Abstract
Chemically modified biofunctional chitosan derivatives may open up new applications in functional food and nutraceuticals development. Two new conjugates, namely vanillic acid and coumaric acid grafted chitosan derivatives were developed and comparative evaluation of their antioxidant and antimicrobial activities was carried out with ferulic acid and gallic acid grafted chitosan. The synthesized water soluble (1.0–4.5 mg/mL) chitosan-phenolic acid conjugates showed characteristic FTIR-spectroscopy band at around 1644 and 2933 cm−1. Free phenolic acid was not observed in TLC plate of the chitosan-phenolic acid conjugates and UV–vis spectra showed primary absorption peak of the corresponding phenolic acids confirming the grafting reaction. Total antioxidant activity of the chitosan-phenolic acid derivatives ranged from ~12 to 29 g ascorbic acid equivalent/100 g of the conjugate. Minute quantity of the derivatives (~57–162 μg and 82–109 μg respectively) could give 50 % inhibition of 2, 2′-diphenyl-1-picrylhydrazyl radical and hydroxyl free radical. Broad spectrum antibacterial activity was observed for all four derivatives against an array of foodborne pathogens and spoilage bacteria. Coumaric acid grafted chitosan showed promise as a food preservative as it inhibited the growth of several foodborne pathogens and spoilage bacteria, including Staphylococcus aureus. Among all the four derivatives, ferulic acid and gallic acid grafted chitosan had lowest minimum inhibitory concentration against Staphylococcus aureus and Pseudomonas aeruginosa respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Applications of chitosan in agricultural, food and pharmaceutical industries are well documented. Chitosan is often claimed to possess antioxidant activity (Kamil et al. 2002; Shahidi et al. 2002). However, the mechanism of antioxidant activity of chitosan is still disputable. Many studies have clearly shown low or no antioxidant activity of native chitosan, although the activity significantly increased with appropriate chemical modifications of the biopolymer (Casettari et al. 2012; Xie et al. 2001).
Biofunctional chitosan derivatives, resulting from graft co-polymerization of phenolic acids on chitosan are of recent interest to chitosan chemistry. Grafting of phenolic acids like gallic acid, caffeic acid and ferulic acid to chitosan, thereby imparting primary antioxidant activity to the molecule, have been reported (Cho et al. 2011; Aytekin et al. 2011; Woranuch and Yoksan 2013). These biomaterials are being promoted as multifunctional packaging materials (Schreiber et al. 2013) and novel antioxidants (Pasanphan et al. 2010).
In recent literature, phenolic acids have been proposed as a potential treatment for many disorders including Alzheimer’s disease, cancer, cardiovascular diseases, diabetes mellitus and skin diseases (Mancuso and Santangelo 2014; Prince and Roy 2013; Prince et al. 2011). However, these compounds are immediately metabolized and excessive antioxidants are disposed from the body. Grafting phenolic acids on chitosan may provide these compounds with the much needed stability and slow release. In consonance to this, recently, antidiabetic potential of chitosan gallic acid conjugate has been documented (Liu et al. 2013). Vanillic acid, the oxidized form of vanillin is found at high concentrations in vanilla beans. Besides being a licensed food additive with pleasant creamy smell (FAO/WHO Expert Committee on Food Additives, JECFA no. 959), vanillic acid has been associated with a variety of pharmacological activities (Gitzinger et al. 2012). Similarly coumaric acid was reported to protect against oxidative stress and genotoxicity (Ferguson et al. 2005). Grafting of vanillic acid and coumaric acid with amino functionality of chitosan has not been reported so far and it is apparent that these chitosan derivatives will open up new applications in innovative functional food and nutrient delivery. Hence, there is a need for detailed study on bioactivity of these new chitosan derivatives. Antioxidant and antibacterial potential of phenolic acid grafted chitosan derivatives as functional food ingredient have not been studied extensively. Only a few reports of antioxidant and antibacterial activity of gallic acid grafted chitosan, ferulic acid grafted chitosan and caffeic acid grafted chitosan are available (Cho et al. 2011; Aytekin et al. 2011; Woranuch and Yoksan 2013). However antibacterial activity has been screened against very limited number of bacteria.
This paper reports free radical mediated grafting of vanillic acid, coumaric acid, gallic acid, and ferulic acid with chitosan. To the best of our knowledge, no literature is so far available on grafting of vanillic and coumaric acid onto amino groups of chitosan. This study also achieved higher grafting ratio of the phenolic acid on chitosan. Characterization of the grafted derivatives was carried out with the help of UV–vis and FTIR spectroscopy. This study also gives a detailed comparative account of invitro antioxidant activity and antibacterial activity against 18 ATCC pathogenic bacterial strains.
Materials and methods
Materials
Chitosan from shrimp shells (MW = 100 kDa, 90 % degree of deacetylation) was prepared in the pilot plant facility of Central Institute of Fisheries Technology, Cochin, Kerala, India. Gallic acid (Ga), ferulic acid (Fe), vanillic acid (Va), coumaric acid (Co) (Fig. 1), deoxyribose, folin-ciocalteu reagent, ferrous chloride (FeCl2), ethanol (HPLC grade) and linoleic acid were obtained from Sigma-Aldrich (USA).Acetic acid, hydrogen peroxide (H2O2), ascorbic acid, 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), sulfuric acid (96 % w/w), trisodium phosphate, ammonium molybdate, ammonium thiocyanate, ninhydrin, ferric chloride (FeCl3), ethylene diamine tetraacetic acid disodium salt (EDTA), dipotassium hydrogen phosphate, potassium dihydrogen phosphate, butyl hydroxyanisole (BHA), butyl hydroxytoluene (BHT), thiobarbituric acid (TBA) and trichloroacetic acid (TCA) were obtained from Merck Millipore (Germany). ATCC cultures of microrganisms were procured from Microbiologics (HiMedia, Mumbai). All the microbiological media including sterile discs used in the study were procured from Oxoid (UK).
Synthesis of chitosan-phenolic acid conjugates
The synthesis of gallic acid grafted chitosan (Ga-Ch), ferulic acid grafted chitosan (Fe-Ch), vanillic acid grafted chitosan (Va-Ch) and coumaric acid grafted chitosan (Co-Ch) was performed employing free radical mediated grafting method as reported by Curcio et al. (2009) with some modifications. Briefly, 10 g of chitosan was dissolved in 2 L of 2 % acetic acid solution (v/v) in a 5 L three necked round bottom flask. 20 mL of 1 M H2O2 containing 1.08 g of ascorbic acid was added dropwise to the chitosan solution. The reaction vessel was then flushed with nitrogen for 30 min with continuous stirring, followed by addition of 10 g phenolic acid (gallic acid/ferulic acid/vanillic acid/coumaric acid) dissolved in 100 mL ethanol. The reaction vessel was stoppered and parafilm was applied at the edges to minimize exposure to oxygen. Finally the reaction was carried out for 24 h at 25 °C with constant stirring over a magnetic stirrer. The reaction mixture was dialyzed against distilled water (with 12 changes of water) with a 14,000 Da molecular weight cutoff membrane for 72 h to remove unreacted phenolic acids. The dialyzate was spray dried (inlet temperature 140 °C, outlet temperature 77 °C) in a pilot plant spray drier to afford individual derivatives.
Characterization of chitosan-phenolic acid conjugates
To verify whether phenolic acids were grafted onto chitosan, TLC analysis was performed. Individual phenolic acids, native chitosan, and chitosan phenolic acid conjugates were developed on a silica gel plate with chloroform–ethyl acetate–acetic acid (50:50:1) as mobile phase (Cho et al. 2011). The developed TLC plate was observed using 30 % H2SO4 followed by heating at 100 °C for 10 min. Absorbance maxima of the four chitosan-phenolic acid conjugates were determined by recording UV–vis absorption spectra with a Hitachi U-2910 spectrophotometer. Individual samples were dissolved in de-ionized water, appropriately diluted and UV–vis absorption spectra were recorded in full scan mode from 200 to 500 nm. FTIR spectra were recorded using a Thermo Nicolet, Avatar 370 spectrometer as KBr pallets over a spectral range of 4000–400 cm−1 at a resolution of 4 cm−1.
Determination of water solubility
Saturated solutions of the chitosan-phenolic acid conjugates were prepared by overnight stirring of the samples (200 mg) in 20 mL deionised water. The aliquots were filtered through a 0.22 μm PTFE disk filter. The samples were serially diluted and absorbance was recorded in a spectrophotometer at corresponding absorbance maxima. Solubility was determined using Eq. (1):
Where S is the solubility of derivatives in mg/mL, Ax is the absorbance of the saturated solution at wavelength x nm (corresponding absorbance maxima) and B is the co-efficient of absorbance.
Estimation of free amino group by ninhydrin assay
A ninhydrin assay method, modified from Sabnis and Block (2000) was used to determine free amino groups of chitosan, Ga-Ch, Fe-Ch, Va-Ch and Co-Ch. Briefly, the samples were dissolved in 1 % (v/v) aqueous acetic acid solution (1 mg/mL) with constant stirring at ambient temperature for 24 h. Acetate buffer (pH 5.5, 1 mL) and freshly prepared ninhydrin reagent (10 mg/mL in dimethylformamide, 1 mL) were added to the sample solution (2 mL). The reaction mixture was incubated in a boiling water bath for 30 min, and then cooled to room temperature prior to UV–vis absorbance measurement at 570 nm. Decrease in the free amino group was expressed in terms of decrease in absorbance of the solution.
Estimation of grafted phenolic group on chitosan
Quantification of grafted phenolic groups on chitosan was carried out using Folin-Ciocalteu reagent procedure, with some modification (Curcio et al. 2009). Briefly, Folin-Ciocalteu reagent (1 mL) was added to sample solution of each chitosan phenolic acid conjugate (100 μg in 0.4 mL distilled water). The contents of the test tubes were mixed thoroughly for 3 min, followed by addition of 3 mL of Na2CO3 (2 %) solution. Then the mixture was allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm against a control (without polymer under the same reaction conditions). The amount of grafted phenolic group in each chitosan derivative was expressed as its corresponding phenolic acid equivalent concentration. For plotting the calibration curve 10, 20, 30, 40, 50 μg of each phenolic acid was processed under the same reaction condition. The corresponding phenolic acid equivalent was calculated from the linear regression equation.
Determination of total antioxidant activity
Total antioxidant activity of the chitosan phenolic acid conjugates was determined through a phosphomolybdenum complex formation method with some modifications (Prieto et al. 1999). Briefly, each chitosan phenolic acid conjugate (100 μg in 1 mL distilled water) was mixed with 2.4 mL of reagent solution (0.6 M H2SO4, 28 mM sodium phosphate, 4 mM ammonium molybdate) and 0.6 mL of methanol in a test tube. The reaction mixture was incubated at 95 °C for 150 min. After cooling to room temperature, the absorbance of the mixture was measured at 695 nm against a control (without polymer under the same reaction conditions). The total antioxidant activity of each chitosan phenolic acid conjugate was expressed as equivalent concentration of ascorbic acid. Different concentrations of ascorbic acid (10, 20, 30, 40 and 50 μg) were processed under the same reaction conditions and a calibration curve was plotted. Ascorbic acid equivalent concentration was calculated from the linear regression equation.
Evaluation of 2,2′-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity
Free radical scavenging activity of all four chitosan phenolic acid conjugates was evaluated by DPPH free radical scavenging method as per literature with some modifications (Woranuch and Yoksan 2013). Sample of each chitosan phenolic acid conjugate (50 μg in 0.9 mL distilled water) was mixed with ethanol (0.4 mL) and ethanolic solution of DPPH (1 mM, 0.5 mL) in a test tube. Then the mixture was incubated in the dark for 8 h. The absorbance of the solution was then measured at 517 nm against a control (without polymer). The radical scavenging activity was defined as a decrease in the absorbance of DPPH and was calculated using Eq. (2):
where Acontrol is the absorbance of control and Asample is the absorbance of the sample.
Evaluation of hydroxyl radical (OH·) scavenging activity
Hydroxyl radical scavenging activity of the chitosan phenolic acid conjugates was determined by deoxyribose assay (Halliwell et al. 1987). Briefly, samples of each chitosan phenolic acid conjugate (100 μg in 0.5 mL distilled water) was mixed with 95 % ethanol (0.5 mL) and incubated with deoxyribose (3.75 mM, 0.5 mL), H2O2 (1 mM, 0.5 mL), FeCl3 (100 mM, 0.5 mL), EDTA (100 mM, 0.5 mL), ascorbic acid (100 mM, 0.5 mL) and potassium phosphate buffer (20 mM, pH 7.4, 2 mL) for 60 min at 37 °C. The samples were filtered and TCA (1 mL, 2 % w/v) and TBA (1 mL, 2 % w/v) were added to the filtrate (1 mL) followed by heating in a boiling water bath for 15 min. The contents were cooled and absorbance was read at 535 nm against a blank (without polymer). Hydroxyl radical scavenging activity of the conjugates was calculated as per Eq. (2).
Screening of antibacterial activity
Antibacterial activity of each grafted chitosan-phenolic acid conjugates was tested against 18ATCC bacterial strains by disc diffusion assay according to the CLSI protocol (CLSI 2012). Selected ATCC strains of bacteria were grown 18–24 h in Brain Heart Infusion (BHI) broth. After centrifugation at 8000 rpm for 15 min, the cultures were washed in sterile phosphate buffer and finally suspended in the same buffer. The turbidity of the suspension was adjusted to 0.5 McFarland standard, corresponding to 2 × 108 cfu/mL. Within 15 min of preparation of suspension, a sterile cotton swab was dipped into the suspension and streaked on to the entire surface of pre-dried Mueller Hinton Agar (MHA) plates. This procedure is repeated by streaking two more times, rotating the plate approximately 60° each time to ensure an even distribution of inoculum. The discs containing the chitosan-phenolic acid conjugates were prepared beforehand. Briefly, 5 % (W/V) solution of the compound was prepared in 0.5 % acetic acid in sterile water. The solution (70 μl) was transferred to each disc of 10 mm diameter and dried. The dried discs were placed on the surface of pre-dried Mueller Hinton Agar (MHA) plates. Control discs containing broad spectrum antibiotic and 0.5 % acetic acid in sterile water were also placed on each plate. The plates were incubated at 37 °C for 18–24 h following which the diameter of the inhibition zone was measured. Antibacterial activity of chitosan and the pure compounds (coumaric acid, vanillic acid, ferulic acid and gallic acid) were also tested separately employing the same procedure.
Determination of minimum inhibitory concentration (MIC) of chitosan-phenolic acid conjugates against selected bacteria
Minimum inhibitory concentrations (MIC) of Ga-Ch, Fe-Ch, Va-Ch and Co-Ch were determined against Pseudomonas aeruginosa and Staphylococcus aureus by a microtitre plate-based antibacterial assay method incorporated with resazurin indicator (Sarker et al. 2007). Prior to conducting microtitre assay, bacterial cultures were grown overnight in isosensitest broth (Oxoid), centrifuged, washed and adjusted to optical density of 0.5–1.0. To a sterile microtitre plate, 100 μl of the test compound in 10 % (v/v) sterile water was pipetted into the first row. To other wells, 50 μL of nutrient broth was added, followed by serial dilution from first row onwards. Then to each well, 10 μl of resazurin indicator (0.68 %) was added followed by 30 μl of 3.3× isosensitest broth. Finally, 10 μl of the bacterial suspension was added to all wells and the plate was wrapped with cling film and incubated at 37 °C for 18–24 h. All the plates contained columns of positive and negative control and any change of colour from purple to pink were considered as positive. The lowest concentration at which colour change was observed was taken as MIC for that compound.
Results and discussion
Synthesis and characterization of chitosan-phenolic acid conjugates
Four different chitosan-phenolic acid conjugates were successfully synthesized by free radical mediated reaction of corresponding phenolic acid with chitosan (1:1 w/w) for 24 h under inert environment at 25 °C. Different synthetic approaches have been reported for grafting phenolic acids with chitosan, such as 1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) reaction (Pasanphan et al. 2010; Yu et al. 2011), laccase catalyzed polymerization (Božič et al. 2012), and free radical mediated grafting (Curcio et al. 2009). However in laccase catalyzed polymerization, phenolic acids are oxidized into o-quinones which further react with the primary amines of chitosan. The loss of the active phenol hydroxyl groups may suppress the bioactivity of the derivatives (Liu et al. 2014). EDC and NHS reagents are usually required in large amounts, considered environmentally disadvantageous and difficult to remove completely from the reaction media. As compared to these approaches, free radical mediated grafting of phenolic acids is considered as rapid and ecofriendly (Curcio et al. 2009). However, this approach has not been evaluated widely for grafting different phenolic acids on chitosan.
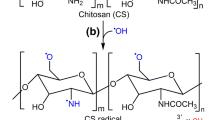
It has been suggested that in acetic acid solution ascorbic acid is present as a di-acid and can further react with H2O2 to generate hydroxyl (OH·) radical. Then the OH· abstracts hydrogen from amino and hydroxyl group of chitosan resulting in chitosan macro radicals. The corresponding phenolic acid molecules become acceptors of chitosan macro radicals, thus forming chitosan-phenolic acid conjugates (Liu et al. 2013). Chitosan solution was sufficiently diluted (5 g/L) for optimum accessibility of phenolic acid monomers to the active sites of chitosan. Earlier it has been observed that with increasing viscosity of chitosan solution, grafting ratio of phenolic acids to chitosan decreases (Liu et al. 2013). The yield of chitosan-phenolic acid conjugates varied between 58 and 65 %. Grafting of phenolic acids on chitosan was confirmed by TLC, FTIR spectroscopy and UV–vis spectrophotometry. No spots corresponding to the individual phenolic acids were observed on the developed TLC plates for chitosan phenolic acid conjugates confirming absence of free phenolic acid and successful grafting of the phenolic acids on chitosan (Fig. 2). Further, FTIR spectroscopy of native chitosan and chitosan-phenolic acid conjugates (Fig. 3) confirmed formation of new covalent bonds. Chitosan exhibited major characteristic bands at around 3428 cm−1 (OH), 2883 cm−1 (C-H stretching), 1650 cm−1 (amide I), 1550 cm−1 (amide II), 1072 cm−1 (COC) and 899 cm−1 (pyranose ring) (Fig. 3a) (Bobu et al. 2011). As compared to chitosan, intensity of amide I (at around 1644 cm−1) and amide II (at around 1549 cm−1) bands in Ga-Ch increased, indicating formation of new amide linkage (Fig. 3b). It was also observed that integral ratio of the CH stretching band (at around 2933 cm−1, belonging to chitosan and gallic acid) to the pyranose band (at around 896 cm−1, belonging to chitosan), i.e. I2933/I896 was higher in Ga-Ch as compared to chitosan (Woranuch and Yoksan 2013). Similar observations were made in case of Fe-Ch, Va-Ch and Co-Ch. This implies that phenolic acids were grafted onto chitosan. Compared to chitosan, Fe-Ch (Fig. 3c) and Co-Ch (Fig. 3d) showed new bands at around 1515 and 1518 cm−1 (C=C aromatic ring) respectively. However these bands were not observed in Ga-Ch (Fig. 3b) and Va-Ch (Fig. 3e) due to broadening of amide I and amide II bands. UV–vis absorption spectra of four chitosan-phenolic acid conjugates (Fig. 4) were recorded between 200 and 500 nm and compared to that of chitosan and individual phenolic acids (data not shown here). Chitosan in 1 % acetic acid (v/v) showed no absorption peak between 200 and 500 nm. Chitosan phenolic acid conjugates showed primary absorption peak at 262, 323, 258 and 286 nm which was same as that of gallic acid, ferulic acid, vanillic acid and coumaric acid respectively. This signifies the grafting of the corresponding phenolic acids onto chitosan. Similar observation was made by Woranuch and Yoksan (2013). However some authors have recorded a slight red shift in absorption wavelength of the conjugates than that of corresponding phenolic acids (Liu et al. 2014).
Water solubility, free amino group and grafting ratio
Further, characterization of the chitosan-phenolic acid conjugates was carried out by estimating their water solubility, free amino groups and grafting ratio of phenolic groups on chitosan. Highest water solubility was observed for Ga-Ch (4.5 mg/mL) followed by Va-Ch (2.2 mg/mL), Co-Ch (1.7 mg/mL) and Fe-Ch (1.0 mg/mL). Available free amino groups in chitosan and chitosan-phenolic acid conjugates were estimated by ninhydrin method. This provides an indirect confirmation of grafting of phenolic acids at amino groups of chitosan. The primary amino groups of chitosan react with ninhydrin reagent to form deep blue colour chitosan–ninhydrin complexes, resulting in an increase in absorbance at 570 nm (Sabnis and Block 2000). Under the same reaction conditions, the absorbance of chitosan at 570 nm was 0.069 whereas for Ga-Ch, Fe-Ch, Va-Ch and Co-Ch it was 0.022, 0.035, 0.030 and 0.041 respectively. The reduction of absorbance implied the decreased free amino group content of chitosan phenolic acid conjugates and confirms the grafting of phenolic acids to amino groups of chitosan. Hence highest degree of substitution was observed in Ga-Ch followed by Va-Ch, Fe-Ch and Co-Ch. The grafting ratios of Ga-Ch, Fe-Ch, Va-Ch and Co-Ch were 393 mg Ga equivalent/g polymer, 263 mg Fe equivalent/g polymer, 305 Va equivalent/g polymer and 129 mg Co equivalent/g polymer respectively. Elsewhere it was reported that the grafting ratio of ferulic acid grafted chitosan and gallic acid grafted chitosan by free radical mediated method was 66.7 mg Fe equivalent/g polymer (Liu et al. 2014) and 7 mg/g polymer (Curcio et al. 2009). Higher grafting ratio in the present study may be due to longer reaction time and inert reaction environment. The reaction for gallic acid grafted chitosan was carried out for 36 h to evaluate any further improvement of grafting ratio with increased reaction time. However no further significant improvement (397 mg Ga equivalent/g polymer) in grafting ratio was observed.
Total antioxidant activity of chitosan-phenolic acid conjugates
Total antioxidant activities of chitosan-phenolic acid conjugates were compared in terms of grams of ascorbic acid equivalent (AsE) per 100 g of conjugate. Ga-Ch showed highest total antioxidant activity (29.33 ± 0.28 g AsE/100 g conjugate) followed by Va-Ch (24.86 ± 0.22 g AsE/100 g conjugate), Fe-Ch (13.88 ± 0.15 g AsE/100 g conjugate) and Co-Ch (12.51 ± 0.25 g AsE/100 g conjugate). This can be explained by the higher grafting ratio achieved for Ga-Ch as compared to other conjugates. This also implies that the antioxidant activities of the chitosan-phenolic acid conjugates are due to phenolic acids grafted onto it.
Free radical scavenging activity of chitosan-phenolic acid conjugates
DPPH and hydroxyl free radical scavenging activity of the chitosan-phenolic acid conjugates correlated well with grafting ratio of corresponding phenolic acid on chitosan. The results are presented in Table 1 and expressed in terms of μg of chitosan/chitosan-phenolic acid conjugate/gallic acid required for 50 % inhibition of DPPH and hydroxyl free radical. Chitosan under the same reaction condition, showed no or negligible scavenging activity against both DPPH and hydroxyl free radical. Among the chitosan-phenolic acid conjugates Ga-Ch showed the best free radical scavenging activity followed by Va-Ch, Fe-Ch and Co-Ch. For 50 % inhibition of DPPH free radical, 57 ± 0.25, 79 ± 0.56, 98 ± 0.21 and 162 ± 0.41 μg of Ga-Ch, Va-Ch, Fe-Ch and Co-Ch was required respectively. Similarly for 50 % inhibition of hydroxyl free radical, 82 ± 0.57, 92 ± 0.37, 102 ± 0.38 and 109 ± 0.71 μg of Ga-Ch, Va-Ch, Fe-Ch and Co-Ch was required respectively. It was reported previously that 20 mg of gallic acid grafted chitosan (Curcio et al. 2009) and 1 mg of ferulic acid grafted chitosan (Woranuch and Yoksan 2013) showed 92 and 97 % inhibition of DPPH free radical respectively.
Antioxidant capacity of a compound depends on its reducing capabilities. In case of DPPH scavenging assay, the antioxidants reduce DPPH radical to diphenylpicrylhydrazine (yellow colored compound). Hence the chitosan-phenolic acid conjugates scavenging ability were evaluated in terms of DPPH reduction.
The deoxyribose assay is considered as most suitable method for determination of hydroxyl radical scavenging properties of antioxidants. Hydroxyl radicals are generated by decomposition of H2O2 by EDTA-Fe2+ complex, which in turn reacts with deoxyribose to form thiobarbituric acid reactive substances (TBARS). Antioxidants donate hydrogen or electron to the hydroxyl radical resulting in decreased TBARS formation. The hydroxyl radical scavenging ability of the conjugates was measured in terms of reduction in TBARS formation.
Antibacterial activity of chitosan-phenolic acid conjugates
Antibacterial activity for different chitosan-phenolic acid conjugates was tested against an array of seafood borne human pathogenic bacteria and spoilage bacteria. Pure forms of phenolic acids were also tested to determine the inhibition patterns against these bacterial groups. Native chitosan showed no antibacterial activity against the strains studied. As shown in Table 2, out of the 18 bacterial groups evaluated, Fe-Ch and Ga-Ch exhibited antibacterial activity against 13 groups; Co-Ch and Va-Ch had activity against 12 and 8 bacterial groups respectively. Fe-Ch had maximum activity against Staphylococcus aureus, followed by Morganella morganii and Enterococcus faecalis. A maximum inhibition zone diameter of 29 mm was observed against Enterococcus faecalis for Co-Ch, followed by Staphylococcus aureus (28.5 mm) and Pseudomonas aeruginosa (26.5 mm). Va-Ch showed maximum activity against Enterococcus faecalis (27 mm) and Staphylococcus aureus (24.5 mm). Similarly, Ga-Ch exhibited maximum activity against Pseudomonas aeruginosa (33 mm), followed by Vibrio alginolyticus (30 mm).
Comparative results were obtained when pure forms of these phenolic acids were individually tested, confirming augmentation of antibacterial activity in grafted chitosan (Table 3). In the present study, both ferulic acid and Fe-Ch showed antibacterial activity against Listeria monocytogenes. Listeria monocytogenesis a known food safety hazard in tropical fish (Karunasagar and Karunasagar 2000) and a causative agent in many disease outbreaks related to consumption of ready to eat seafood (Gombas et al. 2003). In earlier studies, ferulic acid was shown to inhibit pathogens like Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, Escherichia coli, Salmonella enterica and Pseudomonas fluorescens (Takahasi et al. 2013). Chitosan-ferulic acid conjugates was reported to inhibit different clinical isolates of methicillin resistant Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Listeria monocytogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Salmonella typhimurium (Lee et al. 2014).
In the present study, Ga-Ch at 0.5 % level showed broad spectrum antibacterial activity against both Gram positive and Gram negative bacteria. Chitosan films incorporated with 1.5 % of gallic acid were reported to possess strong antimicrobial activity against Escherichia coli, Salmonella typhimurium, Listeria innocua and Bacillus subtilis (Sun et al. 2014). Božič et al. (2012) demonstrated the effectiveness of gallic acid functionalized chitosan at pH 4.5 in reducing the load of Escherichia coli by 97 % and Listeria monocytogenes by 44 % and no activity against Salmonella enterica. Similar results were obtained in present study, as 0.5 % Ga-Ch was found to inhibit E. coli and Listeria monocytogenes, but no activity was observed against 3 serovars of Salmonella enterica, except Paratyphi A.
Naturally occurring p-coumaric acid found in peanuts, tomatoes, carrots, garlic, wine, vinegar, etc. is known to possess antimicrobial activity against potential pathogens such as Staphylococcus aureus in food systems (Stojković et al. 2013). Present study showed promising application of Co-Ch as food preservative as many foodborne pathogens and spoilage bacteria, including Staphylococcus aureus were inhibited.
Minimum inhibitory concentration of the grafted chitosan types were estimated by a novel microtire assay (Sarker et al. 2007). As shown in Table 4, Fe-Ch possessed the lowest MIC (250 ppm) against Staphylococcus aureus and Ga-Ch had lowest MIC (625 ppm) against Pseudomonas aeruginosa. The minimum inhibitory concentration determined for Fe-Ch against Pseudomonas aeruginosa and Staphylococcus aureus was determined to be 5000 and 250 ppm respectively. MIC of ferulic acid was reported as 1500 ppm against Staphylococcus aureus ATCC 12600 (Takahasi et al. 2013).
Conclusion
Free radical mediated synthesis of four phenolic acid functionalized chitosan derivatives was successfully achieved. Improved water solubility, antioxidant and antibacterial activities of these chitosan-phenolic acid conjugates as compared to chitosan may open up new applications in functional food and health food development. Among the four derivatives, highest antioxidant activity was observed for Ga-Ch followed by Va-Ch, Fe-Ch and Co-Ch. Gallic acid grafted chitosan showed broad spectrum antibacterial activity against both Gram positive and Gram negative bacteria. Fe-Ch, Co-Ch and Va-Ch also showed significant activity against several food spoilage bacteria. To the best of our knowledge this is the first report of antioxidant and antibacterial activities of vanillic acid grafted chitosan and coumaric acid grafted chitosan.
References
Aytekin AO, Morimura S, Kida K (2011) Synthesis of chitosan–caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 111:212–216
Bobu E, Nicu R, Lupei M, Ciolacu FL, Desbrières J (2011) Synthesis and characterization of n-alkyl chitosan for papermaking applications. Cellul Chem Technol 45:619–625
Božič M, Gorgieva S, Kokol V (2012) Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr Polym 87:2388–2398
Casettari L, Gennari L, Angelino D, Ninfali P, Castagnino E (2012) ORAC of chitosan and its derivatives. Food Hydrocoll 28:243–247
Cho Y, Kim S, Ahn C, Je J (2011) Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr Polym 83:1617–1622
CLSI (2012) Performance standards for antimicrobial disk susceptibility tests; approved standard—eleventh edition, Vol 32 (1). Clinical and Laboratory Standards Institute, Wayne, 58p
Curcio M, Puoci F, Iemma F, Parisi OI, Cirillo G, Spizzirri UG, Picci N (2009) Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J Agric Food Chem 57:5933–5938
Ferguson LR, Zhu S, Harris PJ (2005) Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol Nutr Food Res 49:585–593
Gitzinger M, Kemmer C, Fluri DA, ElBaba MD, Weber W, Fussenegger M (2012) The food additive vanillic acid controls transgene expression in mammalian cells and mice. Nucleic Acids Res 40:37–50
Gombas DE, ChenY CRS, Scott VN (2003) Survey of Listeria monocytogenes in ready-to-eat foods. J Food Prot 66:559–569
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: a simple “test tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Kamil J, Jeon Y, Shahidi F (2002) Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (clupea harengus). Food Chem 79:69–77
Karunasagar I, Karunasagar I (2000) Listeria in tropical fish and fishery products. Int J Food Microbiol 62:177–181
Lee DS, Woo JY, Ahn CB, Je JY (2014) Chitosan–hydroxycinnamic acid conjugates: preparation, antioxidant and antimicrobial activity. Food Chem 148:97–104
Liu J, Lu J, Kan J, Jin C (2013) Synthesis of chitosan-gallic acid conjugate: structure characterization and in vitro anti-diabetic potential. Int J Biol Macromol 62:321–329
Liu J, Wen X, Lu J, Kan J, Jin C (2014) Free radical mediated grafting of chitosan with caffeic and ferulic acids: structures and antioxidant activity. Int J Biol Macromol 65:97–106
Mancuso C, Santangelo R (2014) Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol 65:185–195
Pasanphan W, Buettner GR, Chirachanchai S (2010) Chitosan gallate as a novel potential polysaccharide antioxidant: an EPR study. Carbohydr Res 345:132–140
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Prince PSM, Roy AJ (2013) p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int J Cardiol 168:3259–3266
Prince PSM, Rajakumar S, Dhanasekar K (2011) Protective effects of vanillic acid on electrocardiogram, lipidperoxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol 668:233–240
Sabnis S, Block LH (2000) Chitosan as an enabling excipient for drug delivery systems. I. Molecular modifications. Int J Biol Macromol 27:181–186
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Schreiber SB, Bozell JJ, Hayes DG, Zivanovic S (2013) Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll 33:207–214
Shahidi F, Kamil J, Jeon Y, Kim S (2002) Antioxidant role of chitosan in a cooked cod (gadus mornua) model system. J Food Lipids 9:57–64
Stojković D, Petrović J, Soković M, Glamočlija J, Kukić-Marković J, Petrović S (2013) In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J Sci Food Agric 93:3205–3208
Sun X, Wang Z, Kadouh H, Zhou K (2014) The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT Food Sci Technol 57:83–89
Takahasi H, Kashimura M, Koiso H, Kuda T, Kimura B (2013) Use of ferulic acid as a novel candidate of growth inhibiting agent against Listeria monocytogenes in ready-to-eat food. Food Control 33:244–248
Woranuch S, Yoksan R (2013) Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr Polym 96:495–502
Xie W, Xu P, Liu Q (2001) Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett 11:1699–1701
Yu SH, Mi FL, Pang JC, Jiang SC, Kuo TH, Wu SJ, et al (2011) Preparation and characterization of radical and pH-responsive chitosan-gallic acid conjugate drug carriers. Carbohydr Polym 84:794–802
Acknowledgments
The authors sincerely acknowledge analytical services provided by “Sophisticated test and instrumentation centre” of Cochin University of Science and Technology, Cochin, Kerala, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Niladri Sekhar Chatterjee, Satyen Kumar Panda and Mary Navitha contributed equally.
Highlights
First report of vanillic acid and coumaric acid functionalization of chitosan
Higher grafting ratio of phenolic acids on chitosan
Better antioxidant activity than earlier reports
Broad spectrum antibacterial activity against foodborne pathogens Potential application in functional food
Rights and permissions
About this article
Cite this article
Chatterjee, N.S., Panda, S.K., Navitha, M. et al. Vanillic acid and coumaric acid grafted chitosan derivatives: improved grafting ratio and potential application in functional food. J Food Sci Technol 52, 7153–7162 (2015). https://doi.org/10.1007/s13197-015-1874-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1874-4