Abstract
The present manuscript deals with the synthesis of novel chitosan-based semi-interpenetrating networks (semi-CIPNs) and explores their potential for removal of Cd2+ ions from solution. Microwave radiation-induced polymerization was carried out using biopolymer chitosan (CS) and acrylic acid (AA) monomer, in the presence of initiator (K2S2O8) and cross-linker thiourea (CH4N2S). The effect of polymerization variables such as the amount of solvent, concentrations of initiator, monomer and cross-linker and the reaction time were optimized as a function of percentage grafting (Pg). Under optimized conditions, the liquid uptake potential of the synthesized semi-CIPN was studied in terms of percentage swelling (Ps). The semi-CIPN showed the maximum percentage grafting (3845%) and percentage swelling (311%) under the optimized conditions. The physico-chemical techniques such as Fourier transform infrared spectroscopy (FTIR), thermal analysis (TGA/DTG/DTA), scanning electron microscopy (SEM), and X-ray diffractometry (XRD) provided the evidence for formation of semi-CIPN from chitosan. Semi-CIPN was found to be an efficient device for facile sequestering of Cd2+ ions on the basis of batch experimental studies as 98.1% efficiency was observed for removal of Cd2+ from 100 mg/L solution. Reusability of polymeric material was evaluated for its sorption performance in consecutive adsorption and desorption cycle and it was observed that the regeneration efficiency decreases slowly up to eighth adsorption/desorption cycles, thereafter, it decreases significantly in the 9th and 10th cycles and 40% efficiency has been observed after 12th cycle. Regeneration of the synthesized semi-CIPN explores the reusability and economic feasibility of biopolymer material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal-laden wastes are often released into the environment from different industries such as tanneries, electroplating, galvanizing, pigments and dyes, metallurgical and other industries. They are responsible for acute metal toxicity in the aquatic systems. Metals once introduced into any environmental segment remain for years due to their persistence and tendency to slowly crawl into the food chain and thereby leading to detrimental effects on human health and the environment. The various processes such as chemical precipitation, redox system, ion exchange, electrochemical treatment, and osmosis and membrane technologies are employed as treatment options for removal of heavy metals from wastewater [1,2,3]. However, these processes have limitations such as high cost, insufficient capacity to meet regulatory requirements and a generation of by-products which are toxic in nature and difficult to treat [4,5,6]. Heavy metals such as cadmium, chromium, arsenic, and nickel are of special concern because of (I) toxicity even at very low concentrations and (II) tendency to become accumulated in various environmental segments [7,8,9]. Cadmium is released into the environment from electroplating and battery industries, mining activities, pigments, alloys and phosphate fertilizers [10, 11]. The permissible limit for cadmium in the wastewater discharge is 0.1 mg/L in India. The adverse effects of cadmium on human beings include high blood pressure, kidney damage and destruction of testicular tissues and red blood cells. To combat the menace of heavy metal pollution, there is a need to develop efficient and environmental friendly adsorbents for treatment of wastewater at an acceptable level of economic costs.
For the last 3 decades, the research has been diverted to the utilization of natural materials having high sorption potential for the removal of heavy metals. Nowadays, the researchers’ efforts are directed to develop the utilization of cellulosic materials as a potential candidate for removal of metals [12, 13]. Chitin is a biomaterial which is found in the exoskeleton of crabs and others, such as arthropod prawns, lobsters and shrimps [14, 15]. Chitosan, a heteropolymer, is obtained by partial deacetylation of chitin in alkaline medium. Chitosan shows interesting properties such as hydrophilicity, biodegradability, and antibacterial activity, and has the potential for sorption of heavy metals [16]. Chemical modification of chitosan helps to improve solubility characteristics and metal adsorption properties of chitosan [17,18,19]. The adsorption capacity of chitosan depends upon porosity, crystalline nature, swelling, degree of deacetylation and amine content [20]. However, its low porosity, weak mechanical strength, less stability and crumbling tendency impair its applications for wastewater treatment. The efforts are directed towards improvement in dissolution behavior, metal sorption capacities and stability of chitosan. Attempts have been made to improve the metal adsorption capacity through grafting technique [21, 22].
Interpenetrating networks (IPNs) are three-dimensional networks formed by intimate combination of two or more polymers in which at least one has been polymerized in the presence of other. IPNs have gained attention because of their mechanical strength, sorptive characteristics and they offer a possibility to design polymers with the desired range of specificity and properties. Polymeric hydrogels are 3D networks of hydrophilic polymeric chains presenting high water permeability to a variety of molecules and good biocompatibility [23, 24].
In continuation of our earlier works on sequestering of heavy metal ions using natural polymeric materials [25, 26], the focus of the present work is to explore the potential of biopolymer chitosan-based semi-CIPN for facile removal of heavy metal ions. Semi-CIPN is prepared from natural biopolymer chitosan using as backbone, vinyl monomer acrylic acid and cross-linker thiourea. The synthesized polymeric network is assessed for its swelling capacity and potential to sequester Cd2+ ions from cadmium solution.
Experimental
Materials
Chitosan, analytical grade, from Hi-Media (Mumbai, India), acetic acid and acrylic acid from SD Fine Chemicals (Mumbai, India) and thiourea from Merck were used without further purification. Cadmium stock solution was prepared by dissolving synthetic cadmium nitrate (Merck, Germany) in Millipore water. Adjustment was made on pH of the solution by adding 0.1 M NaOH and 0.1 M HCl (Fluka, USA) solutions. Millipore water was used in preparation of solutions, buffers and other polymeric reactions.
Synthesis and characterization of synthesized semi-CIPN
Microwave radiation assisted the polymerization reaction to synthesize semi-CIPN. The required amount of acrylic acid (5 mol/L) was added drop by drop to the filtered homogenized viscous chitosan solution under continuous stirring condition. The solution was stirred for 20 min at room temperature (25 °C), followed by the addition of appropriate amount of potassium persulfate to the homogeneous mixture to initiate the reaction under continuous stirring for 20 min. The cross-linker thiourea was added drop wise to the milky white solution, and the mixture was kept to remove homopolymer. The synthesized semi-CIPN was dried in an oven at 60 °C to reach a constant weight. Various reaction variables such as the amount of solvent, initiator concentration, reaction time and concentrations of monomer and cross-linker were optimized as functions of percentage grafting (Pg). The maximum liquid uptake capacity of synthesized semi-CIPN was evaluated in terms of percent swelling (Ps). The percentage grafting (Pg) and percentage swelling (Ps) were calculated using the following equations, respectively [27, 28]:
where \({w_1}\) and \({w_2}\) are the weights of chitosan and chitosan-based semi-CIPN, respectively,
where \({\text{ws}}\) and \({\text{wd}}\) are the weights of swollen semi-CIPN and dry semi-CIPN, respectively.
FTIR spectra of pure chitosan and synthesized semi-CIPN were recorded on Perkin Elmer-RXI FTIR. Thermogravimetric analysis (TGA), derivative thermogravimetric analysis (DTG) and differential thermal analysis (DTA) of the semi-CIPN were carried out using TG/DTA 6300, SII EXSTAR 6000 in the temperature range of 50–700 °C at the heating rate of 10 °C/min. X-Ray diffraction (XRD) data were recorded using a Philips Xpert diffractometer (Almelo, Netherlands) with monochromatic CuKα radiation operating at 40 kV and 20 mA. SEM analysis was carried out on a Joel (Model JSM6100) scanning electron microscope. Before focusing the electron beam on the samples, the samples were gold sputtered to make them conductive.
Potential of semi-CIPN for removal of Cd2+ from aqueous solution
Batch experiments were carried out for investigating the potential of semi-CIPN for removal of cadmium ions from aqueous solution. For each batch experiment, the required dose of semi-CIPN was added to 50 mL of 25 mg/L of Cd2+solution which was agitated on a mechanical shaker for 120 min. Thereafter, the adsorbent was removed by filtration and the residual concentration of the supernatant was determined using Cd2+ ion selective electrode. All the experimental results were averages of three repeated performances.
Experiments were also performed by varying the adsorbent dose (50–700 mg), concentration of Cd2+ solution (10–100 mg/L) and contact time (30–240 min). The removal percentage (R%) of Cd2+ ions was estimated for each run using the following equation:
where \({C_{\text{i}}}\) and \({C_{\text{e}}}\) are the initial and final concentrations of cadmium ions, respectively.
The adsorption capacity of semi-CIPN was obtained experimentally for each equilibrium concentrations of Cd2+ ions for solution volume (V) through the following equation:
where Ci and Ce are the initial and final concentrations of cadmium ions in the solution, and V and M are the volume in (L) and mass of semi-CIPN in (g), respectively.
Results and discussion
Chitosan-based semi-CIPN was synthesized using monomer acrylic acid, initiator K2S2O8 and thiourea as a cross-linker under the influence of microwave radiations. The –OH and –NH2 functional groups of the chitosan are the active sites where the grafting of poly (acrylic acid) chains could take place to form semi-CIPN in presence of thiourea as a cross-linking agent. The possible mechanism of grafting of poly (vinyl) chains onto chitosan is shown in Schemes 1 and 2.
Different reaction parameters such as the amount of solvent, concentrations of initiator, cross-linker, monomer and reaction time are optimized as a function of percentage grafting (Pg).
Amount of solvent and percentage grafting (Pg) on semi-CIPN
The effect of the amount of solvent on percentage grafting (Pg) is studied by varying the amount of 3% acetic acid solution from 10 to 30 mL for a fixed amount of chitosan (0.5 g), monomer (0.05 mol/L), initiator (0.25 × 10− 2 mol/L) and cross-linker (1.25 × 10− 4 mol/L). Figure 1 shows that the maximum Pg (714%) is observed with 10 mL solvent (acetic acid) and the Pg decreased with increasing the amount of solvent. The 638% Pg is observed with 25 mL of 3% acetic acid solution and thereafter not much decrease is evident in percentage grafting with an increase in the amount of solvent. The polymerization reaction involves the OH* free radicals along with the formation of SO4−* and solvent volume facilitated the movement of ions towards the backbone and acrylic acid moieties, thereby generating more active sites both on vinyl monomer molecules and chitosan. Thus, it facilitated the movement of live poly (acrylic acid) chains towards the active sites of chitosan (backbone) resulted in high Pg.
Initiator (K2S2O8) concentration and the percentage grafting (Pg) of semi-CIPN
The polymerization reaction was carried out by varying the concentration of initiator (K2S2O8) from 0.25 × 10− 2 to 0.5 × 10− 2 mol/L keeping all other variables constant such as monomer (0.05 mol/L), cross-linker (1.25 × 10− 4 mol/L) for the fixed amount of chitosan (0.5 g). Figure 2 shows a drop in percentage grafting with an increase in the initiator concentration. The maximum Pg is observed with 0.25 × 10− 2 mol/L of initiator concentration. The role of initiator is to initiate the polymerization reaction by formation of SO4 in the reaction medium. These reactive species generate a large number of active sites on the chitosan backbone and monomer molecules which ultimately gives rise to higher grafting of live poly (vinyl) chains onto chitosan and hence high Pg is obtained. However, an increase in initiator concentration resulted in the predominance of homopolymerization over graft copolymerization due to the formation of a large number of live polyacrylic acid chains in the reaction medium.
Cross-linker concentration and percentage grafting (Pg) on semi-CIPN
The polymerization reaction to synthesize semi-CIPN was carried out by varying the amount of cross-linker thiourea from 0.25 × 10− 4 to 2.75 × 10− 4 mol/L keeping other parameters constant, i.e., chitosan (0.5 g), initiator (0.25 × 10− 2 mol//L), monomer (0.05 mol/L) and 10 mL of solvent. Initially, with an increase in thiourea concentration, an increase in percentage grafting (Pg) (714%) was observed with 1.25 × 10− 4 mol/L concentration of cross-linker (Fig. 3). Thereafter, a continuous drop was observed in percentage grafting and for 2.75 × 10− 4 mol/L of cross-linker, the value of Pg dropped to 104%. The increase in cross-linker concentration beyond an optimum level resulted in decreased Pg because the cross-linker provides stiffness and rigidity to the polymer structure and the penetration of grafting material into chitosan network becomes difficult.
Monomer concentration and the percentage grafting (Pg) on semi-CIPN
Figure 4 shows the effect of variation of concentration of acrylic acid on the percentage of grafting. It has been observed that with an increase in the concentration of monomer (acrylic acid), the percentage grafting increases continuously and reaches the maximum value of 3588% with 0.225 mol/L and thereafter Pg is decreased with further increase in acrylic acid concentration. This behavior can be initially explained as an increase in monomer concentration, which leads to the accumulation of monomer molecules in close proximity to the chitosan backbone. However, after saturation, a decrease in percentage grafting can be associated with depletion in the available acrylic acid concentration, as well as reduction in the active sites of chitosan backbone. At higher concentrations of monomer, the primary free radicals attack the monomer chains instead of reacting with the backbone of chitosan which may result in the formation of homopolymer. It can also be noted that, once the graft copolymer radical is formed, the excess monomer would shield the graft copolymer, which may slow down the rate of graft copolymerization. In addition to this, the excess monomer becomes available for initiator radicals to initiate the homopolymerization reaction and thus, results in lower grafting percentage (Pg).
Polymerization time versus grafting percentage (Pg) on semi-CIPN
The effect of polymerization on percentage grafting is presented in Fig. 5. Experiments were performed by varying the polymerization time from 25 to 65 min, under exposure to microwave radiation at 100 W keeping other parameters constant (i.e., chitosan 0.5 g; acrylic acid 0.225 mol/L; initiator 0.25 × 10− 2 mol/L and thiourea 1.25 × 10− 4 mol/L). Initially, increase in reaction time exhibited an increase in Pg till 45 min and maximum Pg (3845%) was attained. This is due to increased interaction between live polyacrylic acid chains and active sites of chitosan giving rise to higher grafting yield. However, the decrease in Pg beyond the optimum reaction time (45 min) is due to the predominance of polyacrylic acid formation over poly (acrylic acid)-g-chitosan graft copolymer, hence, a decrease in Pg is observed.
The obtained optimized reaction conditions for maximum semi-CIPN grafting yield are concentration of acrylic acid 0.225 mol/L, initiator concentration 0.25 × 10− 2 mol/L, cross-linker concentration 1.25 × 10− 4 mol/L and reaction time of 45 min. The percentage grafting for semi-CIPN synthesized under optimized conditions is found to be 3845%. The swelling percentage (311%), Pg, of the synthesized semi-CIPN was in agreement with earlier findings on swelling percentage [29].
Fourier transform infrared spectrum (FTIR)
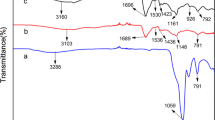
FTIR spectroscopy was used to determine the interactions amongst chitosan, acrylic acid and thiourea molecules. The FTIR spectrum of chitosan, shown in Fig. 6a, demonstrates a strong absorption band at 3365 cm−1 which may be attributed to the symmetrical stretching vibrations of –OH group. The characteristic absorption peaks of the chitosan observed at 1662 and 1598 cm− 1 are due to the stretching vibration of amino group. A peak corresponding to symmetric –CH2 stretching vibration of pyranose ring is observed at 2883 cm− 1 which is in line with the literature [30]. The small peak at ~ 890 cm−1 is due to wagging of the saccharide structure of chitosan. In case of synthesized semi-CIPN (Fig. 6b), a shift in absorption band is observed at 3426 cm− 1corresponding to –OH group. The increased intensity of this peak observed in semi-CIPN is attributed to the polymerization and grafting of acrylic acid chains onto chitosan. The smaller breath in this peak is due to the group’s involvement in polymerization. A characteristic peak at 1018 cm− 1 appeared for C=S stretching of semi-CIPN. Peaks at 670 cm− 1, 510 cm− 1 and 465 cm− 1 correspond to bending vibrations of N–H, CH2 rocking and C=S band. FTIR spectrum is also recorded for the Cd2+-loaded semi-CIPN and is presented in Fig. 6c. Disappearance of small peaks and decrease in the intensity of peaks indicate the bonding between Cd2+ ions and the synthesized semi-CIPN.
Thermal analysis
Thermal properties of chitosan and synthesized semi-IPN have been studied with the help of TGA, DTG and DTA and the results are presented in Fig. 7a, b. The first stage showed 6.7% weight loss of chitosan which corresponded to evaporation of free and bound water molecules along with other volatile compounds at 104 °C, while in semi-CIPN this step was observed at higher temperature, i.e., 157.7 °C, probably due to the entrapment of small molecules in the network. The second stage for chitosan was 225–325.5 °C and showed 38% weight loss probably due to breakage of linkages present in chitosan. The final decomposition stage demonstrated gradual loss of weight by approaching 631.6 °C due to thermal decomposition of polysaccharide structure. The TGA and DTG curves of semi-CIPN exhibited well-differentiated weight loss steps. The initial decomposition temperature of semi-CIPN, observed at 259.2 °C in DTG and TGA graph, occurred at 281.8 °C corresponded to 46.6% weight loss. The second step showed the gradual loss at temperature 285–450 °C and final decomposition occurred at 579 °C with 3.8% polymeric mass left. Decomposition at these temperatures was attributed to the breakdown of grafted chains and backbone along with other side chains and the polysaccharide structure. Glass transition temperature was observed at 158.8 °C for semi-CIPN which was apparent from the peak at 157.7 °C in DTG graph. DTA graph of semi-CIPN showed exothermic peak at 533.8 °C which confirmed the formation of cross-linked structure in semi-CIPN.
Scanning electron microscopy (SEM)
SEM analysis was used to determine the surface morphology of chitosan and semi-CIPN which are shown in Fig. 8a, b, respectively. One can observe a clear demarcation in surface morphology of chitosan and semi-CIPN. The morphology of chitosan displays more rigid and compact-structured network as depicted in the Fig. 8a. Whereas the morphology of the synthesized semi-CIPN indicates a more exfoliated cloud-like structure. This may be due to the grafting of acrylic acid onto chitosan backbone which has produced an amorphous surface morphology. Thus, the surface area of the polymer for the adsorption of Cd2+ ions is increased after grafting.
X-ray diffraction studies
The X-ray diffraction patterns of chitosan and semi-CIPN are presented in Fig. 9a, b, respectively. Generally, the polymers contain both amorphous as well as crystalline regions. It is common to find the presence of an amorphous region leading to the appearance of characteristic halo in diffraction pattern. A strong peak of chitosan at 2θ = 21.9° is observed in X-ray diffractogram which reflects the crystalline nature of chitosan. The X-ray diffractogram of semi-CIPN shows a broad peak at around 22° (2θ). The intensity of this peak indicates the amorphous nature of semi-CIPN. In other words, semi-CIPN showed disruption along with a characteristic peak of reduced intensity, thereby it was established that the grafting of the chains onto backbone has led to reduced crystallinity to form the amorphous structure.
Adsorption potential of semi-CIPN for removal of Cd2+ ions
Microwave-assisted synthesized semi-CIPN adsorbent’s ability to remove metal ions from aqueous solutions is assessed by performing experiments and varying the parameters such as pH, adsorbent dose, concentration of Cd2+ ions and contact time.
Effect of pH on removal of Cd2+ ions
To optimize the pH value, adsorption experiments were carried out at pH from 2 to 7 for constant concentration of cadmium ion solution (25 mg/L) and semi-CIPN dose (1 g/L) with continuous stirring at 100 rpm for 120 min contact time. Figure 10 depicts the effect of pH on percentage removal of Cd2+ ions. It can be noted from the graph that the percentage removal of Cd2+ ions is increased with an increase in pH of the solution with uniform pattern. From the experimental data, it is observed that the maximum 99.9% removal is achieved at pH 6 and pH 7. Experiments could not be performed beyond pH 7 due to the occurrence of hydrolysis of cadmium ions. For further optimization of other parameters, pH 6 was fixed for present research work.
Effect of contact time on removal of Cd2+ ions
The study of the effect of contact time on percentage removal is required to establish adsorption equilibrium. The experiments were carried out using 25 mg/L metal ion concentration solution of pH 6 with 1.0 g/L of semi-CIPN at stirring speed of 100 rpm and the results are presented in Fig. 11. The initial fast increase in percent removal is observed up to 60 min of contact time and after that, there is not much change detected by observations. Maximum percentage removal (98.22%) was achieved after 120 min of contact time which was selected to optimize the remaining parameters.
Effect of adsorbent dose on removal of Cd2+ ions
The Cd2+ removal efficiency was studied by varying adsorbent dose from 25 to 700 mg keeping Cd2+ ion concentration 25 mg/L, pH 6, stirring speed 100 rpm and contact time 120 min and results are plotted in Fig. 12. The graphical values indicate that with 25 mg of semi-CIPN dose 49.4% removal of cadmium is achieved and for 50 mg semi-CIPN dose 98.1% Cd2+ ions removal is obtained. With further increase in adsorbent dose, no significant change is observed. For further experiments 1.0 g of adsorbent dose was fixed.
Effect of metal ion concentration on removal of Cd2+ ions
To study the effect of metal ion concentration on semi-CIPN adsorption efficiency, experiments were performed by varying Cd2+ ion concentration from 10 to 100 mg/L, under constant condition of adsorbent dose at 1.0 g/L, stirring speed of 100 rpm, pH 6, temperature at 25 °C and contact time (120 min). The Cd2+ ion concentration was varied from 10 to 100 mg/L. As shown in Fig. 13, the cadmium removal efficiency of the semi-CIPN is decreased with an increase in the initial concentration of metal ions. With 10 mg/L of Cd2+ ion concentration in solution, 99.9% removal is achieved and with 100 mg/L, the percentage removal is found to be 90%. This is due to the fact that initially the metal ions have sufficient binding sites for the adsorption on semi-CIPN and as the concentration of metal ions increases the binding sites become saturated and thus, removal efficiency decreases.
Adsorption isotherms
The adsorption isotherms analysis correlates the quantity of adsorbate in the bulk and the amount adsorbed at the interface [31]. The adsorption isotherm analysis of experimental data relies on the equivalence of every adsorption sites of the adsorbent surface and the adsorbate particles bind independently of the occupancy of adjacent sites [32]. The experimental data obtained from batch adsorption studies is often subjected to commonly used Langmuir and Freundlich isotherm to understand the nature of the surface. The Langmuir adsorption isotherm is based on the formation of a layer of molecules on an adsorbent surface as a function of the concentration of the adsorbed material in the liquid in which it is in contact. Moreover, there is no interaction between molecules of metal adsorbed on adjacent binding sites [33]. The shape of the isotherm is a gradual positive curve that flattens to a constant value.
According to Langmuir isotherm model:
where Qo and b are the Langmuir constants related to adsorption capacity and energy of adsorption.
The Freundlich expression is an empirical equation based on sorption on a heterogeneous surface and according to Freundlich isotherm model:
where x/m is the amount of metal ions adsorbed at equilibrium per gram of adsorbent (mg/g), Ce is the equilibrium concentration of metal ions in the solution (mg/L), KF, and n are the Freundlich model constants.
The results obtained for removal of cadmium ions are analyzed using adsorption isotherm models shown in Fig. 14a, b. A better fit is obtained with Freundlich isotherm model and the values of KF, n and R2 obtained are 1.041, 3.02 and 0.999, respectively.
Semi-CIPN is an interlinked polymeric network containing a plethora of functional groups viz. COOH, OH, NH2 and C=S which act as binding sites for the sequestering of cadmium ions from solution. Semi-CIPN’s high affinity for the Cd2+ can be attributed to the binding of the metal ion through rather complex process which involves chemisorption, adsorption on surface and pores, complexation, chelation and entrapment into the polymeric networks. FTIR spectral studies (Fig. 6c) provide the evidence of chemisorption along with the physical adsorption of Cd2+ from aqueous solutions. The involvement of functional groups such as –NH and CS groups is also manifested from subduing effect on the IR peaks in the spectra. Cadmium being soft acid preferred complexation with soft base sulfur present in semi-CIPN and supports the high removal efficiency even at the higher Cd2+ concentration (100 mg/L).
Reusability potential of semi-CIPN
Regeneration of synthesized semi-CIPN is important to explore the reusability and economic feasibility of biopolymer material. For present study, reusability of polymeric material was evaluated for its sorption performance in consecutive adsorption and desorption cycle. Regeneration of synthesized material was carried out with 0.1 M HNO3 acid solution.
The regeneration efficiency (RE%) is calculated using the following equation:
where qreg is the adsorptive capacity of the regenerated material and qorg is the original capacity of the adsorbent.
The studies were carried out up to 12 cycles. As seen in Fig. 15, regeneration efficiency decreases slowly up to 8th adsorption/desorption cycles, thereafter, it decreases significantly in the 9th and10th cycles and 40% efficiency has been observed after 12th cycle. This may be due to repeated contact with desorption agent leading to loss of sorption performance and also due to disintegration or loss of strength of cross-linked networks.
Conclusion
Biopolymer chitosan-based semi-CIPN synthesized under the influence of microwave radiations is found to be an efficient device for facile sequestering of Cd2+ ions from aqueous solution. CIPN polymeric networks offer a plethora of sites for metal chelation due to the presence of functional groups along with networks voids for entrapment of metal ion. Potential Cd2+ removal efficiency is observed even for high cadmium ion concentration (100 mg/L). Further investigations on the semi-CIPN efficacy for the removal of heavy metals ions will provide an insight into the mechanistic aspects and synthetic development of newer materials for wastewater treatment.
References
Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination: a review. Sci Total Environ 409:4141–4166
Wang H, Ren ZJ (2014) Bioelectrochemical metal recovery from wastewater: a review. Water Res 66:219–232
Park JY, Yoo YJ (2009) Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose. Appl Microbiol Biotechnol 82:415–429
Kurniawan TA, Chan GY, Lo WH, Babel S (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling: an overview. Rsc Adv 2:6380–6388
Hu H (2002) Human health and heavy metals life support: the environment and human health. MIT Press, Cambridge
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79:241–244
MatisKA ZouboulisAI, Hancock IC (1994) Waste microbial biomass for cadmium ion removal: application of flotation for downstream separation. Bioresour Techno l49:253–259
Ramachandra TV, Sudarshan PB, Mahesh MK,VinayS (2018) Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J Environ Manage 206:1204–1210
Çelik S, Akar ST, Şölener M, Akar T (2017) Anionically reinforced hydrogel network entrapped fungal cells for retention of cadmium in the contaminated aquatic media. J Environ Manage 204:583–593
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Hubbe MA, Hasan SH, Ducoste JJ (2011) Cellulosic substrates for removal of pollutants from aqueous systems: A review 1 Metals. Bio Resour 6:2161–2287
Yeul VS, Rayalu SS (2013) Unprecedented chitin and chitosan: a chemical overview. J Polym Environ 21:606–614
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43:401–414
Mourya VK, Inamdar NN (2008) Chitosan-modifications and applications: opportunities galore. React Funct Polym 68:1013–1051
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Kołodyńska D (2012) Adsorption characteristics of chitosan modified by chelating agents of a new generation. Chem Eng J 179:33–43
Miretzky P, Cirelli AF (2009) Hg (II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167:10–23
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Manson JA (2012) Polymer blends and composites. Springer Science & Business Media, New York
Jing G, Wang L, Yu H, Amer WA, Zhang L (2013) Recent progress on study of hybrid hydrogels for water treatment. Colloids Surfac A Physicochem Eng Aspts 416:86–94
Garg K, Kaur MP, Sud D (2008) Removal of nickel [II] from aqueous solution by adsorption on agricultural waste biomass using a response methodological approach. Bioresour Technol 99:1325–1331
Mahajan G, Garg U, Sud D, Garg V (2013) Utilization Properties of Jatropha De-oiled cake for removal of Nickel (ii) from aqueous solutions. Bio Resour 8:5596–5611
Kumar K, Kaith BS, Mittal H (2012) A study on effect of different reaction conditions on grafting of psyllium and acrylic acid-based hydrogels. J Appl Polym Sci 123:1874–1883
Maiti M, Jindal R, Kaith BS, Jana AK (2011) Synthesis of graft copolymers of binary vinyl monomer mixtures onto acetylated Saccharum spontaneum L and characterization. J Appl Polym Sci 121:2060–2071
BhullarN KumariK, Sud D (2018) A biopolymer-based composite hydrogel for rhodamine 6G dye removal: its synthesis, adsorption isotherms and kinetics. Iran Polym J l27:527–535
Kweon H, Um IC, Park YH (2001) Structural and thermal characteristics of Antheraea pernyi silk fibroin/chitosan blend film. Polymer 42:6651–6656
Demirbaş O, Alkan M, Doğan M (2002) The removal of Victoria blue from aqueous solution by adsorption on a low-cost material. Adsorption 8:341–349
Shukla SS, Yu LJ, Dorris KL, Shukla A (2005) Removal of nickel from aqueous solutions by sawdust. J Hazard Mater 121:243–246
Liu HL, Chen BY, Lan YW, Cheng YC (2004) Biosorption of Zn (II) and Cu (II) by the indigenous Thio bacillus thio oxidans. Chem Eng J 97:195–201
Acknowledgements
This research project could not have been accomplished without the support of Sant Longowal Institute of Engineering and Technology, Longowal. We are pleased to acknowledge the facilities provided by National Institute of Technology Jalandhar and Panjab University, Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This project is self-financed and the authors have no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Bhullar, N.K., Kumari, K. & Sud, D. Semi-interpenetrating networks of biopolymer chitosan/acrylic acid and thiourea hydrogels: synthesis, characterization and their potential for removal of cadmium. Iran Polym J 28, 225–236 (2019). https://doi.org/10.1007/s13726-019-00693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00693-8