Abstract
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF) that is used to treat patients with various cancers. However, it is known to be associated with adverse events, such as hypertension and proteinuria. The histology of bevacizumab-induced nephropathy is known as thrombotic microangiopathy or minimal change nephrotic syndrome. Recently, however, the terms “bevacizumab-associated glomerular microangiopathy” and “anti-VEGF therapy-induced glomerular microangiopathy” have been proposed. We present a case of a 68-year-old woman who was administered postoperative chemotherapy (carboplatin, paclitaxel, and bevacizumab) for stage IV ovarian cancer. Proteinuria and hypertension appeared after three courses; however, six courses were completed. Then, gemcitabine and carboplatin were administered for recurrence of her cancer. She was diagnosed with nephrotic syndrome after eight courses. Renal biopsy showed accumulation of periodic acid-Schiff (PAS)-positive substances in the capillary walls and para-mesangial areas. Double contouring of basement membranes was also observed. Immunofluorescence microscopy revealed positive staining for IgG, IgA, IgM, C3, C4, and C1q. Immunosuppressive therapy was administered, but was ineffective. Further examination by electron microscopy and immunostaining led to a diagnosis of bevacizumab-associated glomerular microangiopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF) that is used to treat various cancers. Adverse events caused by this agent include hypertension and proteinuria; notably, severe proteinuria (grade 3 or ≥ 3.5 g/day) is observed in approximately 3% of patients [1, 2]. The histological types of this type of nephropathy include thrombotic microangiopathy (TMA) [3, 4] and minimal change nephrotic syndrome [5].
However, bevacizumab-associated glomerular microangiopathy or anti-VEGF therapy-induced glomerular microangiopathy have been proposed as renal lesions that differ from TMA and were previously considered forms of ‘glomerular TMA’ [6, 7]. Here, we report our experience with a patient whose presentation appeared to match this concept.
Case report
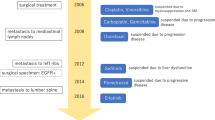
A 68-year-old woman with a history of advanced ovarian cancer was admitted to our hospital for nephrotic syndrome. In June 2016, hysterectomy and bilateral salpingo-oophorectomy with dissection of the omental, umbilical, and right inguinal lymph nodes were performed for ovarian cancer. The postoperative diagnosis was high-grade serous adenocarcinoma, pT3N1M1, stage IVB. Preoperative urinary examinations showed no abnormal findings. Postoperative chemotherapy with carboplatin, paclitaxel, and bevacizumab was initiated in July 2016. After three courses, proteinuria and elevated blood pressure were observed. The proteinuria was still present after six courses at a level of 2 + , but the hypertension trended toward improvement. The patient was placed under observation.
In June 2017, ovarian cancer recurrence was detected as peritoneal dissemination and left lymph node metastasis, and administration of gemcitabine and carboplatin was initiated. The proteinuria was sustained at a level of 1 + to 2 + during the second chemotherapy. In March 2018, after the eighth course, the patient noticed edema of the lower legs, and was admitted to our hospital.
On admission, physical examination revealed a slightly elevated blood pressure of 144/89 mmHg. Her palpebral conjunctiva was pale. Surgical scars were observed on her abdomen, lower abdominal midline, and right lower abdomen. Her limbs exhibited mild pitting edema. No abnormal signs were observed in her lungs, heart, or skin.
Laboratory findings on admission are listed in Table 1. Nephrotic syndrome was significant with urinary protein 6.75 g/gCr and serum albumin 2.7 g/dL, accompanied by urinary erythrocytes (30–40/high-powered field), and granular casts. Antinuclear antibodies were positive, while antidouble stranded-DNA antibodies were negative. Cryoglobulin was also negative.
Percutaneous renal biopsy was performed. A total of 29 glomeruli were observed in a specimen by light microscopy; of these, 4 showed global sclerosis. The other glomeruli exhibited periodic acid-Schiff (PAS)-positive lesions in the capillary walls and para-mesangial area, and the affected capillary lumen was almost occluded. Podocyte detachment was found in some glomerular capillaries (Fig. 1a). Periodic acid-methenamine silver staining showed extensive double contouring of the glomerular basement membrane and spike formation in restricted area (Fig. 1b). Some dilation of proximal tubules, absence of the brush border, and mild interstitial infiltration of lymphocytes were observed (Fig. 1c). Immunohistochemical staining revealed that the PAS-positive lesions were CD61-negative (anti CD61 antibody; Clone Y2/51, Dako, dilution 1:100), which indicated that the lesion contained no platelets (Fig. 1d). VEGF (anti VEGF antibody; SC7269, Santa Cruz, dilution 1:250) expression was decreased in glomeruli (Fig. 1e). Staining for phospholipase A2 receptor (Anti-PLA2R1; HPA012657, Atlas Antibodies, Poly IgG, × 2000) and thrombospondin type-1 domain-containing 7A (Anti-THSD7A; HPA000923, SIGMA, Atlas Antibodies, Poly IgG, × 800) were negative. The samples tested positive for IgG, IgA, IgM, C3, C4, and C1q on immunofluorescence microscopy (Fig. 2). Electron microscopy revealed the presence of electron dense materials in the subendothelial and para-mesangial area. Disappearance of the endothelial fenestrations and extensive foot process effacement were also noted in glomerular capillaries (Fig. 3a). Subepithelial deposits were observed in a limited area of the glomerular basement membrane (Fig. 3b).
Light microscopy findings and immunohistochemical analysis. a Periodic acid-Schiff (PAS)-positive hemispherical lesions in the glomerular capillary walls and para-mesangial area. The affected capillary lumen is almost occluded. Podocyte detachment is found in some glomerular capillaries. b Periodic acid-methenamine silver staining showing double contouring of the glomerular basement membrane and spike formation (inset). c Mild tubulointerstitial change with some dilation of the proximal tubules, absence of the brush border, and interstitial infiltration of lymphocytes is observed. d Immunohistochemical analysis showing that the PAS-positive lesions were CD61 negative. e VEGF expression is markedly reduced in glomeruli. (a, b, d, and f: original magnification, 400 × ; c: original magnification, 100 ×)
Electron microscopy. a Electron-dense materials in the subendothelial space and paramesangial area are shown. Some electron-dense material in the subendothelial space contained cell debris (asterisk). Extensive foot process effacement is present in glomerular capillaries. Microvillous changes in podocytes are seen. Disappearance of the endothelial fenestrations is also noted (original magnification, 1500 ×). b Subepithelial deposits are observed in a limited area (arrow; original magnification, 5000 ×)
Immune complex-mediated glomerulopathy was suspected based on the renal biopsy findings. Oral corticosteroids (prednisolone) were administered, but were ineffective; hence, mycophenolate mofetil (MMF) was included in the regimen. There was no response after approximately 3 months of immunosuppressive therapy, following which prednisolone and MMF were tapered and symptomatic treatment was administered. Approximately 10 months after biopsy (28 months after the last bevacizumab administration and 12 months after the last gemcitabine administration), the patient exhibited partial remission (type 2). No further progression of the ovarian cancer was observed during this time.
Discussion
Glomerular TMA caused by bevacizumab is thought to result from a decrease in VEGF secretion from glomerular podocytes; thus, preventing the stimulation of endothelial cells and disrupting the maintenance function of the capillaries [4]. Renal biopsies from patients with TMA generally reveal subendothelial edema, double contouring of basement membranes, mesangiolysis, and podocyte effacement. However, there have been several reports of bevacizumab-induced nephropathy, in which immune complex involvement is suspected. In such cases, positive staining for immunoglobulins (commonly for IgA), complements on immunofluorescence microscopy, or exhibition of electron dense deposits in the subendothelium or paramesangial area were thought to represent immune complexes [8,9,10], these are often interpreted as reflecting IgA nephropathy alone or in combination with TMA.
Pfister et al. performed histological comparisons between membranoproliferative glomerulonephritis, acute TMA, and nephropathy associated with anti-VEGF agents, including bevacizumab [6]. They reported that the features of anti-VEGF-associated nephropathy include glomerular capillary microaneurysms and segmental hyalinosis. Furthermore, signs of acute TMA, such as fibrin thrombi and fragmented erythrocytes are not common in patients with anti-VEGF-associated nephropathy. The term “anti-VEGF therapy-induced glomerular microangiopathy” was proposed instead of glomerular TMA.
Person et al. compared renal biopsy findings in patients with nephropathy due to bevacizumab to those of patients with TMA from other causes [7] and found that PAS-positive pseudothrombi are characteristic of bevacizumab-associated nephropathy. Unlike the thrombi observed in TMA, immunohistochemical assays revealed that pseudothrombi did not contain platelets or fibrin. Moreover, IgG, IgA, IgM, C3, and C1q were positive at varying intensities on immunohistochemical staining, although the results of proximity ligation assays indicated no formation of immune complexes. Based on these findings, they argued that inhibition of VEGF by bevacizumab enhances endothelial cell permeability and allows serum proteins to accumulate in the subendothelium without forming immune complexes.
In our patient, PAS-positive lesions similar to the pseudothrombi reported by Person et al. were observed, although these areas did not contain platelets. Double contouring of the basement membranes was also observed, and immunofluorescence microscopy revealed “full house” staining. These are the characteristics of bevacizumab-associated glomerular microangiopathy.
However, our patient exhibited certain characteristics that were distinct from those reported previously. First, the onset of nephrotic syndrome was 17 months after the end of bevacizumab administration and that gemcitabine was administered after bevacizumab. Proteinuria as an adverse event of bevacizumab administration generally decreased after discontinuing this medication; however, sustained proteinuria over 10 months after cessation has been reported [8]. In addition, gemcitabine also causes TMA, and the possibility that it might have contributed to the development of glomerular endothelial cell damage and nephrotic syndrome in our patient cannot be ruled out.
Second, our case revealed extensive effacement of the foot process. Foot process effacement in bevacizumab-associated glomerular microangiopathy has been reported to have varying intensity, ranging from mild to subtotal [6]. VEGF is generally expressed in podocytes and considered to have a protective effect on podocytes by reducing apoptosis and regulating slit diaphragm proteins [11]. VEGF blockade in mice is reported to induce proteinuria and foot process effacement via down-regulation of nephrin, one of slit diaphragm proteins [12, 13]. Zhao et al. also reported that bevacizumab administration in rats reduced VEGF expression and induced an extremely fused foot process of podocytes [14]. In our case, VEGF expression in glomeruli was markedly reduced (Fig. 1e), and bevacizumab was considered the possible cause of this podocyte damage.
Third, we considered the possibility of paraneoplastic syndrome. The histological types of paraneoplastic glomerulopathies associated with ovarian tumor have been reported as membranous nephropathy, minimal change nephrotic syndrome, and membranoproliferative glomerulonephritis (MPGN) [11]. In our clinical case, both spike formation in the glomerular basement membranes as well as subepithelial deposits observed in restricted areas cannot be explained by either TMA or bevacizumab-associated glomerular microangiopathy. These may have been complications of membranous nephropathy caused by the cancer itself. THSD7A is known as a target antigen of idiopathic membranous nephropathy, but it is also associated with malignancy in 33.3% [15]. However, THSD7A staining was negative in our case. Our case also had the characteristics of MPGN such as double contouring of the basement membranes and dense deposit-like lesions in the subendothelial and mesangial areas. However, the clinical course of nephrotic syndrome was not parallel to that of ovarian cancer, indicating that this glomerulopathy was not paraneoplastic syndrome.
Conclusion
We encountered a patient with bevacizumab-associated glomerular microangiopathy who presented with nephrotic syndrome after postoperative chemotherapy for stage IV ovarian cancer. Our examinations revealed that nephropathy caused by bevacizumab exhibits PAS-positive pseudothrombi and “full house” staining determined by immunofluorescence microscopy findings, as well as electron dense deposit-like findings in the pseudothrombi as observed by electron microscopy. These findings are similar to those of immune complex-mediated glomerulopathy, such as lupus nephritis. In recent years, the use of bevacizumab and other forms of anti-VEGF therapy have become widespread. To avoid unnecessary immunosuppressive therapy, nephrologists should be aware of bevacizumab-associated glomerular microangiopathy as a separate type of drug-induced nephropathy.
References
Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21(8):1381–9. https://doi.org/10.1681/asn.2010020167.
Izzedine H. Anti-vegf cancer therapy in nephrology practice. Int J Nephrol. 2014. https://doi.org/10.1155/2014/143426.
Frangié C, Lefaucheur C, Medioni J, Jacquot C, Hill GS, Nochy D. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. 2007. https://oncology.thelancet.comvol.
Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–36. https://doi.org/10.1056/nejmoa0707330.
Hanna RM, Lopez E, Wilson J, Barathan S, Cohen AH. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016;9(2):239–44. https://doi.org/10.1093/ckj/sfv139.
Pfister F, Amann K, Daniel C, Klewer M, Büttner A, Büttner-Herold M. Characteristic morphological changes in anti-VEGF therapy-induced glomerular microangiopathy. Histopathology. 2018;73(6):990–1001. https://doi.org/10.1111/his.13716.
Person F, Rinschen MM, Brix SR, et al. Bevacizumab-associated glomerular microangiopathy. Mod Pathol. 2019;32(5):684–700. https://doi.org/10.1038/s41379-018-0186-4.
Roncone D. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat Clin Pr Nephrol. 2007;3:287.
Tomita M, Ochiai M, Shu S, et al. A case of thrombotic microangiopathy with glomerular subendothelial IgA deposition due to bevacizumab. Jpn J Nephrol. 2014;56(5):612–7.
Yahata M, Nakaya I, Sakuma T, Sato H, Aoki S, Soma J. Immunoglobulin A nephropathy with massive paramesangial deposits caused by anti-vascular endothelial growth factor therapy for metastatic rectal cancer: a case report and review of the literature. BMC Res Notes. 2013. https://doi.org/10.1186/1756-0500-6-450.
Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF—a system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol - Ren Physiol. 2006;291(2):422–8. https://doi.org/10.1152/ajprenal.00448.2005.
Sugimoto H, Hamanog Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278(15):12605–8. https://doi.org/10.1074/jbc.C300012200.
Hara S, Tsuji T, Fukasawa Y, et al. Clinicopathological characteristics of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. Virchows Arch. 2019. https://doi.org/10.1007/s00428-019-02558-0.
Zhao N, Xu Q, Wang M, Fei X, Pan Y, Chen X, Ma S. Mechanism of kidney injury caused by bevacizumab in rats. Int J Clin Exp Pathol. 2004;7:8675–83.
Hoxha E, Beck LH, Wiech T, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7a-specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28(2):520–31. https://doi.org/10.1681/ASN.2016010050.
Acknowledgements
We thank Dr. Yoshihiko Ueda (emeritus professor of Dokkyo Medical University) and Dr. Kazushi Konma (Sakai City Medical Center) for useful advice. We also thank Mr. Takuya Okamura (Dokkyo Medical University Saitama Medical Center) for excellent technical assistance.
Funding
There were no specific funding sources relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient in the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Morimoto, M., Arai, T., Matsuura, M. et al. Bevacizumab-associated glomerular microangiopathy that occurred after postoperative chemotherapy for ovarian cancer. CEN Case Rep 10, 6–11 (2021). https://doi.org/10.1007/s13730-020-00504-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00504-7