Abstract
Carboxymethyl chitosan (CMCS) is a water soluble derivate of chitosan, the unique natural cationic polysaccharide with versatile functions. However, the weak chain-breaking antioxidant capacity is still a shortage of CMCS in practical applications. To enhance the antioxidant capacity, CMCS was functionalized with chlorogenic acid (CA), a natural antioxidant compound, by a free radical grafting method in this study. The successful formation of CA-grafted CMCS (CA-CMCS) was confirmed by UV–Vis, FTIR, and 1H NMR analyses, and CA-CMCS had a grafting ratio of 58.6 ± 1.5 mg CAE/g and a water-solubility of 21.0 ± 1.1 mg/mL. Due to CA presence, CA-CMCS showed different physicochemical and biological properties compared with CMCS. The crystallinity and thermal stability of CA-CMCS were lower than those of CMCS. More importantly, the DPPH and ABTS radical scavenging activities of CA-CMCS reached 92.4% and 99.4%, respectively, being much higher than those of CMCS. Also, better hydroxyl and superoxide radical scavenging activities as well as reducing power were obtained for CA-CMCS relative to CMCS. In addition, CA-CMCS revealed good stability, with its higher percentage in phenolic content and DPPH radical scavenging activity compared to CA. Our results suggest that CA-CMCS could be a promising antioxidant used in the fields of food and health care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free radicals, especially reactive oxygen species (ROS), are continuously generated in the human body by normal metabolic processes. The free radicals at low levels have beneficial effects on cellular responses and immune functions, and the human body has various mechanisms to balance the formation of free radicals [1]. However, the overload of free radicals occasionally occurs in cells, resulting in severe damage to biomolecules, including lipids, proteins, and DNA, and subsequent various diseases such as aging, cancer, and cardiovascular disease [2]. To eliminate the excessive free radicals, intake of dietary antioxidants has been proposed, and positive effects are proved [3]. Among various natural compounds, chitosan is considered as a suitable candidate for dietary antioxidant supplements due to its good biocompatibility and health effects on the human body [4]. However, chitosan is insoluble in water and has weak hydrogen-donating ability, which would limit its practical applications. Fortunately, chitosan has numerous active hydroxyl and amino groups [5], which can be easily chemically modified. More importantly, the chemically modified derivatives can partially offset the limitations of chitosan [6]. For example, by introducing carboxymethyl groups onto the hydroxyl and amino sites of chitosan, a water soluble chitosan derivative, carboxymethyl chitosan (CMCS), can be obtained [7]. Interestingly, CMCS remains the attractive biological properties of chitosan, such as anti-microbial activity and non-cytotoxicity. However, like chitosan, the antioxidant capacity of CMCS is still insufficient for practical applications. Therefore, some efforts have been paid on enhancing the antioxidant capacity of CMCS in recent years, and a promising approach, introducing natural antioxidants onto CMCS, has been developed [8].
Polyphenols, the secondary metabolites of plants, are considered suitable moieties combined with CMCS, because they have not only the potent antioxidant capacity but also the ability to prevent some human diseases [9]. Moreover, the stability and bioavailability of polyphenols are improved by combining with polysaccharides, which strengthens their health effects [10,11,12,13]. To date, some polyphenols, such as gallic acid, caffeic acid, ferulic acid, and quercetin, have been successfully inserted onto CMCS, and the resulting conjugates exhibit the excellent antioxidant capacity and anti-tumor activity [8, 14, 15]. Nevertheless, the physical and biological properties of polyphenols depend on chemical structure, leading to different properties in the resulting conjugates. Therefore, it is worth while devoting more efforts to study the conjugates of CMCS with other polyphenols. Chlorogenic acid (CA), one of the most available polyphenols in fruit and vegetable, is considered as a well-known antioxidant agent. CA also has beneficial effects on immune systems and can reduce the risk of many infectious diseases [16]. Thus, CA may be a suitable polyphenol to functionalize polysaccharides. Recently, inserting CA onto chitosan has been achieved by enzymatic and carbodiimide-coupling methods [17, 18]. However, the enzymatic procedure involves the oxidization of polyphenols to quinones, reducing the biological activities. The carbodiimide-coupling method usually requires chemical crosslinking reagents that are not eco-friendly. Thus, another effective approach for the preparation of polyphenol-polysaccharide conjugates, the free radical grafting procedure, has attracted more attention recently due to its safe and environmentally friendly properties. However, to the best of our knowledge, little information regarding CA-grafted CMCS (CA-CMCS) synthesized by the free radical grafting procedure is found in the literature.
Therefore, this work aimed to prepare CA-CMCS by a free radical grafting procedure, optimize the grafting ratio, characterize its physicochemical properties, and evaluate its antioxidant activity and stability.
Experimental
Materials
CMCS with a carboxylation degree of 80% was purchased from Aoxing Biotechnology Company, China. CA was obtained from Ciyuan Biotechnology Company, China. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonicacid) (ABTS) were purchased from Solarbio, China. Other analytical-grade chemicals were provided by Fengchuan Chemicals, China.
Preparation of CA-CMCS
The synthesis of CA-CMCS was performed according to the method of Hu et al. [19]. Briefly, 0.5 g of CMCS was fully dissolved in 100 mL of acetic acid solution (0.5%, v/v) for 30 min with magnetic agitation, followed by adding 4 mL of 1.0 mol/L hydrogen peroxide solution containing 0.2 g of ascorbic acid. After stirring for another 30 min, CA (the molar ratio of CA to CMCS monomer: 0.025:1, 0.05:1, 0.1:1, 0.2:1, 0.3:1 or 0.4:1) was added in the CMCS solution. The mixture was then stirred until CA was completely dissolved, followed by resting at room temperature for 12 h. Afterward, the reaction solution was poured into a dialysis tubing (MWCO 8000–10,000 Da) and dialyzed against distilled water (with 8 changes of water) at room temperature for 48 h to remove unreacted CA and other chemicals. Finally, the reaction solution was centrifuged at 10,000 rpm for 5 min and freeze-dried to obtain the powdered CA-CMCS.
Determination of grafting ratio
The grafting ratio of CA-CMCS was measured by Folin-Ciocalteu method [20]. CA-CMCS was dissolved in distilled water to a concentration of 1 mg/mL. Then, 1 mL of CA-CMCS solution was mixed with 2.5 mL of Folin-Ciocalteu reagent. The mixture was maintained in the dark for 5 min and then added 4 mL of Na2CO3 solution (7.5%, w/v). Afterward, the mixture was adjusted to a volume of 10 mL with distilled water and incubated at 30 ºC for 2 h. Finally, the absorbance of the mixture was measured at 760 nm by a spectrophotometer (UV-1100, Mapada, China). The grafting ratio of CA-CMCS was calculated using CA as a standard and expressed as mg of CA equivalents per g of CA-CMCS, mg CAE/g.
Characterization of CA-CMCS
The structure of CA-CMCS was characterized by ultraviolet–visible (UV–Vis) absorbance, Fourier transform infrared (FTIR) spectroscopy, and proton nuclear magnetic resonance (1H NMR). The UV–Vis spectrum was recorded from the wavelength of 250–450 nm by a spectrometer (Evolution 300, Thermo, America). The FTIR spectrum was measured in a wavenumber range of 4000–500 cm−1 by a spectrophotometer (Vector 22, Bruker, Germany). The 1H NMR spectrum was recorded by a spectrometer (Avance 400, Bruker, Germany), by dissolving CA-CMCS in 2% (v/v) CD3COOD/D2O.
The crystallography of CA-CMCS was determined by X-ray diffraction (XRD), which was performed in a 2θ range of 5–70° by an X-ray diffractometer (D8 Advance, Bruker, Germany). The thermogravimetry (TG), differential thermogravimetry (DTG), and differential scanning calorimetry (DSC) of CA-CMCS were analyzed by a DSC-TGA (SDT/Q600, TA, USA) under the protection of nitrogen, with heating from 40 to 550 ºC at a rate of 10 ºC/min.
Determination of solubility of CA-CMCS
The water-solubility of CA-CMCS was evaluated according to the previous work [21]. In brief, a saturated solution of CA-CMCS was prepared by dissolving 0.5 g of CA-CMCS in 10 mL of distilled water at room temperature for 12–16 h. The saturated CA-CMCS solution was centrifuged at 5000 rpm for 10 min. Then, the supernatant was withdrawn, diluted, and subjected to the measurement of optical density at the absorbance maxima. The solubility was calculated using the following equation:
where S is the solubility (mg/mL), Ax is the absorbance of the saturated solution and B is the coefficient of absorbance.
Assay of antioxidant activity
DPPH radical scavenging assay
The DPPH radical scavenging activity was evaluated according to the previous report [20]. A 2 mL aliquot of CA-CMCS solution was mixed with 2 mL of DPPH solution (0.1 mmol/L) in ethanol. After being vigorously shaken, the mixture was maintained in the dark at room temperature for 30 min. The absorbance of the mixture was then measured at 517 nm. The DPPH radical scavenging activity was calculated by the following equation:
where As and A0 are the absorbance of the sample and blank (without the CA-CMCS solution).
ABTS radical scavenging assay
The ABTS radical scavenging activity was analyzed as previously described by Lee and Je [22]. In brief, ABTS stock was prepared by mixing an ABTS solution (7 mmol/L) in phosphate-buffered saline (PBS, 10 mmol/L, pH 7.4) with an equal volume of potassium persulfate (2.75 mmol/L). The ABTS stock was maintained in the dark at room temperature for 12–16 h and then diluted with PBS (10 mmol/L, pH 7.4) to an absorbance of 0.70 ± 0.02 at 734 nm to obtain the ABTS radical solution. Afterward, 50 μL of CA-CMCS solution was added in 3 mL of ABTS radical solution followed by maintaining in the dark for 6 min. Finally, the absorbance of the mixture was measured at 734 nm. The ABTS radical scavenging activity was calculated using the following equation:
where As and A0 are the absorbance of the sample and blank, respectively.
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity was determined according to the previous report [21]. Typically, 1.5 mL of CA-CMCS solution was well mixed with 0.5 mL of 1,10-phenanthroline (1.5 mmol/L) and 1 mL of PBS (20 mmol/L, pH 7.4). Then, 0.5 mL of FeSO4 solution (0.5 mmol/L) and 0.5 mL of H2O2 solution (25 mmol/L) were added into the mixture. After incubation at 37 ºC for 50 min, the absorbance of the mixture was evaluated at 536 nm. The hydroxyl radical scavenging activity was calculated using the following equation:
where As, Ac, and A0 are the absorbance of the sample, control (without the H2O2 solution), and blank, respectively.
Superoxide radical scavenging assay
The superoxide radical scavenging activity was measured as previously described by Jing et al. [23]. A 1 mL aliquot of CA-CMCS solution was mixed with 4.5 mL of Tris–HCl buffer (50 mmol/L, pH 8.2) and 3.2 mL of distilled water followed by incubation at 25 ºC for 20 min. After adding 0.3 mL of pyrogallol solution (60 mmol/L), the optical density of the mixture was immediately recorded at 420 nm at 30-s interval within 5 min. The superoxide radical scavenging activity was calculated using the following equation:
where ΔAs and ΔA0 are the absorbance change of the sample and blank, respectively.
Determination of reducing power
The reducing power was assayed according to the previous report [24]. First, 1 mL of CA-CMCS solution was mixed with 2.5 mL of PBS (20 mmol/L, pH 6.6) and 2.5 mL of potassium ferricyanide (1%, w/v) followed by incubation at 50 ºC for 20 min. Second, the reaction solution was cooled on ice for 5 min, added 2.5 mL of trichloroacetic acid solution (10%, v/v), and centrifuged at 3000 rpm for 10 min. Third, 2.5 mL of the supernatant was withdrawn and added 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%, w/v). After incubation for 10 min, the absorbance of the reaction solution was measured at 700 nm, which was considered the reducing power.
Determination of stability of CA-CMCS
The stability of CA-CMCS was determined according to the previous method [19] with modifications. CA-CMCS was fully dissolved in distilled water at a concentration of 1 mg/mL. The CA-CMCS solution was then stored at 25 ºC and 4 ºC for 12 days. During storage, the phenolic content and DPPH radical scavenging activity were measured every 2 days. The percent remaining of phenolic content and DPPH radical scavenging activity was calculated and applied to evaluate the stability of CA-CMCS.
Statistical analysis
All experiments were conducted in triplicates; data were expressed as mean values with standard deviation. One-way analysis of variance was used to analyze significant differences (P < 0.05); multiple comparisons were carried out using a Tukey multiple range test. All statistical analyses were performed using SPSS software (Version 20.0, SPSS Inc., USA).
Results and discussion
Preparation of CA-CMCS
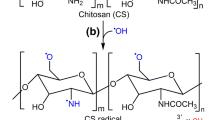
In this study, to improve the antioxidant capacity, CMCS was functionalized with CA by free radical grafting procedure using the ascorbic acid/hydrogen peroxide redox pair as an initiator system. Liu et al. [25] studied the mechanism of the grafting procedure and found that this procedure was mediated by ascorbate radicals generated by the reaction between ascorbic acid and hydrogen peroxide. Accordingly, a possible reaction mechanism for the synthesis of CA-CMCS is herein proposed (Fig. 1). First, an oxidant-reduction reaction occurs in the ascorbic acid/hydrogen peroxide redox system, which generates hydroxyl and ascorbate radicals. Second, the ascorbate radicals abstract hydrogen atoms from the hydroxyl and/or amino groups of CMCS, producing macromolecular CMCS radicals. Third, the CMCS radicals react with CA, resulting in the formation of CA-CMCS.
The grafting ratio, i.e., the phenolic content, is crucial for polyphenol-polysaccharide conjugates, because the phenolic moieties can bring beneficial properties for the conjugates, such as the strong antioxidant capacity. However, the grafting ratio is influenced by several factors such as the molar ratio of polyphenol to polysaccharide monomer, the ascorbic acid and hydrogen peroxide concentrations, and the reaction time. Of these factors, the most notable one is the molar ratio of polyphenol to polysaccharide monomer [26]. Thus, in this study, a series of molar ratios of CA to CMCS monomer were used to obtain a better grafting ratio of CA-CMCS. When the molar ratio of CA to CMCS monomer rose from 0.025:1 to 0.2:1, CA-CMCS showed a significant increase (P < 0.05) in the grafting ratio (Fig. 2), which is probably due to the accumulation of CA moieties on CMCS chains. However, no significant improvement (P > 0.05) in the grafting ratio was observed as the molar ratio further increased from 0.2:1 to 0.4:1, which may be because the available reactive sites of CMCS radicals have been fully occupied by CA moieties. Consequently, the optimal molar ratio of CA to CMCS monomer was 0.2:1. Under this condition, a grafting ratio of 58.6 ± 1.5 mg CAE/g was obtained for CA-CMCS.
Characterization of CA-CMCS
UV–Vis spectrum of CA-CMCS
The UV–Vis analysis is a useful tool to confirm the successful conjugation of polysaccharides with polyphenols. Herein, the UV–Vis spectra of CA, CMCS, and CA-CMCS were measured to validate the grafting of CA onto CMCS. The CA molecule contains an aromatic ring, the π system of which has UV–Vis absorbance in the wavelength range of 250–450 nm [10]. This UV–Vis absorbance characteristic of CA was also observed in this work, with confirmation by a typical absorption peak at 322 nm (Fig. 3). In general, CMCS does not have UV–Vis absorbance within 250–450 nm [8]. Accordingly, no absorption peaks were observed herein in the spectrum of CMCS. In contrast, CA-CMCS exhibited the characteristic absorption peak of the aromatic ring at a slight short wavelength of 320 nm, revealing the successful insertion of CA onto CMCS. In a previous study, Wei and Gao [27] also observed a similar shift in the absorption peak of CA-chitosan. In another previous study [17]; however, both CA and CA-chitosan showed a characteristic peak at 322 nm. After grafting, a red-shift in the absorption peak frequently takes place in some polyphenol-polysaccharide conjugates due to a decrease in energy for the π − π* transition of the aromatic ring [22]. Nevertheless, no red-shift is observed in the absorption peak of CA-grafted polysaccharides, which could be a result of the distance between the grafting site and the aromatic ring in CA [17].
FTIR spectrum of CA-CMCS
The FTIR spectra of CA, CMCS, and CA-CMCS were measured to further confirm the formation of CA-CMCS. CA exhibited the typical phenolic characteristics with the C=O stretching vibration at 1201 cm−1 and the C=C stretching vibration of the aromatic ring in the range of 1442–1600 cm−1 (Fig. 4), as suggested by Rui et al. [17]. For CMCS, the band at 1597 cm−1 is ascribed to the overlap of N–H bending vibration of amino groups and asymmetrical stretching vibration of COO−groups [28]. The band at 1413 cm−1 is attributed to the symmetrical stretching vibration of COO− groups [29]. The band at 1072 cm−1 is the characteristic of hydroxyl groups. Compared with CMCS, CA-CMCS showed a similar FTIR spectrum, revealing that CMCS is the backbone of CA-CMCS. Furthermore, some alterations appeared in the spectrum of CA-CMCS. First, a small band occurred at 1203 cm−1, which is owing to the C=O stretching vibration of CA, indicating the insertion of CA onto CMCS. Second, CA-CMCS exhibited a band at 1732 cm−1, indicating the formation of ester bonds between the hydroxyl groups of CMCS and the carboxyl groups of CA. Third, the band of the overlap of amino and COO− groups shifted to 1591 cm−1 in the spectrum of CA-CMCS, suggesting that the amino groups of CMCS may be also involved in the grafting. These alterations provide evidence for the successful formation of CA-CMCS, and CA molecules are probably grafted onto the hydroxyl and amino groups of CMCS.
1H NMR spectrum of CA-CMCS
In this work, the characterization of CA, CMCS, and CA-CMCS was also carried out by 1H NMR spectrum. In the spectrum of CA, several typical signals appeared in the region of 5.0–7.5 ppm (Fig. 5), which represented the phenyl protons of the aromatic ring of CA [30]. The spectrum of CMCS displayed a single peak at 3.0 ppm, being attributed to the H-2 of the pyranose ring. CMCS also exhibited multiple peaks at 3.3–4.4 ppm, which are assigned to the H-3–H-6 of the pyranose ring. After grafting, CA-CMCS showed the typical signals of both the aromatic and pyranose rings in their respective regions, demonstrating again the successful synthesis of CA-CMCS. Similar results were also observed by Rui et al. [17] in CA-chitosan prepared by carbodiimide-coupling.
Crystallographic structure of CA-CMCS
The XRD pattern was measured to determine the crystallographic structure of CA, CMCS, and CA-CMCS. The XRD pattern of CA displayed multiple intense and sharp peaks from 2θ = 9.6° to 30.3° (Fig. 6), indicating its crystalline form [31]. The original CMCS showed a main peak at 2θ = 20.4° related to crystal form II and a small peak at 2θ = 9.2° assigned to crystal form I, which agrees with the previous report [7]. The crystal form of CMCS is mainly owing to the inter- and intra-molecular hydrogen bonds generated by the hydroxyl and amino groups. After grafting, however, the insertion of CA may hinder the formation of inter- and intra-molecular hydrogen bonds of CMCS, resulting in a loose packing structure of CA-CMCS. Accordingly, CA-CMCS showed a broad peak at 2θ = 14.5° and a weak peak at 2θ = 28.6°, suggesting that CA-CMCS had a lower crystallinity than CMCS. The decrease in the crystallinity was also observed in other polyphenol-polysaccharide conjugates. For example, the introduction of gallic acid onto chitosan decreased the crystallinity of the resultant conjugate [32].
Thermal properties of CA-CMCS
The thermal properties of CA, CMCS, and CA-CMCS were analyzed by TG, DTG, and DSC. For the TG analysis, CA, CMCS, and CA-CMCS showed similar behavior (Fig. 7a), i.e., two stages in weight loss. The first stage with a minor weight loss was assigned to the loss of the residue and bound water on materials. The second stage with a major weight loss was attributed to the decomposition of polymers. Herein, the second stage of CMCS started from 230 °C, which could be considered the initial temperature of the decomposition of CMCS. In contrast, the initial temperature of decomposition for CA-CMCS was only 120 °C. The sharp decrease in the initial temperature of decomposition revealed that CA-CMCS had much lower thermal stability than CMCS. The stability of a polymer has been generally related to its inter- and intra-molecular hydrogen bonds. The lower thermal stability of CA-CMCS is mainly due to the damage in the inter- and intra-molecular hydrogen bonds we discussed above. In addition, although CA-CMCS has a lower initial temperature of decomposition than CMCS, higher peak and final temperatures of decomposition are observed for CA-CMCS (290 °C and 390 °C, respectively) relative to CMCS (270 °C and 310 °C, respectively) (Fig. 7b), which is probably because CA-CMCS contains the CA moieties with high thermal stability.
The DSC thermogram of CA displayed an endothermic melting peak at 200 °C (Fig. 7c), which is assigned to the degradation of this compound. CMCS showed an exothermic peak at 280 °C, representing the crosslinking reactions of its decomposed compositions [33]. In contrast, both the endothermal peak of CA and the exothermal peak of CMCS disappeared in the DSC thermogram of CA-CMCS, indicating the conjugation of CMCS with CA again. Similar results were also observed in hydroxycinnamic acid-chitosan [24] and gallic acid-chitosan [32].
Water-solubility of CA-CMCS
After grafting, CA-CMCS showed a good solubility of 21.0 ± 1.1 mg/mL in water. The water-solubility of CA-CMCS was much better than that of other reported polyphenol-polysaccharide conjugates, e.g., gallic acid-chitosan (4.5 mg/mL), vanillic acid-chitosan (2.2 mg/mL), coumaric acid-chitosan (1.7 mg/mL) [21], ferulic acid-chitosan (1.3 mg/mL) [34], CA-chitosan (4.1 mg/mL) [17], and tannic acid-chitosan (15.0 mg/mL) [23]. The favorable water-solubility of CA-CMCS is probably a result of the synergistic effects of the hydrophilic groups of CA and the carboxyl groups of CMCS. In addition, the introduction of CA reduces the inter- and intra-molecular hydrogen bonds of CMCS, which also has a positive effect on water-solubility of CA-CMCS. Importantly, good water-solubility could endow CA-CMCS with more extensive applications in various fields.
Antioxidant capacity of CA-CMCS
CMCS is not a good chain-breaking antioxidant due to the lack of H-atom donors. Inserting polyphenols onto CMCS can produce CMCS-based conjugates with the strong antioxidant capacity [8]. The antioxidant capacity of compounds generally includes radical scavenging ability, ROS quenching ability, and reducing power. Accordingly, multiple assays were performed to evaluate the antioxidant capacity of CA-CMCS in this work.
Radical scavenging activity
The primary antioxidant capacity of CA-CMCS was evaluated by determining the scavenging capacity against DPPH and ABTS radicals. The DPPH and ABTS radicals are hydrophobic and hydrophilic, respectively. The DPPH and ABTS radical solutions have the absorbance maxima at 517 nm and 734 nm, respectively [20, 22]. Antioxidants are able to scavenge DPPH and ATBS radicals by donating H-atoms, leading to lowering absorbance of the solution. Therefore, the DPPH and ABTS assays are regularly used to determine the chain-breaking antioxidant capacity of compounds [23]. As a natural antioxidant compound, CA showed strong scavenging capacity against DPPH and ABTS radicals, with the scavenging activities of > 89.4% and > 96.0% at tested concentrations, respectively (Fig. 8). However, very weak DPPH and ABTS radical scavenging activities (< 9.5%) were observed for CMCS due to the lack of H-atom donors. Compared with CMCS, a significant improvement (P < 0.05) in the scavenging activity was obtained for CA-CMCS. With increasing concentration, both the DPPH and ABTS radical scavenging activities of CA-CMCS increased significantly (P < 0.05). At the concentrations of 110 μg/mL and 60 μg/mL, the maximum scavenging activities of CA-CMCS were 92.4% and 99.4% for DPPH and ABTS radicals, respectively. These values were close to those of CA, demonstrating the strong radical scavenging capacity of CA-CMCS. Moreover, the DPPH and ABTS radical scavenging activities of CA-CMCS were higher than those of other reported conjugates formed with CMCS or CA. For example, the DPPH radical scavenging activities of ferulic acid-CMCS, caffeic acid-CMCS, and gallic acid-CMCS were only 59.26%, 64.81%, and 74.54%, respectively, at the concentration of 1 mg/mL [8]. The ABTS radical scavenging activity of CA-chitosan was < 90% at the concentration of 0.5 mg/mL [17]. In addition, the half-inhibition concentrations (IC50) of CA-CMCS against DPPH and ABTS radicals were 35.0 μg/mL and 23.1 μg/mL, respectively, confirming again the strong radical scavenging activity of CA-CMCS.
Our results confirm the formation of CA-CMCS with strong antioxidant capacity by introducing CA onto CMCS. Since CA has available phenolic hydroxyl groups with great H-atom donating ability, it is considered a strong antioxidant compound [16]. When CA is inserted onto CMCS, the phenolic hydroxyl groups are brought into the resultant CA-CMCS, which is supported by UV–Vis, FTIR, and 1H NMR discussed above. Therefore, CA endows CA-CMCS with the strong primary antioxidant capacity, as suggested by Božič et al. [35] in gallic acid-chitosan and caffeic acid-chitosan. Moreover, CA-CMCS had a higher maximum scavenging activity and a lower IC50 value against DPPH radicals than ABTS radicals, indicating that CA-CMCS may have better scavenging capacity against hydrophilic radicals than hydrophobic radicals. These results also suggest that CA-CMCS may have better efficiency in aqueous solution than an organic environment, which agrees with the previous report [36].
ROS quenching ability
The hydroxyl radical is the most active ROS in living cells. It can damage the adjacent biomolecules, including DNA, proteins, and lipids, causing many serious human diseases [1]. Antioxidants are able to quench ROS through electron-donating, which is beneficial to human health [3]. Thus, the hydroxyl radical scavenging activities of CA, CMCS, and CA-CMCS were assayed in this work. CA and CA-CMCS showed a continuous increase in the hydroxyl radical scavenging activity with the concentration (Fig. 9a). The maximum scavenging activities of CA-CMCS and CA reached 83.9% and 90.5%, respectively, at the concentration of 31.5 μg/mL. In contrast, the hydroxyl radical scavenging activity of CMCS increased to 61.8% as the concentration increased to 13.5 μg/mL but had no significant increase thereafter (P > 0.05). Moreover, CA-CMCS had a lower IC50 value of 12.7 μg/mL against hydroxyl radicals than other reported polyphenol-CMCS conjugates, e.g., ferulic acid-CMCS (1.46 mg/mL), caffeic acid-CMCS (0.65 mg/mL), and gallic acid-CMCS (0.36 mg/mL) [8], suggesting that CA-CMCS had better ROS quenching ability.
The superoxide radical is considered the primary ROS; it can further interact with other molecules to generate secondary ROS such as the hydroxyl radical [2]. The superoxide radical scavenging activities of CA,CMCS, and CA-CMCS are presented in Fig. 9b. Three samples showed similar behavior in the superoxide radical scavenging activity, that is, their scavenging activities all increased with the concentration. Nevertheless, at each tested concentration, the scavenging activity of CA-CMCS was higher than that of CMCS. At the concentration of 100 μg/mL, the scavenging activity of CA-CMCS reached a maximum of 85.0%, whereas CMCS only had a maximum of 66.4%. Furthermore, the superoxide radical scavenging activity of CA-CMCS was also higher than that of ferulic acid-CMCS, caffeic acid-CMCS, and gallic acid-CMCS [8], indicating again its better ROS quenching ability.
In this study, both CA and CMCS exhibited scavenging activity against hydroxyl and superoxide radicals, confirming their ROS quenching ability. After grafting, CA-CMCS showed better hydroxyl and superoxide scavenging activities than CMCS. These results illustrate that both CA and CMCS have contributions to the quenching ability of CA-CMCS against ROS.
Reducing power
Besides radical scavenging and ROS quenching abilities, reducing power is also frequently used to evaluate the antioxidant capacity of compounds [37]. According to the previous literature [38], the reducing power of antioxidants generally associates with the electron-donating ability. Herein, CMCS showed low reducing power (0.054–0.072) in all tested concentrations (Fig. 10), reflecting its weak electron-donating ability. Due to containing the phenolic hydroxyl group that has the excellent electron-donating ability, high reducing power (1.74–2.15) was obtained for CA. Introducing CA onto CMCS brings phenolic hydroxyl groups to the resultant copolymer. As a result, CA-CMCS exhibited significant improvement (P < 0.05) in reducing power compared with CMCS. At the concentration of 96 μg/mL, the reducing power of CA-CMCS increased to 0.57, which was higher than that of CMCS and other reported polyphenol-CMCS conjugates [8, 14]. In addition, the improvement in reducing power was also observed in other graft copolymers [19, 26].
Stability of CA-CMCS
Although polyphenols have strong antioxidant activity, they are unstable in aqueous solution and susceptible to oxygen and temperature [39]. Thus, the degradation of polyphenols occurs normally during storage, resulting in a reduction in the antioxidant capacity. Herein, the stability of CA and CA-CMCS in aqueous solution was evaluated by measuring the percent remaining of phenolic content and DPPH radical scavenging activity during 12 days storage at 25 ºC and 4 ºC. As expected, the phenolic content of both CA and CA-CMCS gradually decreased with storage time (Fig. 11). Nevertheless, after 12 days of storage, the percent remaining of phenolic content was significantly higher (P < 0.05) for CA-CMCS with respect to CA. Similar results were also observed in the DPPH radical scavenging activity. The percent remaining of 80.1% and 83.6% was obtained for the DPPH radical scavenging activity of CA after 12 days of storage at 25 ºC and 4 ºC, respectively, whereas the percent remaining was > 98.3% for that of CA-CMCS. These results suggest that CA-CMCS had better stability than CA. In a previous study, Hu et al. [19] evaluated the stability of gallic acid-chitosan by measuring the phenolic content and found a similar result. In addition, CA showed higher percent remaining of phenolic content and DPPH radical scavenging activity at 4 ºC than 25 ºC, whereas no significant differences (P > 0.05) between 25 ºC and 4 ºC were observed for CA-CMCS after 12 days of storage. These results also reflect the good stability of CA-CMCS.
Conclusion
In the present study, CA-CMCS was successfully synthesized by combining CMCS with CA by a safe and ecofriendly procedure, the free radical grafting procedure using the ascorbic acid/hydrogen peroxide pair redox as an initiator system. Due to the introduction of CA, CA-CMCS exhibited different physicochemical and biological properties compared with CMCS. The crystallinity and thermal stability CA-CMCS were lower than those of CMCS. Notably, stronger radical scavenging ability, ROS quenching ability, and reducing power were obtained for CA-CMCS relative to CMCS. In addition, CA-CMCS had a good water-solubility and showed good stability during storage. Therefore, CA-CMCS could become a promising antioxidant used in the fields of food and healthcare.
References
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4:89–96
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Seifried HE, Anderson DE, Fisher EI, Milner JA (2007) A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem 18:567–579
Anraku M, Fujii T, Kondo Y, Kojima E, Hata T, Tabuchi N, Tsuchiya D, Goromaru T, Tsutsumi H, Kadowaki D, Maruyama T, Otagiri M, Tomida H (2011) Antioxidant properties of high molecular weight dietary chitosan in vitro and in vivo. Carbohyd Polym 83:501–505
Yu J, Lu Q, Zheng J, Li Y (2019) Chitosan/attapulgite/poly(acrylic acid) hydrogel prepared by glow-discharge electrolysis plasma as a reusable adsorbent for selective removal of Pb2+ ions. Iran Polym J 28:881–893
Bhullar NK, Kumari K, Sud D (2019) Semi-interpenetrating networks of biopolymer chitosan/acrylic acid and thiourea hydrogels: synthesis, characterization and their potential for removal of cadmium. Iran Polym J 28:225–236
Bukzem AL, Signini R, Dos Santos DM, Liao LM, Ascheri DP (2016) Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int J Biol Macromol 85:615–624
Liu J, Lu JF, Kan J, Tang YQ, Jin CH (2013) Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int J Biol Macromol 62:85–93
El Gharras H (2009) Polyphenols: food sources, properties and applications—a review. Int J Food Sci Technol 44:2512–2518
Yu SH, Hsieh HY, Pang JC, Tang DW, Shih CM, Tsai ML, Tsai YC, Mi FL (2013) Active films from water-soluble chitosan/cellulose composites incorporating releasable caffeic acid for inhibition of lipid oxidation in fish oil emulsions. Food Hydrocolloid 32:9–19
Cirillo G, Curcio M, Vittorio O, Iemma F, Restuccia D, Spizzirri UG, Puoci F, Picci N (2016) Polyphenol conjugates and human health: a perspective review. Crit Rev Food Sci Nutr 56:326–337
Liu J, Pu H, Liu S, Kan J, Jin C (2017) Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: a review. Carbohydr Polym 174:999–1017
Hu Q, Luo Y (2016) Polyphenol-chitosan conjugates: synthesis, characterization, and applications. Carbohydr Polym 151:624–639
Bai R, Yong H, Zhang X, Liu J, Liu J (2020) Structural characterization and protective effect of gallic acid grafted O-carboxymethyl chitosan against hydrogen peroxide-induced oxidative damage. Int J Biol Macromol 143:49–59
Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J (2014) Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 35:7654–7665
Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, Ahmad F, Babazadeh D, Xia F, Modarresi-Ghazani F, Li W, Zhou X (2018) Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother 97:67–74
Rui L, Xie M, Hu B, Zhou L, Saeeduddin M, Zeng X (2017) Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr Polym 170:206–216
Yang C, Zhou Y, Zheng Y, Li C, Sheng S, Wang J, Wu F (2016) Enzymatic modification of chitosan by cinnamic acids: antibacterial activity against Ralstonia solanacearum. Int J Biol Macromol 87:577–585
Hu Q, Wang T, Zhou M, Xue J, Luo Y (2016) In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J Agric Food Chem 64:5893–5900
Curcio M, Puoci F, Iemma F, Parisi OI, Cirillo G, Spizzirri UG, Picci N (2009) Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J Agric Food Chem 57:5933–5938
Chatterjee NS, Panda SK, Navitha M, Asha KK, Anandan R, Mathew S (2015) Vanillic acid and coumaric acid grafted chitosan derivatives: improved grafting ratio and potential application in functional food. J Food Sci Technol 52:7153–7162
Lee DS, Je JY (2013) Gallic acid-grafted-chitosan inhibits foodborne pathogens by a membrane damage mechanism. J Agric Food Chem 61:6574–6579
Jing Y, Diao Y, Yu X (2019) Free radical-mediated conjugation of chitosan with tannic acid: characterization and antioxidant capacity. React Funct Polym 135:16–22
Lee DS, Woo JY, Ahn CB, Je JY (2014) Chitosan-hydroxycinnamic acid conjugates: preparation, antioxidant and antimicrobial activity. Food Chem 148:97–104
Liu J, Pu H, Chen C, Liu Y, Bai R, Kan J, Jin C (2018) Reaction mechanisms and structural and physicochemical properties of caffeic acid grafted chitosan synthesized in ascorbic acid and hydroxyl peroxide redox system. J Agric Food Chem 66:279–289
Xie M, Hu B, Wang Y, Zeng X (2014) Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J Agric Food Chem 62:9128–9136
Wei Z, Gao Y (2016) Evaluation of structural and functional properties of chitosan-chlorogenic acid complexes. Int J Biol Macromol 86:376–382
Moaddab M, Nourmohammadi J, Rezayan AH (2018) Bioactive composite scaffolds of carboxymethyl chitosan-silk fibroin containing chitosan nanoparticles for sustained release of ascorbic acid. Eur Polym J 103:40–50
Medeiros Borsagli FGL, Mansur AAP, Chagas P, Oliveira LCA, Mansur HS (2015) O-carboxymethyl functionalization of chitosan: complexation and adsorption of Cd (II) and Cr (VI) as heavy metal pollutant ions. React Funct Polym 97:37–47
Berregi I, Santos JI, Campo Gd, Miranda JI, Aizpurua JM (2003) Quantitation determination of chlorogenic acid in cider apple juices by 1H NMR spectrometry. Anal Chim Acta 486:269–274
Shao P, Zhang J, Fang Z, Sun P (2014) Complexing of chlorogenic acid with β-cyclodextrins: inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocolloid 41:132–139
Pasanphan W, Chirachanchai S (2008) Conjugation of gallic acid onto chitosan: an approach for green and water-based antioxidant. Carbohydr Polym 72:169–177
Aelenei N, Popa MI, Novac O, Lisa G, Balaita L (2009) Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J Mater Sci Mater Med 20:1095–1102
Woranuch S, Yoksan R (2013) Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr Polym 96:495–502
Božič M, Štrancar J, Kokol V (2013) Laccase-initiated reaction between phenolic acids and chitosan. React Funct Polym 73:1377–1383
Cirillo G, Curcio M, Spizzirri UG, Vittorio O, Valli E, Farfalla A, Leggio A, Nicoletta FP, Iemma F (2019) Chitosan-quercetin bioconjugate as multi-functional component of antioxidants and dual-responsive hydrogel networks. Macromol Mater Eng 304:1800728
Eom TK, Senevirathne M, Kim SK (2012) Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ Toxicol Pharmacol 34:519–527
Siddaraju MN, Dharmesh SM (2007) Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res 51:324–332
Wang J, Li H, Chen Z, Liu W, Chen H (2016) Characterization and storage properties of a new microencapsulation of tea polyphenols. Ind Crop Prod 89:152–156
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province (No. B2016202111).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Yu, X., Diao, Y. et al. Functionalization of carboxymethyl chitosan with chlorogenic acid: preparation, characterization, and antioxidant capacity. Iran Polym J 30, 81–91 (2021). https://doi.org/10.1007/s13726-020-00875-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00875-9