Abstract
A fast and economical method has been developed for the preconcentration of the carbamates propoxur, pirimicarb and promecarb by using magnetic molecularly imprinted polymers (m-MIPs). Propoxur was applied as the template for the analytes, and carbon nanotubes as a supporting template. The effects of the amount of magnetic sorbent, shaking time and rate, eluting solution on the extraction efficiency were studied. The processes of adsorption and desorption occur within 25 min, and the m-MIPs can be collected by applying an external magnetic field within 15 s. The m-MIPs were characterized by using FTIR, transmission electron microscopy, and with a vibrating sample magnetometer. The pesticides were quantified by HPLC with UV detection. Recoveries of spiked samples range from 90.5 to 98.6 %, and the lower detection limits range from 9.7 to 12.0 μg kg−1. The method is selective and convenient. The m-MIPs were successfully applied to enrich and determine the carbamates in spiked homogenates of apples, oranges and pears.

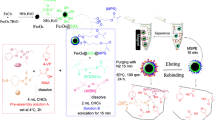
Extraction process using magnetic molecularly imprinted polymers. The m-MIPs were prepared using proporxur as the template molecular, carbon nanotubes as supporter and Fe3O4 as the magnetic component. The m-MIPs were successfully applied to determine the carbamates in fruit samples with recoveries that range from 90.5 to 98.6 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbamates are toxic to human beings because they act as inhibitors of the enzyme acetylcholine esterase [1, 2]. It is necessary to develop an analytical method with high sensitivity for monitoring of carbamate residue. Many studies have been reported based on high performance liquid chromatography (HPLC) with UV detector [2–6] or mass spectrometry (MS) [7–9] and gas chromatography mass spectrometry (GC-MS) [10]. However, the thermal instability of carbamates limits their direct determination by GC unless they are derived into thermally stable derivatives. HPLC is effective in separating of non-volatile and thermally labile compounds, it has become a routine analytical technique for the determination of carbamates residues [3].
The sample preparation technique is the bottleneck for determination carbamates in complex matrix. Liquid-liquid extraction (LLE), solid phase extraction (SPE) [6, 9], solid-phase microextraction (SPME) [3, 10], cloud point extraction (CPE) [4], ionic liquid magnetic bar microextraction (ILMB-ME) [2] and dispersive liquid-liquid microextraction (DLLME) [6] were used for extraction of carbamates. In these methods, large amount of organic solvents were consumed by LLE. As for SPE, the analytes recovery may be decreased when the loading flow rate is too high due to the decrease of the interaction time [11]. Meanwhile, the fiber used in SPME is too fragile, the thermal instability compounds cannot be analyzed. Micelle in CPE can adversely affect HPLC.
Molecularly imprinted polymers (MIPs) have been developed more than 40 years [12]. With the development of MIPs, a technique based on magnetic polymer has received more increasing attention. The magnetic MIPs (m-MIPs) can be dispersed into the solution directly and then easily separated from the matrix by using an external magnetic field. However, m-MIPs which were prepared by bulk polymerization exhibited poor accessibility to the template, as the template molecules were embedded inside the thick polymer network. In order to overcome this drawback, surface molecular imprinting method that attempts to build molecular recognition systems on the support materials surface with nanoscale dimensions was introduced [13]. Some problems such as sensitivity [14] and stability [15, 16] have not been resolved yet. Among the supporters, carbon nanotubes (CNTs) have been proved to be available support materials in surface imprinting process [17, 18].
We describe magnetic propoxur-imprinted polymers which synthesized with CNTs as the supporter, Fe3O4 as the magnetic component, (3-Aminopropyl) triethoxysilane (APTES) as the functional monomer and tetraethyl orthosilicate (TEOS) as the cross-linker. The m-MIPs applied as selective materials coupled with HPLC to determination of carbamates in fruit samples (apples, oranges and pears).
Materials and methods
Chemicals and equipment
The CNTs were purchased from Nanoport (Shenzhen, China, http://www.nanotubes.com.cn). The standards of propoxur, pirimicarb, promecarb, dimethoate, APTES, ferrocene and TEOS were purchased from Aladdin (Shanghai, China, http://www.aladdin-reagent.com). Ammonia hydroxide, nitric acid (65–68 wt.%) and acetic acid were purchased from Guangfu (Tianjin, China, http://www.guangfu-chem.com). Methanol, hexadecy ltrimethyl ammonium bromide (CTAB), acetonitrile (ACN) and ethanol were obtained from Kermel (Tianjin, China, http://www.chemreagent.com). Chromatographic grade methanol was obtained from Fisher (Pittsburgh, PA, USA, http://www.fishersci.com.cn). High purity water was obtained from a Milli-Q Water System (Millipore, Billerica, MA, USA, http://www.millipore.com). All other reagents were of analytical grade.
Chromatographic analysis was performed on a LC-15C high performance liquid chromatograph with a UV detector (Shimadzu, Kyoto, Japan,http://www.shimadzu.com.cn). A Zorbax SB-C18 column (250 × 4.6 mm I.D., 5 μm) was used as an analytical column (Agilent, Palo Alto, CA, USA, http://www.home.agilent.com). A KQ5200E ultrasonic apparatus (Kunshan Instrument, Kunshan, China, http://www.ks-csyq.com), DZKW-C thermostatic bath (Shanghai, China, http://shuliyiqi.cn.china.cn), DF-101S thermostatic oil bath (Yarong Instrument, Zhengzhou, henan, China, http://www.zzyarong.com), TG 16-WS centrifuge (Xiangyi, Changsha, China, http://xylxj.testmart.cn), SHA-B shaking table (Shengtang, Jintan, China, http://www.jtstyq.com), FT-IR 360 Fourier transform infrared spectroscopy (Nicolet, Madison, WI, USA, http://www.artisan-scientific.com/69062.htm), Hitachi H-7650 TEM (Matsudo, Japan, http://www.hitachi-hitec.com), SK2.5-13 furnace tube (Shuli instrument, Shanghai, China, http://www.shuliyiqi.com) and vibrating sample magnetometer (Quantum Design Instrument, San Diego, CA, USA, http://www.qdusa.com) were used.
Preparation of fruits samples and standard solution
For the development and validation of the method, three kinds of fresh fruits (apples, oranges and pears) were randomly collected from a market in the Harbin of China. One apple sample was checked to be free of the studied carbamates pesticides by National standard method used in China [19]. This sample was used as the blank sample for calibration and validation purposes.
The stock solutions (1.0 mg mL−1) of propoxur, pirimicarb and promecarb were prepared by dissolving 10.0 mg of propoxur, pirimicarb and promecarb in 10.0 mL ACN. Working solutions of propoxur, pirimicarb and promecarb were prepared by diluting the stock solution with LC mobile phase. All propoxur, pirimicarb and promecarb standard solutions were stored at 4 °C.
Preparation of m-MIPs
The m-MIPs were prepared as follows.
-
Step 1
was the activation of the CNTs. The impurities such as amorphous carbon and metallic catalyst in the CNTs were removed by using HNO3 (65 %) solution at 90 °C for 6 h. Then the CNTs were washed with water.
-
Step 2
was the synthesis of magnetic CNTs (m-CNTs). The activated CNTs were mixed with ferrocene at the ratio of 1:5 and dispersed with ethanol. The mixture was calcined at 400 °C in the tubular furnace for 4 h, the m-CNTs was successfully synthesized [20, 21].
-
Step 3
was the synthesis of m-CNTs@SiO2. The SiO2 coating on the m-CNTs was performed according to the reported method [22]. First, m-CNTs (250 mg), APTES (0.5 mL), CTAB (100 mg) and water (48 mL) were added in one conical flask. Second, TEOS (4 mL), water (3 mL) and ethanol (50 mL) were added in the other conical flask. Then the two conical flasks were sonicated for 20 min and stirred (300 rpm) for another 3 h. The substance in the two flasks was mixed. Ammonia hydroxide was added dropwise into the mixture until the pH reached 9.5. The reaction was allowed to be continued for 24 h. The magnetic compounds were magnetically separated, washed and dried.
-
Step 4
was the synthesis of the m-MIPs. Propoxur (0.2 g) was dissolved in 60 mL ethanol, mixed with APTES (4 mL) by stirring (300 rpm) 20 min. m-CNTs@SiO2 (1.2 g), acetic acid (0.12 mL) as catalyst and water (2 mL) were added. After stirring (300 rpm) 10 h, the product was collected magnetically, washed by a mixture of methanol/acetic acid (9:1, v/v) to remove the template (propoxur).
The magnetic non-imprinted polymers (m-NIPs) were also prepared for comparison. The preparation process was similar to the synthesis of m-MIPs without adding propoxur.
Characterization
The m-MIPs were characterized by using Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopy (TEM), and with a vibrating sample magnetometer (VSM).
Binding experiment
-
(a)
Static adsorption experiments
The static adsorption experiments were carried out by adding 30 mg m-MIPs or m-NIPs in a glass tube containing 5.0 mL of propoxur standard solution with concentrations varying from 1 to 200 μg mL−1. Then the mixture was stirred for 12 h. Finally the magnetic sorbent was separated by a magnet and the supernatant was detected by HPLC.
-
(b)
Kinetic adsorption experiments
To investigate the adsorption kinetics of the sorbent, 30 mg of m-MIPs was added to a 5 mL of 50 μg mL−1 of propoxur standard solution, and was shaken for 1–150 min. The propoxur residue was determined by HPLC.
-
(c)
Selectivity experiments
The rebinding selectivity experiments were carried out by using dimethoate as the structural similarity during the binding process. The m-MIPs or m-NIPs (30 mg) were placed in 5 mL of 50 μg mL−1 of propoxur or dimethoate standard solution. The mixture was stirred at room temperature for 20 min. Then the magnetic nanoparticles were isolated by a magnet. The supernatant was detected by HPLC.
Extraction of carbamates by m-MIPs
A representative portion of these samples was chopped and homogenised in a food chopper. Fruit samples (10 g) were added into a centrifuge tube. The carbamates in the samples were extracted by homogenizing with 40 mL ACN. The extract solution was separated by centrifuging, evaporated under nitrogen gas for decreasing the volume to 5 mL. Water (5 mL) was added into the extract. The m-MIPs (30 mg) were added into the solution, the mixture was shaken at room temperature for 20 min. Subsequently, the m-MIPs with absorbed carbamates were separated by a permanent magnet. The carbamates were eluted from the m-MIPs with 3 × 2.0 mL ACN/acetic acid (95:5, v/v, 2.0 mL every time and eluted 3 times) under ultrasonic for 30 s. All eluents were combined and evaporated to dryness under nitrogen gas at 40 °C. The residue was reconstituted with 0.5 mL of 70 % methanol aqueous solution for HPLC analysis. The experiment process was shown in Fig. S1 in Electronic Supplementary Material.
Liquid chromatography analysis
Separation was achieved on C18 column. The mobile phase was 70 % methanol aqueous solution. The flow rate was 1.0 ml min−1. Detection wavelength was set at 222 nm and the injection volume was 20 μL.
Results and discussion
Choice of materials
During the synthesis process of m-MIPs, the supporter is the key. The most commonly used supporter was SiO2 [23]. However nano-SiO2 was easy to agglomerate. CNTs which have unique atomic structures are ideal support materials because they have stable under acidic conditions, no swelling and large surface area, and easy to be modified [24, 25]. These properties reveal that they can be used for preparing reinforced polymer composites. CTAB was used for dispersing CNTs. APTES we used had two promising features. First, it acted as functionalized monomer for molecular imprinting due to its amino group, which can interact with the template propoxur via hydrogen bonds. Second, it provided Si-O bonds via hydrolysis and condensation to link the MIP to the supporter [26]. TEOS acted as cross-linker to form a polymeric network around the template [26]. Different carbamates including propoxur, pirimicarb and promecarb were investigated as template molecule to prepare the m-MIPs. The result showed that the higher imprinting factor was obtained when using propoxur as template molecule. The imprinting factor is the ratio of the Qm-MIPs/Qm-NIPs.
Characterization results
The m-MIPs were investigated by FTIR spectroscopy (Fig. 1a). The -OH vibration was detected at 3,424 and 1,637 cm−1, the absorbance peaks of C-H stretching vibration at 2,924 and 2,854 cm−1 [27], the peak at 1,388 cm−1 is attributed to the existence of O-H bending vibration, the strong peak at 1,059 and 460 cm−1 are attributed to the stretch of Si-O-Si. The peak at 1,578 cm−1 indicates N-H bond could be grafted onto the surface of m-MIPs [22]. The peak at 551 cm−1 is attributed to the stretch of Fe-O [28]. The m-MIPs were characterized by TEM in order to know the surface morphological image. Figure 1b shows Fe3O4 and MIPs were coated on CNTs successfully. The magnetic property of m-MIPs was studied by using VSM at room temperature. As Fig. 1c suggested, saturation magnetization of m-MIPs is 17.66 emu g−1. Obviously, m-MIPs have enough magnetic field strength to be separated.
Adsorption isotherm
Propoxur was selected as a representative of three carbamates for studying of Scatchard and kinetics adsorption experiment.
Scatchard model was used to normalize the adsorption data. In the Scatchard analysis, the amount of propoxur bound to the magnetic adsorbent was obtained by subtracting the free concentration from the initial concentration of propoxur. When the propoxur concentration was varied, the Scatchard plot was obtained according the following equation:
where Q (mg g−1) is the amount of propoxur bound to the magnetic adsorbent at equilibrium, C e (mg L−1) is the free propoxur concentration at equilibrium, K d is the dissociation constant, Q max (mg g−1) is the maximum binding amount. The K d and Q max are estimated from the slope and intercept of the linear plot of Q vs Q/C e [29, 30].
As can be observed from Fig. 2a, the adsorption capacities increase with increasing initial propoxur concentration for both m-MIPs and m-NIPs, but m-MIPs exhibited a much higher adsorption capacity than that of m-NIPs because of the imprinting effect [31]. The Scatchard analysis of m-MIPs and m-NIPs were performed.
It is observed that two straight lines were obtained in the plot region (Fig. 2b), which indicated that there are two kinds of binding sites for m-MIPs [32]. The linear regression equations for the left and right slope of the biphasic curve is Q/C = 0.38–0.10Q (R 2 = 0.997) and Q/C = 0.24–0.018Q (R 2 = 0.998). From the slope and the intercept of the biphasic curve, the K d were 10.0 and 55.6 g L−1, the Q max were 3.8 and 13.3 mg g−1, respectively.
As can be seen from Fig. 2c, m-NIPs showed one kind of binding site. The linear regression equation is Q/C = 0.21–0.032Q (R 2 = 0.997), the K d was 31.2 g L−1 and the Q max was 6.6 mg g−1.
Adsorption kinetics and selectivity
More information on adsorption kinetics and selectivity was shown in Electronic supplementary material.
Optimization of extraction conditions
Apples were selected as a representative for studying of optimization of sorption experiment. The apple sample was checked to be free of carbamates by National Standard method used in China [19], then 0.5 mg kg−1 carbamates were added in the apple. According to previous reports [19], ACN was often used for extracting carbamates from fruits. ACN was also selected as the extraction solvent. After extraction, the extract obtained was diluted with water, in order to subsequently adsorb carbamates with m-MIPs conveniently. More detailed information about the optimization of other conditions effecting the adsorption and desorption of carbamates with m-MIPs was shown in Electronic supplementary material.
Method validation
To evaluate this method, experiments with regard to the linearity, limit of detection (LOD), limit of quantification (LOQ) and precision were performed. LODs and LOQs in fruit samples were defined as the concentration providing three and ten times of signal-to noise ratio, respectively [33]. LODs, LOQs and liner range were listed in Table 1.
Five parallel extractions of a sample over a day gave the within-day relative standard deviations (RSDs). The between-day RSDs were determined by extracting sample that had been independently prepared for a continuous 5 days. RSDs of within- and between- day ranging from 0.2 to 2.3 % and from 6.8 to 9.6 % were obtained, respectively.
To evaluate the analytical performance of this method, a comprehensive comparison of this m-MIPs method with other reported methods for the analysis of carbamates in the fruit samples was presented in Table 2. It can be seen that this method had a similar linearity, recovery and LOD. It is conceivable that the sensitivity may be improved by using HPLC-MS. However, in some previous studies, sample purification technology was not used [7, 8], which would interfere with the determination of HPLC. The adsorbent we used can selectively adsorption of pesticides and reduction of the interference of matrix. In addition, the magnetic adsorbents can facilitate separation process.
Application to fruit samples
Under the optimized conditions, this method was successfully applied to analysis the fruit samples, including three apple samples, three orange samples and three pear samples. Pirimicarb residue was detected in one pear sample with the concentration of 0.018 mg kg−1. But the concentration of the pesticide residue was no more than the maximum residue limits (0.05 mg kg−1) [34]. The chromatograms of spiked apple, blank apple and one pear sample were shown in Fig. 3.
The accuracy of the method was confirmed by analyzing the spiked samples prepared by adding suitable amounts of standard solution to homogenized fruit samples with known contents (0.05, 0.5 and 5.0 mg kg−1) of these target compounds. The recoveries of target compounds were between 90.5 and 98.6 %.
Conclusion
We describe a simple method to synthesize CNTs based m-MIPs which used for the extraction of carbamates (propoxur, pirimicarb and promecarb) from fruit samples. The analytes were detected by HPLC. The method was shown good recoveries and precisions. The pseudo-second-order kinetic model was more accurate to describe the adsorption behavior of propoxur onto m-MIPs. In Scatchard analysis there are two kinds of binding sites for this material. The m-MIPs could provide a relatively rapid and convenient way for withdrawal of magnetic polymers from sample matrices by a magnet without additional centrifugation or filtration.
This approach in which imprinted polymer was built on CNTs seems to be promising as it provides more binding sites for template recognition. It is not limited to the determination of carbamates in fruit sample. The m-MIPs synthesized by changing temples may be used for the selective adsorption and determination of other pesticides, pigment or food additives in other food samples like vegetable. This method provided a technological support for food safety.
References
Fernández M, Picó Y, Mañes J (2000) Determination of carbamate residues in fruits and vegetables by matrix solid-phase dispersion and liquid chromatography–mass spectrometry. J Chromatogr A 871(1–2):43–56
Peng S, Xiao J, Cheng J, Zhang M, Li X, Cheng M (2012) Ionic liquid magnetic bar microextraction and HPLC determination of carbamate pesticides in real water samples. Microchim Acta 179(3–4):193–199
Ma X, Wang J, Wu Q, Wang C, Wang Z (2014) Extraction of carbamate pesticides in fruit samples by graphene reinforced hollow fibre liquid microextraction followed by high performance liquid chromatographic detection. Food Chem 157:119–124
Santalad A, Srijaranai S, Burakham R, Glennon JD, Deming RL (2009) Cloud-point extraction and reversed-phase high-performance liquid chromatography for the determination of carbamate insecticide residues in fruits. Anal Bioanal Chem 394(5):1307–1317
Zhang T, Ma C, Wu M, Ye Y, Chen H, Huang J (2013) Selective microextraction of carbaryl and naproxen using organic–inorganic monolithic columns containing a double molecular imprint. Microchim Acta 180(7–8):695–702
Zhou S, Chen H, Wu B, Ma C, Ye Y (2011) Sensitive determination of carbamates in fruit and vegetables by a combination of solid-phase extraction and dispersive liquid-liquid microextraction prior to HPLC. Microchim Acta 176(3–4):419–427
Goto T, Ito Y, Yamada S, Matsumoto H, Oka H, Nagase H (2006) The high throughput analysis of N-methyl carbamate pesticides in fruits and vegetables by liquid chromatography electrospray ionization tandem mass spectrometry using a short column. Anal Chim Acta 555(2):225–232
Granby K, Andersen JH, Christensen HB (2004) Analysis of pesticides in fruit, vegetables and cereals using methanolic extraction and detection by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 520(1–2):165–176
Liu M, Hashi Y, Song Y, Lin JM (2005) Simultaneous determination of carbamate and organophosphorus pesticides in fruits and vegetables by liquid chromatography-mass spectrometry. J Chromatogr A 1097(1–2):183–187
Lachenmeier DW, Nerlich U, Kuballa T (2006) Automated determination of ethyl carbamate in stone-fruit spirits using headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry. J Chromatogr A 1108(1):116–120
Chen L, Zhang X, Xu Y, Du X, Sun X, Sun L, Wang H, Zhao Q, Yu A, Zhang H, Ding L (2010) Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 662(1):31–38
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchim Acta 180(15–16):1359–1370
Chen X, Zhang Z, Yang X, Li J, Liu Y, Chen H, Rao W, Yao S (2012) Molecularly imprinted polymers based on multi-walled carbon nanotubes for selective solid-phase extraction of oleanolic acid from the roots of kiwi fruit samples. Talanta 99:959–965
Apodaca DC, Pernites RB, Ponnapati R, Del Mundo FR, Advincula RC (2011) Electropolymerized molecularly imprinted polymer film: EIS sensing of bisphenol A. Macromolecules 44(17):6669–6682
Huang J, Zhang X, Lin Q, He X, Xing X, Huai H, Lian W, Zhu H (2011) Electrochemical sensor based on imprinted sol–gel and nanomaterials for sensitive determination of bisphenol A. Food Control 22(5):786–791
Kubo I, Yokota N, Fuchiwaki Y, Nakane Y (2012) Characteristics of molecularly imprinted polymer thin layer for bisphenol A and response of the MIP-modified sensor. ISRN Mater Sc 2012:1–6
Zhang M, Huang J, Yu P, Chen X (2010) Preparation and characteristics of protein molecularly imprinted membranes on the surface of multiwalled carbon nanotubes. Talanta 81(1–2):162–166
Ma G, Chen L (2014) Development of magnetic molecularly imprinted polymers based on carbon nanotubes — application for trace analysis of pyrethroids in fruit matrices. J Chromatogr A 1329:1–9
GB/T 19648–2006 method for determination of 500 pesticides and related chemicals residues in fruits and vegetables-GC-MS method
Cheng J, Zou XP, Zhu G, Wang MF, Su Y, Yang GQ, Lü XM (2009) Synthesis of iron-filled carbon nanotubes with a great excess of ferrocene and their magnetic properties. Solid State Commun 149(39–40):1619–1622
Liu Q, Chen Z-G, Liu B, Ren W, Li F, Cong H, Cheng H-M (2008) Synthesis of different magnetic carbon nanostructures by the pyrolysis of ferrocene at different sublimation temperatures. Carbon 46(14):1892–1902
Gao R, Kong X, Su F, He X, Chen L, Zhang Y (2010) Synthesis and evaluation of molecularly imprinted core-shell carbon nanotubes for the determination of triclosan in environmental water samples. J Chromatogr A 1217(52):8095–8102
Xu L, Pan J, Dai J, Li X, Hang H, Cao Z, Yan Y (2012) Preparation of thermal-responsive magnetic molecularly imprinted polymers for selective removal of antibiotics from aqueous solution. J Hazard Mater 233–234:48–56
Hirano A, Uda K, Maeda Y, Akasaka T, Shiraki K (2010) One-dimensional protein-based nanoparticles induce lipid bilayer disruption: carbon nanotube conjugates and amyloid fibrils. Langmuir : ACS J Surf Colloids 26(22):17256–17259
Zhang Z, Zhang H, Hu Y, Yang X, Yao S (2010) Novel surface molecularly imprinted material modified multi-walled carbon nanotubes as solid-phase extraction sorbent for selective extraction gallium ion from fly ash. Talanta 82(1):304–311
Gao N, Xu Z, Wang F, Dong S (2007) Sensitive biomimetic sensor based on molecular imprinting at functionalized indium tin oxide electrodes. Electroanalysis 19(16):1655–1660
Chen FF, Xie XY, Shi YP (2013) Preparation of magnetic molecularly imprinted polymer for selective recognition of resveratrol in wine. J Chromatogr A 1300:112–118
Kong X, Gao R, He X, Chen L, Zhang Y (2012) Synthesis and characterization of the core-shell magnetic molecularly imprinted polymers (Fe(3)O(4)@MIPs) adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J Chromatogr A 1245:8–16
Ibarra IS, Miranda JM, Rodriguez JA, Nebot C, Cepeda A (2014) Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem 157:511–517
Wang B, Wang Y, Yang H, Wang J, Deng A (2011) Preparation and characterization of molecularly imprinted microspheres for selective extraction of trace melamine from milk samples. Microchim Acta 174(1–2):191–199
Hu C, Deng J, Zhao Y, Xia L, Huang K, Ju S, Xiao N (2014) A novel core-shell magnetic nano-sorbent with surface molecularly imprinted polymer coating for the selective solid phase extraction of dimetridazole. Food Chem 158:366–373
Barri T, Trtic-Petrovic T, Karlsson M, Jonsson JA (2008) Characterization of drug-protein binding process by employing equilibrium sampling through hollow-fiber supported liquid membrane and Bjerrum and Scatchard plots. J Pharm Biomed Anal 48(1):49–56
Shui G, Leong LP (2002) Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A 977(1):89–96
EC Council Directive, Informal coordination of MRLs established in Directives 76/895/EEC, 86/362/EEC, 86/363/EEC, and 90/642/EEC
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21205010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 271 kb)
Rights and permissions
About this article
Cite this article
Gao, L., Chen, L. & Li, X. Magnetic molecularly imprinted polymers based on carbon nanotubes for extraction of carbamates. Microchim Acta 182, 781–787 (2015). https://doi.org/10.1007/s00604-014-1388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1388-1