Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) had clinical success in the treatment of non-small cell lung carcinoma (NSCLC). An effect of this drug on kidney has not been clarified and the occurrence of glomerulonephritis related to EGFR-TKI has rarely been reported. We present the case of a 71-year-old man with NSCLC who developed proteinuria and microscopic hematuria with the rise in a titer of MPO–ANCA, when 2 years and 3 months passed since the initiation of erlotinib, one of oral EGFR-TKI. Two serial biopsies support that ANCA-associated vasculitis may have been modified by the persistent use of erlotinib. We initiated intravenous pulse therapy with methylprednisolone followed by oral prednisone. The proteinuria has decreased and serum CRP was normalized. However, the serum creatinine level and hematuria did not change during the treatment period. While EGFR inhibition is implicated in protective control for glomerulonephritis, it may exacerbate vasculitis. Close monitoring of the kidney function and urinary findings is required during the use of EGFR inhibitors, such as erlotinib, because it may cause renal adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidermal growth factor receptor (EGFR) mutations encourage to tumor progression [1]. EGFR tyrosine kinase inhibitor (TKI) has had remarkable clinical success in the treatment of non-small cell lung carcinoma (NSCLC) with EGFR mutation [2]. Erlotinib is one of the available oral EGFR TKIs. Rash and diarrhea were most common adverse events of erlotinib [2]. The rate of kidney dysfunction and urinary abnormality as adverse event is still unclear because of low frequency. Several reports have recently shown cases of glomerulonephritis, such as crescentic glomerulonephritis and diffuse glomerular endothelial injury in patients treated with EGFR TKIs [3, 4].
In humans, EGF is expressed in the ascending portion of Henle’s loop and the distal tubule in the kidney [5]. Recent studies of animal models of acute kidney injury (AKI) demonstrated that EGFR activation may enhance renal recovery by dedifferentiation of renal tubular cells [6]. Meanwhile, it was proposed that EGFR activation promotes renal fibrosis in chronic kidney disease (CKD) [6]. The relationship between EGFR activation and effects on kidney function remains obscure. We herein report a case of renal-limited ANCA-associated vasculitis (AAV) in a patient treated with erlotinib for NSCLC, who underwent two serial kidney biopsies.
Case report
A 71-year-old man with history of hypertension and metastatic NSCLC was referred to our department for examination of proteinuria and microscopic hematuria. His blood pressure was well controlled to approximately 130/70 mmHg with medication. He was diagnosed with NSCLC of the left lower lobe lung in 2006. He was a smoker with 30 years of more than 40 cigarettes per-day history. He had undergone a left lower lobectomy and lymph node dissection in 2006, and the tumor stage was pT4N2M0 (stage IIIB). He received multiple systemic chemotherapy, which was demonstrated in Fig. 1. He experienced several episodes of acute kidney failure during chemotherapy (Table 1). Peak value of serum creatinine level before use of erlotinib was 2.86 mg/dL, which had been caused by severe diarrhea as adverse event of gefitinib. However, his base serum creatinine level mostly remained normal (0.94–1.01 mg/dL) and no urinary abnormalities were detected before use of erlotinib. As shown in Fig. 1, after pemetrexed was suspended because of progressive disease, chemotherapy was eventually changed to erlotinib (150 mg/day) in 2016. Erlotinib was supposed to be effective, because osteosclerosis of bone metastasis was found 3 months after the initiation of erlotinib. One year after the initiation of erlotinib treatment, the serum creatinine increased to 1.3 mg/dL without urinary abnormality. Thus, erlotinib was decreased to an every other day dose. When 2 years and 3 months passed after erlotinib treatment was started, the patient exhibited proteinuria and microscopic hematuria without fever. His serum creatinine level was 1.23 mg/dL. A urinalysis revealed that his urinary protein was 0.65 g/gCr and his urinary sediment contained 10–19 red blood cells (RBCs) per high power field. Serum C-reactive protein (CRP) was 0.38 mg/dL, and myeloperoxidase–anti-neutrophil cytoplasmic antibody (MPO–ANCA) was elevated (35.4 U/mL; normal range < 3.5 U/mL).
Then, we performed the first kidney biopsy. The laboratory data at the first biopsy is demonstrated in Table 2. The total number of glomeruli was 42, with 23 sclerotic glomeruli. There were no glomerular crescents. Light microscopy revealed mild infiltration of mononuclear and polymorphonuclear leukocytes (Fig. 2A). Mild mesangial matrix expansion was seen in a part of the glomeruli (Fig. 2B). Glomerular basement membrane was disrupted in periodic acid methenamine silver (PAM) stain (Fig. 2C). Approximately 20% of the cortical interstitial area was fibrotic in Masson’s trichrome staining. As for arterial and arteriolar changes, medial thickening was present, reflecting aging and hypertension. Immunofluorescence microscopy showed only non-specific mild deposition of immunoglobulin A(IgA). There were no specific findings on electron microscopy. Thereafter, the patient was diagnosed with renal-limited AAV, because any other extrarenal lesions including skin rush, peripheral neuropathy and pulmonary involvement did not develop.
First kidney biopsy findings. A Mild infiltration of mononuclear and polymorphonuclear leukocytes. (PAS staining × 400; the arrows show cell infiltration, scale bars, 20.0 μm). B Mild mesangial matrix expansion. (PAS staining × 400, scale bars, 20.0 μm). C Destruction of glomerular basement membranes and fibrinoid necrosis. (PAM staining × 400, the yellow arrows show the destruction of glomerular basement membranes, scale bars, 20.0 μm). PAS periodic acid-Sciff, PAM periodic acid silver-methenamin
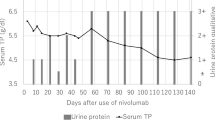
Erlotinib was continued after the first kidney biopsy, considering that discontinuation of erlotinib would worsen the prognosis of NSCLC, while the activity of AAV seemed limited. The patient’s proteinuria remained less than 2.0 g/gCr and his serum creatinine level remained stable (Fig. 3). The level of MPO–ANCA reached peak levels of 67.9 U/mL, and then decreased to 24.0 U/mL (Fig. 3). The level of serum CRP also remained to less than 1.0 mg/dL. He showed no recurrence of NSCLC.
Eleven months after the first biopsy, the level of serum CRP increased (3.29 mg/dL), and MPO–ANCA was elevated (450 U/mL). His serum creatinine level was gradually increased to 2.29 mg/dL. A urinalysis revealed massive proteinuria (3.26 g/gCr) and hematuria (30–49 red blood cells per high-power field).
Thus, we performed the second biopsy to evaluate the activity of vasculitis. The laboratory data at the second biopsy is demonstrated in Table 2. He had intermittent fever, but neither neurological abnormality nor skin lesion including multiple purpura appeared.
The second biopsy specimen contained 40 glomeruli, of which 23 showed global sclerosis. Crescent formation was observed in 11 glomeruli (Fig. 4A). PAS stain revealed that necrosis lesion with the accumulation of fibrinoid material was observed in remaining glomerulus (Fig. 4B). Masson’s trichrome staining showed that the extent of fibrosis was deteriorated, compared to that of previous biopsy. Immunofluorescence stainings were negative. On electron microscopy examination, absence of electron-dense immune deposits was the main finding. The result of the second biopsy was consistent with typical AAV. We initiated intravenous pulse therapy with methylprednisolone followed by oral prednisone (0.6 mg/kg/day after 1000 mg/day of methylprednisolone for 3 days). We judged that the treatment was clinically successful because of reduction of proteinuria and normalization of CRP. The titer of MPO–ANCA decreased to 63.2 U/mL 1 month after steroid pulse therapy (Fig. 3).
However, the serum creatinine level and hematuria did not change during the treatment period. During the steroid therapy, we continued oral erlotinib and closely followed-up the clinical course in consideration of the prognosis of lung cancer. A relapse of NSCLC was not detected so far.
Discussion
We experienced the case of AAV which may have been modified by the persistent use of erlotinib through two serial biopsies. The field of onconephrology has been especially highlighted in recent years, because renal impairment caused by anticancer drugs affects patients’ quality of life, prognosis, and therapeutic strategy. This patient has been exposed to multiple kinds of anticancer drugs for more than 10 years. Therefore, there remains the question about the influence on kidney of these medications. We summarized the anticancer drugs previously administered to this patient and possible nephrotoxicity (Table 1). As shown in Table 1, there was no culprit drug for AAV, though fibrotic cortical interstitial area might be attributed to platinum derivatives.
EGFR-TKIs have shown great promise as therapeutics of lung cancer with EGFR mutation [2]. It is well known that the major toxic effects of EGFR-TKI are skin reactions and diarrhea [2]. There are some reports which support that EGFR inhibition is implicated in protective control for glomerulonephritis. It is not clear how EGFR inhibition is associated with the development of glomerulonephritis. An animal study suggested that heparin-binding epidermal growth factor-like growth factor (HB-EGF) deficient mice, which did not exhibit activation of the EGFR in glomeruli, were notably protected from nephrotoxic serum-induced rapidly progressive glomerulonephritis compared to wild-type mice [7]. In analysis of samples of biobank of cases of glomerulonephritis, the amount of EGF protein in urine was shown to be an independent risk predictor of CKD progression [8].
In contrast, it is presumed that EGFR inhibition may provoke vasculitis in previous reports. There is a case report of leukocytoclastic vasculitis with purpura and renal failure induced by panitumumab, which is one of anti-EGFR antibodies [9]. A 67-year-old Japanese man with advanced colon cancer, who was treated by panitumumab monotherapy, represented bilateral purpura of his forearms 2 days after the second cycle. At the same time, blood test revealed acute kidney injury (Serum creatinine level was 3.1 mg/dL). Kidney biopsy showed only mesangial matrix expansion with segmental mesangial hypercellularity, though skin biopsy of purpura showed leukocytoclastic vasculitis. In addition, there have been the case of pauci-immune crescentic glomerulonephritis in a patient with advanced lung cancer during erlotinib therapy [3]. Authors concluded that it was possible that crescentic glomerulonephritis, which was often observed in leucocytoclastic vasculitis, was induced by erlotinib, because there have been several cases of leucocytoclastic vasculitis during treatment with erlotinib [3]. Wu et al. investigated the association between urinary EGF and outcomes in patients with AAV [10]. They reported that the level of urinary EGF was significantly higher in patients in remission than in patients with active disease [10]. Though little has been reported on the pathophysiologic mechanism underlying the relationship between EGFR TKI and vasculitis, we speculate that EGF inhibition may be associated with harm to vasculitis.
When encountering the coexistence of AAV and a neoplastic disease, several possible explanations for pathophysiology should be considered. First, the elevation of ANCA can be a phenomenon of paraneoplastic syndrome. Mechanism is assumed that cancer cells trigger an immunologic response against blood vessel. In the past reports, the progression of vasculitis was parallel to the cancer [11]. This theory was not applicable to our case, because the lung cancer did not worsen at the timing of two biopsies. Second, infections have also been implicated as triggers AAV. Pathogens work as molecular mimicry, and trigger immunological activation [12]. In this case, when the titer of MPO–ANCA increased, blood culture was negative and whole CT scan did not reveal any infectious findings. Third, the possibility of drug-induced AAV should be taken account of. The often implicated drugs in the published work include propylthiouracil, hydralazine, anti-tumor necrosis factor-α (TNF-α) agents, sulfasalazine, D-penicillamine, and minocycline [13]. These drugs have never been administered to this patient. To the best of our knowledge, there was no reports that had established the association between the elevation of ANCA and every anti-cancer drug given to him which are shown in Fig. 1. Considering that EGF inhibition might be harmful to vasculitis, of the use of erlotinib may be involved in activating MPO–ANCA. However, we do not have the data about the titer of MPO–ANCA before administration of erlotinib, and we could not confirm whether the titer dropped by disconnection of erlotinib, because this patient benefited from erlotinib use to treat NSCLC. Therefore, it is reasonable to think that the initial renal-limited AAV occurred coincidentally and a long-term use of erlotinib contributed to develop the subsequent worsening of AAV or the exacerbation of preexistent CKD.
In conclusion, we reported the detailed clinical course of primary AAV which may have been aggravated by persistent use of erlotinib. EGFR inhibitors, such as erlotinib, may cause renal adverse event, and therefore, careful attention to kidney function and urinary findings should be paid during therapy.
References
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67.
Goto K, Nishio M, Yamamoto N, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer. 2013;82(1):109–14.
Kurita N, Mise N, Fujii A, Ikeda S, Sugimoto T. Crescentic glomerulonephritis in a patient with advanced lung cancer during erlotinib therapy. NDT Plus. 2009;2(6):512–3.
Latcha S, Jaimes EA, Gutgarts V, Seshan S. Case of Proteinuria, Worsening Hypertension, and Glomerular Endotheliosis With Erlotinib and Gefitinib. Kidney Int Rep. 2018;3(6):1477–81.
Gesualdo L, Di Paolo S, Calabró A, Milani S, Maiorano E, Ranieri E, Pannarale G, Schena FP. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int. 1996;49(3):656–65.
Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013;83(5):804–10.
Bollée G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17(10):1242–50.
Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A et al: Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015, 7(316):316ra193.
Kamo H, Shinozaki E, Sugase T, Mizunuma N, Taniguchi S, Gotoh T, Chin K, Tanaka T, Koga K, Yamaguchi K. Leukocytoclastic vasculitis with purpura and renal failure induced by the anti-epidermal growth factor receptor antibody panitumumab: a case report. J Med Case Rep. 2019;13(1):13.
Wu L, Li XQ, Goyal T, Eddy S, Kretzler M, Ju WJ, Chen M, Zhao MH. Urinary epidermal growth factor predicts renal prognosis in antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2018;77(9):1339–44.
Diez-Porres L, Rios-Blanco JJ, Robles-Marhuenda A, Gutiérrez-Molina M, Gil-Aguado A, Vázquez-Rodríguez JJ. ANCA-associated vasculitis as paraneoplastic syndrome with colon cancer: a case report. Lupus. 2005;14(8):632–4.
Flint J, Morgan MD, Savage CO. Pathogenesis of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010;36(3):463–77.
Gao Y, Zhao MH. Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2009;14(1):33–41.
Malyszko J, Kozlowska K, Kozlowski L. Nephrotoxicity of anticancer treatment. Nephrol Dial Transplant. 2017;32(6):924–36.
Takimoto T, Nakabori T, Osa A, Morita S, Terada H, Oseto S, Iwazawa T, Abe K. Tubular nephrotoxicity induced by docetaxel in non-small-cell lung cancer patients. Int J Clin Oncol. 2012;17(4):395–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Oki, R., Hirakawa, Y., Oda, Y. et al. Renal-limited ANCA-associated vasculitis during erlotinib treatment for lung carcinoma. CEN Case Rep 11, 67–72 (2022). https://doi.org/10.1007/s13730-021-00632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-021-00632-8