7 + H2O systems at T = 298.15 K were shown in Fig. 3, thereinto, the binodal curve of the [Bmim]BF4 + (NH4)3C6H5O7 + H2O system [40] at T = 298.15 K has been published. The distance between binodal curves and the origin was in the order of [Bmim]BF4 < [Pmim]BF4 < [Emim]BF4. Due to the more hydrophobic character of ILs with higher molecular weight, the incompatibility of the system components increased. So [Bmim]BF4 was chosen as organic solvent in this work.
Effect of the type of salts
The distribution behaviors of SMX and SD in three ILATPSs were studied. Figures 4 and 5 showed the changes in the extraction efficiency of SD/SMX when adding different salts. After adding the three different salts (NaH2PO4, (NH4)3C6H5O7 and Na2C4H4O6), SD and SMX were directly extracted to the top phase. The results indicated that [Bmim]BF4-NaH2PO4/(NH4)3C6H5O7 ATPSs showed higher extraction efficiency of SD as well as SMX at the same time. [Bmim]BF4/(NH4)3C6H5O7 ATPS was chosen for further study in this work for its strong phase-forming ability and better extraction efficiency as well as its environmental friendly character.
Effect of the concentrations of (NH4)3C6H5O7
The influence of the concentrations of (NH4)3C6H5O7 on the extraction efficiency of SD and SMX was investigated, and the results are also illustrated in Figs. 4 and 5. It was obvious that the extraction efficiency of SMX was about 98 % when the concentration of (NH4)3C6H5O7 reached 0.56 g mL−1, while the extraction efficiency of SD was 92 %, indicating that SMX and SD were almost enriched in the top phase. That the amount of (NH4)3C6H5O7 reached the maximum to induce SMX and SD precipitation is the reason why the extraction efficiency nearly unchanged when the concentration of (NH4)3C6H5O7 was higher than 0.56 g mL−1 and no decomposition of SMX and SD was observed during the extraction process. In order to obtain high recovery and reduce the cost, an appropriate concentration of (NH4)3C6H5O7 with 0.56 g mL−1 was adopted.
Effect of temperature
Temperature is an important factor in SD and SMX partitioning. The effect of temperature was investigated from 15 to 55 °C. When the temperature was below 45 °C, the extraction efficiency of SD and SMX changed indistinctively, indicating that the temperature range from 15 to 45 °C had little influence on the distribution behavior of SD and SMX. However, the extraction efficiency decreased when the temperature was above 45 °C. This phenomenon can be explained from two aspects. On one hand, as the temperature increased, [Bmim]BF4 became more hydrophilic, and the water was driven to the [Bmim]BF4-rich phase so that the [Bmim]BF4 was diluted; as a result, the phase-forming ability of the investigated system declined and the extraction efficiency of SD and SMX also reduced. On the other hand, it may be that SD and SMX began to decompose at high temperature. For convenience, all experiments were done at room temperature.
Effect of SD and SMX concentrations as well as extraction time
The effect of the concentrations of SD and SMX on the extraction efficiency was researched in the range of 50–500 ng mL−1. The extraction efficiency was about 96.0–97.0 % and 91.0–92.0 % for SMX and SD, respectively. This indicated that the influence of SD and SMX concentrations was insensitive to the extraction efficiencies. In the experiment, the concentrations of SD and SMX both were 100 ng mL−1.
The effect of extraction time on the extraction efficiency was also discussed. After centrifugation, the mixtures were placed into a thermostatic waterbath for a period of time to equilibrate. The range of extraction time from 10 to 90 min was studied, and the result was that the extraction efficiency was almost constant. In order to ensure sufficient phase separation and save time, 30 min was chosen as the extraction time in the experiment.
Standard curve
Under the optimal conditions, the calibrations were performed by adding different concentrations of spiked solution of SD and SMX to [Bmim]BF4/(NH4)3C6H5O7 ILATPS. Linearity of the calibration curves was rather good with the correlation coefficient (R 2) higher than 0.999 in the concentration range of 2–160 ng mL−1 (SD) and 4–200 ng mL−1 (SMX). After phase separation, the top phase including SD and SMX was determined by HPLC–UV method as described in “HPLC–UV analysis”. The calibration curves were Area = 0.6421 × c + 0.2178 (SD) and Area = 0.6855 × c − 1.2722 (SMX), where “c” represented the concentrations of SD and SMX with the unit of nanogram per milliliter. To check the repeatability of the chromatographic procedure, analysis of 10 ng mL−1 standard solution of SD and SMX was performed (n = 7) and the relative standard deviation (RSD) was 2.1 %.
The limit of detection (LOD) was obtained from the signal-to-noise ratio (S/N) and the calibration curve. The LOD was a signal value of three times the noise (S/N = 3). The LOD obtained was 0.9 ng mL−1 (SD) and 1.8 ng mL−1 (SMX). The limit of quantification (LOQ) was a signal value of ten times the noise (S/N = 10). The LOQ was 3.0 ng mL−1 (SD) and 6.0 ng mL−1 (SMX). The LOD was well below the maximum residue limit (MRL, 100 ng mL−1) established by European Union [3].
Determination of SD and SMX in water samples and aquaculture products
Water samples collected from Yangzi River, fish farm of Jiangsu University which located in Zhenjiang (China) and two aquaculture products purchased from local retail market were examined by this method. No sulfonamide residues were detected in all samples before the standard solution was added. Figure 6 shows the chromatograms of SD and SMX in the blank sample and of those added with 100 ng mL−1 after ATPS extraction. From Fig. 6a, there was a peak (retention time = 3.041 min) in the aquaculture products that we could not determine SD in the top phase when the concentration of SD was low. However, as shown in Fig. 6c, d, SD was extracted to the top phase. It demonstrated that this method can be applied to the extraction of SD in the aquaculture products. Table 1 shows the results of recoveries of SD and SMX in water samples and aquaculture products. Recovery rates of SD (98.29–99.55 %) were obtained with RSD of 1.1–2.9 % in water samples and SMX (92.09–99.82 %) with RSD of 0.6–2.5 % in water samples and aquaculture products. This indicated that the recoveries of SD and SMX were highly satisfactory. The recoveries and RSD met the Codex criteria for residue analysis (recovery 70–110 % and RSD < 20 %) [44].
HPLC chromatograms with UV detection of a the blank Crucian carp sample after ILATPS extraction; b Fish farm water sample added with 100 ng mL−1 SD and SMX after ILATPS extraction; c Crucian carp sample added with 100 ng mL−1 SD and SMX after ILATPS extraction; d Shrimp sample added with 100 ng mL−1 SD and SMX after ILATPS extraction
Comparison of ATPS extraction and tradition solvent extraction
In order to further verify the advantages of SD and SMX extraction in actual samples by ILATPS, the tradition solvent extraction was used to contrast with ILATPS extraction. The results are also summarized in Table 1. The recoveries of SD and SMX in actual samples using ILATPS extraction were much better than that of tradition solvent extraction. In addition, the enrichment factor of ILATPS extraction was higher than solvent extraction. As is known to all, in comparison with the traditional solvent extraction, ILATPS was free of toxic organic solvents which could address the increasing challenges of environmental protection and product safety.
Conclusions
In this paper, an ionic liquid aqueous two-phase system (ILATPS) of [Bmim]BF4/(NH4)3C6H5O7 coupled with HPLC method was developed for analyzing trace sulfonamide antibiotics (SD and SMX) present in water samples and aquaculture products. The influences of various factors on partitioning behaviors were researched and it was found that the salting-out effect played an important role in the partitioning process. As a viable pretreatment and clean-up technique, this novel extraction method, combined with HPLC, has been successfully used to concentrate and determine trace level of SD and SMX in actual samples. Furthermore, the extraction efficiency and enrichment factor of ILATPS extraction were higher than that of the traditional solvent extraction.
References
A.M. Jacobsen, B. Halling-Sensen, F. Ingerslev, S.H. Hansen, J. Chromatogr. A 1038, 157 (2004)
P. Sukul, M. Lamshot, S. Zühlke, M. Spiteller, Chemosphere 73, 1344 (2008)
C. Hartig, T. Storm, M.J. Jekel, J. Chromatogr. A 854, 163 (1999)
Z. Ye, H.S. Weinberg, Anal. Chem. 79, 1135 (2007)
P.T.P. Hoa, L. Nonaka, P.H. Viet, S. Suzuki, Sci. Total Environ. 405, 377 (2008)
L. Sun, L. Chen, X. Sun, X. Du, Y. Yue, D. He, H. Xu, Q. Zeng, H. Wang, L. Ding, Chemosphere 77, 1306 (2009)
S. Su, M. Zhang, B. Li, H. Zhang, X. Dong, Talanta 76, 1141 (2008)
M. Laurentie, V. Gaudin, J. Chromatogr. B 877, 2375 (2009)
N. Rodrígueza, M.C. Ortiza, L.A. Sarabiab, A. Herreroa, Anal. Chim. Acta 657, 136 (2010)
M. Yang, J. Fang, T. Kuo, D. Wang, Y. Huang, L. Liu, P. Chen, T. Chang, J. Agric. Food Chem. 55, 8851 (2007)
E.M. Costi, M.D. Sicilia, S. Rubio, J. Chromatogr. A 1217, 6250 (2010)
W.H. Tsai, T. Huang, H. Chen, Y. Wu, J. Huang, H. Chuang, J. Chromatogr. A 1217, 250 (2010)
L. Sun, X. Sun, X. Du, Y. Yue, L. Chen, H. Xu, Q. Zeng, H. Wang, L. Ding, Anal. Chim. Acta 665, 185 (2010)
P. Chu, R. Wang, H. Chu, J. Agric. Food Chem. 50, 4452 (2002)
M.T. Tena, M.D. Luque de Castro, M. Valcárcel, Analyst 119, 1625 (1994)
A.R. Medina, M.C.C. García, A.M. Díaz, Anal. Lett. 35, 269 (2002)
A. Espinosa-Mansilla, F. Salinas, I. De Orbe Paya, Anal. Chim. Acta 313, 103 (1995)
S.A. Dhahir, A.H. Mhemeed, Asian J. Chem. 24, 3053 (2012)
K. Takatsuki, T. Kikuchi, J. Assoc. Off. Anal. Chem. 73, 886 (1990)
V.B. Reeves, J. Chromatogr. B 723, 127 (1999)
C.D. Souza, O.C. Braga, I.C. Vieira, A. Spinelli, Sens. Actuators B Chemical 135, 66 (2008)
T.Y. You, X.R. Yang, E.K. Wang, Analyst 123, 2357 (1998)
T.M. Nancy, A.M. Mustafa, J. Chromatogr. A 1127, 154 (2006)
J. He, S. Wang, G. Fang, H. Zhu, Y. Zhang, J. Agric. Food Chem. 56, 2919 (2008)
X. Huang, D. Yuan, B. Huang, Talanta 72, 1298 (2007)
J.L.M. Vidal, M.D.M. Aguilera-Luiz, R. Romero-González, A.G. Frenich, J. Agric. Food Chem. 57, 1760 (2009)
G. Hamscher, S. Sczesny, H. Holper, H. Nau, Anal. Chem. 74, 1509 (2002)
C. Cavaliere, R. Curini, A.D. Corcia, M. Nazzari, R. Samperi, J. Agric. Food Chem. 51, 558 (2003)
J. Bernal, M.J. Nozal, J.J. Jiménez, M.T. Martín, E. Sanz, J. Chromatogr. A 1216, 7275 (2009)
M.S. Díaz-Cruz, M.J. García-Galán, D. Barceló, J. Chromatogr. A 1193, 50 (2008)
F.J. Lara, A.M. García-Campa-na, C. Neusüss, F. Alés-Barrero, J. Chromatogr. A 1216, 3372 (2009)
L.C. Lin, J. Fujian, Agric. Sci. 26, 697 (2011)
K.E. Gutowski, G.A. Broker, H.D. Willauer, J.G. Huddleston, R.P. Swatloski, J.D. Holbrey, R.D. Rogers, J. Am. Chem. Soc. 125, 6632 (2003)
U. Deng, T. Long, D. Zhang, J. Chen, S. Gan, J. Chem. Eng. Data 54, 2470 (2009)
Y. Pei, J. Wang, K. Wu, X. Xuan, X. Lu, Sep. Purif. Technol. 64, 288 (2009)
C.Y. He, S.H. Li, H.W. Liu, K.A. Li, F. Liu, J. Chromatogr. A 1082, 143 (2005)
J. Han, Y. Wang, C.L. Yu, Y.S. Yan, X.Q. Xie, Anal. Bioanal. Chem. 399, 1295 (2011)
S.Y. Won, C.H. Lee, H.S. Chang, S.O. Kim, S.H. Lee, D.S. Kim, Food Control 22, 1101 (2011)
S. Castiglioni, F. Pomati, K. Miller, B.P. Burns, E. Zuccatoa, D. Calamari, B.A. Neilan, Water Res. 42, 4271 (2008)
J. Han, R. Pan, X.Q. Xie, Y. Wang, Y.S. Yan, G.W. Yin, W.X. Guan, J. Chem. Eng. Data 55, 3749 (2010)
C.X. Li, J. Han, Y. Wang, Y.S. Yan, J.M. Pan, X.H. Xu, Z.L. Zhang, J. Chem. Eng. Data 55, 1087 (2010)
J. Han, C.L. Yu, Y. Wang, X.Q. Xie, Y.S. Yan, G.W. Yin, W.X. Guan, Fluid Phase Equilib. 295, 98 (2010)
Y. Marcus, J. Chem. Soc. Faraday Trans. 87, 2995 (1991)
K. Kishida, N. Furusawa, J. Liq. Chromatogr. Relat. Technol. 26, 2931 (2003)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21076098, 21206059 and 21207051), the Natural Science Foundation of Jiangsu Province (No. BK2010349 and BK2011529), China Postdoctoral Science Foundation funded project (No. 20110491352), Jiangsu Postdoctoral Science Foundation funded project (No. 1101036C), and start-up Foundation of Jiangsu University (No. 10JDG070).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Han, J., Yan, Y. et al. Simultaneous extraction and determination of sulfadiazine and sulfamethoxazole in water samples and aquaculture products using [Bmim]BF4/(NH4)3C6H5O7 aqueous two-phase system coupled with HPLC. J IRAN CHEM SOC 10, 339–346 (2013). https://doi.org/10.1007/s13738-012-0164-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0164-6