Abstract
Chitosan (CS) is considered a versatile biopolymer with promising applications. However, it is not a good chain-breaking antioxidant due to the lack of H-atom donors. In this work, CS was combined with quercetin (Q), a natural antioxidant, via a free radical-mediated procedure to strengthen the antioxidant capacity. The successful formation of Q-grafted CS (Q-CS) was confirmed by ultraviolet–visible absorbance and Fourier transform infrared spectroscopy. After combination, the obtained Q-CS had a phenolic content of 13.9 mg QE/g Q-CS and showed a lower crystallinity and thermal stability than the native CS. The 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), superoxide, and hydroxyl radical scavenging activities of Q-CS were higher than those of CS, illustrating that grafting with Q is an available way to improve the antioxidant capacity of CS. In addition, Q-CS showed higher minimal inhibitory concentrations against tested bacteria than CS, suggesting that combining with Q has a negative effect on the antibacterial activity of CS. Our results indicate that Q-CS may have great potential for applications in the fields of food and healthcare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitosan (CS) is a linear polysaccharide commercially obtained by the deacetylation of chitin, the second most abundant biopolymer after cellulose on the earth (Rinaudo 2006). As a natural biopolymer, CS possesses many desired properties such as biocompatibility, biodegradability, and non-toxicity (Qin et al. 2015). Structurally, CS is composed of repeating units of d-glucosamine and N-acetyl-d-glucosamine; the d-glucosamine unit has an amino group on the C2 position, the protonation of which in acid media makes CS being a unique cationic natural biopolymer. As a result, CS possesses favorable biological properties such as antimicrobial, antitumor, and anti-inflammatory activities (Ngo et al. 2015), which endow CS with extensive applications in various fields including food, biomedicines, and nutraceuticals (Sarbon et al. 2015). Nevertheless, CS has a drawback of weak antioxidant activity due to the lack of H-atom donors, which limits its practical applications. Fortunately, CS has reactive amino and hydroxyl groups that can be easily chemical-modified, and the antioxidant capacity of CS can be effectively improved by modification with antioxidant compounds (Curcio et al. 2009).
Polyphenols are natural compounds widely distributed in plants. Most polyphenols have a potent antioxidant capacity due to the presence of phenolic hydroxyl groups. Combining CS with polyphenols is able to enhance antioxidant properties, which is frequently achieved by carbodiimide-based coupling, enzyme-catalyzed grafting, and free radical-mediated grafting. The carbodiimide-based coupling is an effective method to synthesize polyphenol–CS conjugates (Moreno-Vasquez et al. 2017). To date, a number of polyphenols have been successfully inserted onto CS via carbodiimide-based coupling, generating CS derivatives with an improved antioxidant capacity (Hu and Luo 2016). However, this procedure requires chemical coupling reagents that are harmful to the human body; thus, the polyphenol–CS conjugates prepared by carbodiimide-based coupling are not suitable for the use in the fields of food and healthcare. Compared with carbodiimide-based coupling, enzyme-catalyzed grafting is more safe and eco-friendly to prepare polyphenol–CS conjugates (Sousa et al. 2009). Some polyphenol oxidases, such as laccase and tyrosinase, are often used to synthesize polyphenol–CS conjugates. Nevertheless, the enzyme-mediated reaction usually catalyzes phenolic compounds into o-quinones, which reduces biological activities. In contrast, the free radical grafting usually uses the hydrogen peroxide/ascorbic acid redox pair as an initiator system. This system is much less toxic than carbodiimide-based coupling and can avoid the oxidation of polyphenols to o-quinones. Therefore, the free radical-mediated procedure becomes an ideal method for the synthesis of polyphenol–CS conjugates used in the fields of food and healthcare. In recent years, some polyphenols have been grafted onto chitosan via the free radical-mediated procedure, such as catechin (Curcio et al. 2009), epigallocatechin gallate (Lei et al. 2014), and phloroglucinol (Woo and Je 2013). More importantly, the introduction of polyphenols is able to bring specific properties to polyphenol–CS conjugates (Oliver et al. 2016).
Quercetin (Q) is a natural polyphenol in fruit and vegetables. It has a strong antioxidant capacity and numerous other biological properties such as anticarcinogenic, cardioprotective, and antiviral activities (Wang et al. 2016). Accordingly, Q is a strong candidate for preparing polysaccharide-based conjugates with enhanced biological properties. For example, Cirillo et al. (2012) prepared a Q-starch conjugate via the free radical-mediated procedure and found that it has improved ultraviolet stability and retains the antioxidant properties of Q. Wang et al. (2014) synthesized a Q-carboxymethyl CS conjugate via carbodiimide-based coupling and proposed it as a candidate for the oral delivery of water-insoluble anticancer drugs. However, little information regarding Q-grafted CS (Q-CS) prepared by the free radical-mediated procedure is noted in the literature.
Therefore, the aim of this study was to prepare Q-CS via the free radical-mediated procedure, characterize its physicochemical properties, and analyze its antioxidant and antibacterial properties. This research is expected to strengthen the applications of CS and its derivatives in the fields of food and healthcare.
Materials and methods
Materials
CS with a deacetylation degree of 90% and a molecular weight of 57.4 kDa was purchased from Aoxing Biotechnology Co., Ltd., China. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and Q were obtained from Dingguo Biotechnology Co., Ltd., China. Other analytical-grade chemicals were provided by Hengshan Chemicals, China.
Bacteria used in antibacterial assays were as follows: Escherichia coli ATCC 25,922, Proteus vulgaris ATCC 49,132, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538, Micrococcus luteus CMCC(B) 28,001.
Preparation of Q-CS
The preparation of Q-CS was carried out according to the previous literature (Cirillo et al. 2012) with modifications. First, 1 g of CS was completely dissolved in 50 mL of acetic acid solution (1 mol/L) followed by slowly adding 45 mL of ethanol with stirring. Second, 2 mL of H2O2 solution (1 mol/L) containing 0.108 g of ascorbic acid was added in the CS solution. Third, the reaction solution was maintained at 20 °C for 30 min and then added 5 mL of Q solution in ethanol at the following molar ratio of Q to CS monomer: 0.01:1, 0.02:1, 0.03:1, 0.04:1, or 0.05:1. The reaction solution was then maintained in the dark at 20 °C for 24 h. Forth, the reaction solution was dialyzed against distilled water in a dialysis bag (MWCO 8000–10,000 Da) at 20 °C for 48 h with eight changes of water and subsequently centrifuged at 8000×g for 10 min to remove the unreacted Q and other compounds. Finally, the sample solution was freeze-dried at − 60 °C to obtain Q-CS.
Determination of phenolic content
The phenolic content of Q-CS, i.e., the Q equivalent content in Q-CS, was evaluated by the Folin–Ciocalteu procedure (Chatterjee et al. 2015). In brief, 1 mL of Q-CS solution (1.2 mg/mL) was mixed with 2.5 mL of Folin–Ciocalteu reagent. The mixture was maintained in the dark at room temperature for 5 min and then added 4 mL of 7.5% (w/v) Na2CO3 solution and 2.5 mL of distilled water. Afterward, the mixture was maintained in the dark at room temperature for 2 h followed by measuring the absorbance at 760 nm using a spectrophotometer (UV-1100, Mapada, China). Finally, the phenolic content was calculated by using Q as a standard and expressed as mg of Q equivalents per g of Q-CS, mg QE/g Q-CS.
Characterization of Q-CS
The chemical structure of Q-CS was characterized by ultraviolet–visible (UV–Vis) absorbance and Fourier transform infrared (FTIR) spectroscopy. The UV–Vis spectrum was measured by using a spectrophotometer (Evolution 300, Thermo, America) in a wavelength range of 200–500 nm. After being ground with KBr and compressed into a pellet, the FTIR spectrum of the sample was recorded in a wavenumber range of 4000–400 cm−1 by an FTIR spectrometer (VECTOR22, Bruker, Germany).
The thermal gravimetric analysis (TGA) and derivative thermogravimetry (DTG) for determining the thermal stability of Q-CS and the differential scanning calorimetry (DSC) for determining the thermal transition were performed by a simultaneous DSC–TGA (SDT/Q600, TA, USA), with heating from 40 to 600 °C at a heating rate of 10 °C/min under nitrogen atmosphere. The X-ray diffraction (XRD) pattern of Q-CS was measured by an X-ray diffractometer (D8 ADVANCE, Bruker, Germany) in a 2θ range of 5°–80°.
Determination of water-solubility of Q-CS
The water-solubility of Q-CS was measured as described by Chatterjee et al. (2015). Briefly, the saturated solution of Q-CS was prepared by dissolving 400 mg of Q-CS in 20 mL distilled water overnight. The saturated solution was then filtered through a 0.22 μm polytetrafluoroethene membrane and appropriately diluted with distilled water. Finally, the optical density of the diluted solution was measured at the absorbance maxima, and the solubility was estimated using the following equation:
where S is the solubility, mg/mL; Ax is the absorbance of the saturated solution; B is the coefficient of absorbance, the value of which was 0.395 mL/mg in this study.
Antioxidant activity assays
ABTS radical scavenging assay
The ABTS radical scavenging assay was carried out as described by Moreno-Vasquez et al. (2017). An ABTS solution (7.0 mmol/L) was prepared and then blended with an equal volume of K2S2O8 solution (2.75 mmol/L). The mixture was maintained in the dark at 20 °C for 12–16 h to obtain an ABTS radical solution. The ABTS radical solution was then diluted with 10 mmol/L phosphate-buffered saline (PBS, pH 7.4) to an absorbance of 0.70 ± 0.02 at 734 nm. Afterward, 3 mL of ABTS radical solution was mixed with 50 μL of Q-CS solution. After maintenance for 6 min, the absorbance of the mixture was measured at 734 nm. The ABTS radical scavenging activity was calculated using the following equation:
where As and A0 are the absorbance of the sample and blank (replacing the Q-CS solution with PBS), respectively.
DPPH radical scavenging assay
The DPPH radical scavenging assay was performed as described by Chatterjee et al. (2015). In brief, 2 mL of Q-CS solution was mixed with 2 mL of DPPH solution (0.1 mmol/L) in ethanol. The mixture was maintained in the dark for 30 min followed by measuring the absorbance at 517 nm. The DPPH radical scavenging activity was calculated using Eq. (3).
where As and A0 are the absorbance of the sample and blank, respectively.
Superoxide radical scavenging assay
The superoxide radical scavenging assay was evaluated as described by Li et al. (2009). First, 1 mL of Q-CS solution was added in a mixture of 4.5 mL of Tris-HCl buffer (50 mmol/L, pH 8.2) and 3.2 mL of distilled water followed by maintaining at 20 °C for 25 min. Second, 0.3 mL of pyrogallol solution (60 mmol/L) was added in the mixture. Third, the absorbance of the mixture was measured at 420 nm for 5 min. The superoxide radical scavenging activity was calculated by the following equation:
where ΔAs and ΔA0 are the absorbance changes of the sample and blank, respectively.
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity was assayed according to a previous method (Su et al. 2013). Briefly, 1 mL of phenanthroline solution (1.5 mmol/L) was mixed with 2 mL of PBS (20 mmol/L, pH 7.4) and 3 mL of Q-CS solution. The mixture was then added 1 mL of FeSO4 solution (0.5 mmol/L) and 1 mL of H2O2 solution (25 mmol/L) followed by incubating at 37 °C for 50 min. Finally, the absorbance of the mixture was measured at 536 nm. The hydroxyl radical scavenging activity was calculated using Eq. (5).
where As, Ac, and A0 are the absorbance of the sample, control (replacing the H2O2 solution with PBS), and blank, respectively.
Assay of antibacterial activity
To evaluate the antibacterial potential, the minimal inhibitory concentrations (MICs) of Q-CS against five bacteria were determined by a two-fold serial dilution method (Lee et al. 2014). Briefly, the Q-CS solution (pH 5.5) was mixed with Luria–Bertani broth to a concentration of 1024, 512, 256, 128, 64, or 32 μg/mL followed by adding bacterial suspension to a concentration of 106 CFU/mL. Then, the mixture was incubated in a shaker at 37 °C for 24 h. The MIC was defined as the lowest concentration at which the bacteria did not show visible growth.
Statistical analysis
Each experiment was triply repeated, and the data were expressed as mean values with standard deviation. Significant differences (p < 0.05) were determined by one-way analysis of variance; multiple comparisons were analyzed by Tukey multiple range test. All statistical analyses were carried out by using SPSS 19.0 (SPSS Inc., USA).
Result and discussion
Preparation of Q-CS
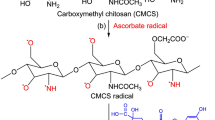
In this work, CS was functionalized with Q, a potent antioxidant, via a free radical grafting procedure. Because of safe and eco-friendly properties, the ascorbic acid–H2O2 redox pair is used as an initiator system for this procedure. According to the previous literature (Oliver et al. 2016), this procedure is mediated by hydroxyl radicals produced via the oxidation–reduction reaction between ascorbic acid and H2O2. Herein, we follow this proposal and present a possible schema for the synthesis of Q-CS in Fig. 1. Initially, ascorbic acid is oxidized by H2O2, forming ascorbate and hydroxyl radicals. The hydroxyl radicals then abstract hydrogen atoms from the hydroxyl and amino groups of CS, producing macromolecular CS radicals. Finally, Q molecules in the close vicinity of the reaction sites become acceptors of CS radicals, resulting in the covalent conjugation of CS with Q. However, the exact reaction mechanism of the free radical grafting procedure still remains unclear. More evidence is needed to illustrate the underlying reaction mechanism of the free radical grafting procedure for the synthesis of polyphenol–CS conjugates.
The antioxidant potential of polyphenol–CS conjugates greatly depends on their phenolic content (Aytekin et al. 2011). Normally, increasing the phenolic dosage in preparation is able to enhance the phenolic content of the resultant conjugates (Aytekin et al. 2011), which was also observed in this study. Increasing the molar ratio of Q to CS monomer from 0.01:1 to 0.02:1 led to a significant improvement (p < 0.05) in the phenolic content of Q-CS (Fig. 2), which is due to the accumulation of Q molecules on CS chains. A similar observation was also reported by Liu et al. (2013) in gallic acid–CS conjugate. However, the further increase in the molar ratio from 0.02:1 to 0.05:1 did not result in continuous improvement (p > 0.05) in the phenolic content, which is probably because the available reactive sites of CS chains are fully occupied by Q molecules. Accordingly, the molar ratio of 0.02:1 between Q and CS monomer was selected for the preparation of Q-CS, and the phenolic content of 13.9 mg QE/g Q-CS was obtained. The phenolic content of Q-CS is lower than that of the conjugates prepared from CS and water-soluble polyphenols such as caffeic acid (73.4 mg CAE/g) and ferulic acid (66.7 FAE/g) (Liu et al. 2014). Because Q is insoluble in water, an ethanol/water mixture was used to dissolve Q, as suggested by Cirillo et al. (2012) in the synthesis of Q-starch conjugate. Ethanol in the system could prevent the hydration of CS, reducing the exposure and availability of active sites (Guo et al. 2016). Therefore, a lower phenolic content was obtained for the conjugate of CS with Q relative to water-soluble polyphenols. A similar result was also reported in a previous study (Cirillo et al. 2012) in which Q-starch conjugate had a low phenolic content of 13.1 mg QE/g.
Characterization of Q-CS
UV–Vis and FTIR spectra
Polyphenols possess one or more aromatic rings, which normally have UV–Vis absorption in a wavelength range of 200–500 nm. However, most polysaccharides have no absorption in the range of 240–400 nm. Thus, comparing the UV–Vis spectra of polyphenols, CS, and their conjugates is able to provide evidence for the successful grafting of polyphenols onto CS. Herein, Q showed two typical peaks at 273 nm and 371 nm (Fig. 3a), being assigned to its A- and B-ring absorption, respectively (Bukhari et al. 2009). The native CS did not exhibit absorption peaks in the tested wavelength range, which agrees with the previous report (Xie et al. 2014). After conjugation, Q-CS showed a broad peak centered at 289 nm, which could be ascribed to the A-ring of Q, demonstrating the successful synthesis of Q-CS. A similar observation was found in a previous study (Torres et al. 2012) in which Q-modified CS by an enzymatic method showed a typical band at 300 nm. In addition, the band for the B-ring of Q did not appear in the spectrum of Q-CS, which indicates that the B-ring may be involved in the grafting with CS. Nevertheless, the grafting position of polyphenols remains unclear to date (Hu and Luo 2016). More efforts are still needed to illustrate the exact grafting position of polyphenols.
FTIR spectrum is a frequently used method to confirm the formation of polyphenol–CS conjugates; it also provides the structure information of the conjugates. To further ensure the formation of Q-CS conjugate, the FTIR spectra of Q-CS and its substrates were measured; the results are presented in Fig. 3b. Q displayed several typical bands in the wavenumber of 1610–1460 cm−1, which are owing to the stretching vibration of its benzoyl rings (Song et al. 2009). For CS, the characteristic bands of C=O stretching vibration of amide I, N–H bending vibration of amide II (–NH2), and C–N stretching vibration of amide III appeared at 1651 cm−1, 1605 cm−1, and 1325 cm−1, respectively, as suggested by Liu et al. (2014). The asymmetric stretching vibration of the C–O–C bridge of CS was characterized by the band at 1155 cm−1. Moreover, the bands at 1080 cm−1 and 1034 cm−1 can be assigned to the C3– and C6–OH groups of CS, respectively. In the grafting reaction, polyphenols are normally inserted onto the amino and hydroxyl groups of CS, leading to distinct differences in the FTIR spectrum (Guo et al. 2016). Herein, the bands of –NH2 and C6–OH groups disappeared in the Q-CS spectrum, which illustrates the involvement of these two groups in grafting. In addition, two new bands occurred at 1630 cm−1 and 1406 cm−1, which are assigned to Q molecules, providing evidence for the successful grafting of Q onto CS. Furthermore, the typical band of the C–O–C bridge also appeared in the Q-CS spectrum (at 1151 cm−1), revealing that the skeleton structure of CS chains remains in Q-CS.
Crystallographic structure and thermal behavior
The crystallographic structures of Q-CS and its substrates were determined by XRD patterns. As shown in Fig. 4a, the XRD pattern of Q showed several sharp diffraction peaks at 2θ = 10.7°, 12.5°, and 27.4°, revealing a high crystallinity of Q (Pralhad and Rajendrakumar 2004). The native CS displayed two characteristic peaks at 2θ = 10.8° and 20.0° corresponded to crystal form I and II, respectively (Khan et al. 2017). In contrast, Q-CS exhibited a broad peak at 2θ = 14.2°, suggesting that it has a lower crystallinity than CS. The crystallinity of a polymer is normally associated with its inter- and intra-molecular hydrogen bonds (Woranuch and Yoksan 2013). The introduction of Q may partially hinder the formation of hydrogen bonds of CS chains, resulting in the loosing of the chain packing of Q-CS. Thus, a lower crystallinity is obtained for Q-CS relative to CS.
The thermal behavior of Q, CS, and Q-CS was analyzed by TGA, DTG, and DSC. TGA is frequently used to study the weight loss of a compound upon heating. Herein, the TGA curves of both Q and CS displayed two distinct stages in weight loss (Fig. 4b). The first and second stages of Q occurred at 70–120 °C with a minor weight loss of 9.5% and 310–370 °C with a major weight loss of 24.5%, respectively. The first and second stages of CS appeared at 40–110 °C with a minor weight loss of 8.6% and 260–340 °C with a major weight loss of 38.6%, respectively. The first stage is due to the loss of free and bound water on the materials, whereas the second stage reflects the degradation and decomposition of the materials. After conjugation, unlike Q and CS, Q-CS exhibited three stages in weight loss. The additional stage of Q-CS was at the temperatures of 100–220 °C, which could be attributed to the preliminary depolymerization of the copolymer (Moreno-Vasquez et al. 2017). The further thermal decomposition of Q-CS occurred at the third stage in a temperature range of 220–350 °C. Furthermore, the temperature for the maximum decomposition rate was determined by DTG; the results are shown in Fig. 4c. CS had a maximum decomposition rate at 300 °C, whereas the maximum decomposition rate of Q-CS appeared at a lower temperature of 289 °C. These results reveal that Q-CS has lower thermal stability than CS.
DSC is a regular method to study the thermal transitions of a polymer (Lee et al. 2014). As shown in Fig. 4d, Q showed an endothermic peak at 120 °C associated with anhydration and a melting endothermic peak at 320 °C related to degradation (Pralhad and Rajendrakumar 2004). CS showed an exothermic peak at 311 °C, which is attributed to the crosslinking reactions of the decomposed compositions (Aelenei et al. 2009). This exothermic peak shifted to a lower temperature at 298 °C in the DSC curve of Q-CS, being probably due to the lower thermal stability of Q-CS (Woranuch and Yoksan 2013).
CS has abundant amino and hydroxyl groups, which can form strong inter- and intra-hydrogen bonds that facilitate thermal stability. However, the amino and hydroxyl groups are also the reaction sites of grafting. The FTIR spectra prove that the Q molecules are inserted onto the amino and hydroxyl groups of CS, which means that the available active sites for the formation of hydrogen bonds decrease in Q-CS. Consequently, a reduction in hydrogen bonds occurs in Q-CS, which can be evidenced by the decrease in the crystallinity of Q-CS discussed above. Thus, compared with CS, the lower thermal stability of Q-CS was found due to the reduction in hydrogen bonds. Similar results were also observed in other polyphenol–CS conjugates (Liu et al. 2014).
Water-solubility of Q-CS
CS is insoluble in water and only soluble in acid media at pH < 6, which becomes a major obstacle in practical applications (Rinaudo 2006). A similar limitation also exists in Q (Smith et al. 2011). Fortunately, the copolymer Q-CS showed a good water-solubility of 11.4 mg/mL, which facilitates its bioavailability and application potential. The improvement in the water-solubility of Q-CS might be a result of the reduction in its crystallinity, as suggested by Woranuch and Yoksan (2013) in ferulic acid-grafted CS.
Antioxidant properties of Q-CS
CS has a weak antioxidant activity due to the lack of H-atom donors. Grafting with natural antioxidants is able to effectively enhance the antioxidant capacity of CS. Thus, in this study, CS was grafted with Q, a natural polyphenol with potent antioxidant activity and other favorable effects on human health, via a free radical-mediated procedure to improve its antioxidant ability.
Antioxidants are able to eliminate ABTS and DPPH radicals via hydrogen-donating. Accordingly, ABTS and DPPH assays are regularly used to evaluate the primary antioxidant ability of compounds. The ABTS and DPPH radical scavenging activities of Q, CS, and Q-CS are shown in Fig. 5a, b, respectively. As a natural antioxidant, Q showed potent scavenging activities against ABTS (> 96.2%) and DPPH (> 93.0%) radicals at tested concentrations. The scavenging activities of CS, however, were less than 14.5% and 26.1% for ABTS and DPPH radicals, respectively. In contrast, the scavenging activities of Q-CS against both ABTS and DPPH radicals increased dramatically with the concentration. Specifically, Q-CS showed a maximum scavenging activity of 88.8% for ABTS radicals and 93.6% for DPPH radicals. These results demonstrate that grafting with Q dramatically enhances the ABTS and DPPH radical scavenging activities of CS. Similar results were also observed in other polyphenol–CS conjugates such as gallic acid-grafted CS (Xie et al. 2014) and ferulic acid-grafted CS (Woranuch and Yoksan 2013). In addition, the half-inhibition concentrations of Q-CS against ABTS and DPPH radicals were 93 μg/mL and 106 μg/mL, respectively, illustrating again its strong radical scavenging ability.
ABTS (a), DPPH (b), superoxide (c), and hydroxyl radical (d) scavenging activities of Q, CS, and Q-CS. Q-CS was prepared at the molar ratio of 0.02:1 between Q and CS monomer. ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH: 2,2-diphenyl-1-picrylhydrazyl; Q: quercetin; CS: chitosan; Q-CS: Q-grafted CS
The antioxidant Q has several phenolic hydrogen groups, which endow it with great hydrogen-donating ability. Thus, the potent scavenging activities of Q against ABTS and DPPH radicals are observed in this study. In contrast, the H-atom donors of CS are insufficient, thus CS exhibits poor scavenging activities against ABTS and DPPH radicals. When Q molecules are grafted onto CS chains via the free radical-mediated procedure, the phenolic hydroxyl groups are introduced into the resultant conjugate. Therefore, improvement in both ABTS and DPPH radical scavenging activities is achieved for Q-CS. In addition, the phenolic content of Q-CS is 13.9 mg QE/g Q-CS; nevertheless, the maximum scavenging activities of Q-CS against ABTS and DPPH radicals are close to those of Q, which may be due to the introduction of Q and the improvement in the water-solubility of the conjugate. According to the analysis of crystallographic structure, grafting reaction causes the loosing of the chain packing and the increase in water-solubility of Q-CS, which exposes more active sites associated with H-atom donating. Therefore, great enhancement in ABTS and DPPH radical scavenging activities is obtained for Q-CS.
Superoxide and hydroxyl radical scavenging activities of Q, CS, and Q-CS are presented in Fig. 5c, d, respectively. For the superoxide radical, Q, CS, and Q-CS showed a similar pattern in the scavenging activity; that is, the scavenging activities of these three compounds increased significantly with the concentration. Nevertheless, due to the introduction of Q, Q-CS showed a higher superoxide radical scavenging activity than CS. Compared with the superoxide radical, different patterns were observed in the hydroxyl radical scavenging activity for Q-CS and its substrates. The antioxidant Q presented strong hydroxyl radical scavenging activities (> 93.5%) at all tested concentrations. In contrast, both CS and Q-CS showed a significant increase in the hydroxyl radical scavenging activity with the concentration. Nevertheless, Q-CS exhibited a higher scavenging activity than CS. Specifically, the scavenging activity of Q-CS was 73.1% at the concentration of 60 μg/mL, which was about 1.5-fold higher than that of CS.
The superoxide radical is considered the primary reactive oxygen species (ROS), and it can be formed by metabolic processes or the addition of an electron to dioxygen. The primary ROS can further react with other molecules, generating secondary ROS such as the hydroxyl radical (Valko et al. 2007). Because excessive ROS may cause damage to cellular molecules such as DNA and proteins in the human body, the elimination of ROS by antioxidants is crucial for human health. Our results show that both CS and Q have the scavenging potential to eliminate ROS, thus they could be used as dietary antioxidants to eliminate the ROS-related damages in the human body (Wang et al. 2016). Furthermore, the copolymer Q-CS exhibits better ROS scavenging ability than unmodified CS. Therefore, Q-CS may have great potential in the fields of food and biomedicine.
Antibacterial activity
CS has abundant amino groups, the protonation of which in an acid environment endows CS with a potent antibacterial activity (Lee et al. 2014). However, the amino groups of CS are involved in grafting with Q, which could reduce free amino groups and impair the antibacterial activity of CS. Thus, the MICs of CS and Q-CS were determined to evaluate the effects of grafting reaction on the antibacterial ability. As seen from Table 1, CS showed great antibacterial activities; the MIC values varied from 32 μg/mL to 512 μg/mL depending on the bacterial species. However, Q-CS showed twofold MIC values than CS for each bacterial strain, revealing that Q-CS had a lower antibacterial activity than CS. These results demonstrate the impairment of grafting reaction on the antibacterial activity of CS. In spite of that, the MIC range of 64–1024 μg/mL illustrated that Q-CS still had an effective antibacterial activity. In addition, both CS and Q-CS had lower MIC values on Gram-positive bacteria than Gram-negative bacteria, indicating that they had better inhibitory ability against Gram-positive bacteria. Similar results were also observed in previous studies (Benhabiles et al. 2012). The discrepancy between the antibacterial activities against Gram-positive bacteria and Gram-negative bacteria may be a result of differences in the composition of the bacterial cell membrane.
As a natural polymer, CS has promising applications in many fields, in particular, food and healthcare, due to its safe and biodegradable properties. Furthermore, the drawback of the low antioxidant capacity of CS can be offset by combining with antioxidants, which extensively enlarges the applications of CS. Thus, CS was herein conjugated with Q, a natural antioxidant polyphenol, by free radical grafting. The resultant Q-CS exhibited dramatically enhanced antioxidant capacity compared with CS. Although the grafting reaction had a negative effect on antibacterial properties, a good antibacterial activity was still observed in Q-CS. Moreover, the polyphenol–CS conjugate has good stability, and the conjugate is able to prevent the polyphenol from degradation over a long period (Hu et al. 2016). This property is favorable for the application of polyphenol–CS conjugates. In addition, CS and its derivatives are able to protect the nutrients in food. For example, CS and CS-monomethyl fumaric acid conjugate prevent the oxidative deterioration of proteins in beef and extend the shelf life (Khan et al. 2017). Therefore, Q-CS with a good antioxidant capacity and antibacterial activity may have promising applications in the fields of food and healthcare. However, additional efforts are needed to evaluate the potential impacts of Q-CS as well as other polyphenol–CS conjugates on the physicochemical stability of food systems, in particular, protein-based formulated food.
Conclusion
In the present study, CS was successfully functionalized with Q, a natural antioxidant, by free radical grafting. The molar ratio of 0.02:1 between Q and CS monomer was optimal for the preparation of Q-CS. The formation of Q-CS was proved by UV–Vis and FTIR spectra, which indicate that the grafting reaction may occur between the B-ring of Q and the –NH2 and C6–OH groups of CS. The XRD pattern shows a decrease in the crystallinity of Q-CS, which may be a cause of the increase in its water-solubility. The TGA, DTG, and DSC profiles suggest a decrease in the thermal stability of Q-CS relative to CS. Importantly, Q-CS exhibited potent ABTS, DPPH, superoxide, and hydroxyl radical scavenging activities, all of which were stronger than those of CS. The enhancement in the antioxidant capacity of Q-CS is probably attributed to the introduction of Q and the improvement in water-solubility. In addition, because grafting with Q reduces the free amino groups of CS, Q-CS exhibited a lower antibacterial activity than CS. Nevertheless, the MIC range of 64–1024 μg/mL illustrates that Q-CS still had an effective antibacterial activity. Therefore, the water-soluble Q-CS synthesized by an eco-friendly procedure could have promising applications in the fields of food and healthcare.
References
Aelenei N, Popa MI, Novac O, Lisa G, Balaita L (2009) Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J Mater Sci Mater Med 20:1095–1102
Aytekin AO, Morimura S, Kida K (2011) Synthesis of chitosan-caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 111:212–216
Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MFA, Mameri N (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29:48–56
Bukhari SB, Memon S, Mahroof-Tahir M, Bhanger MI (2009) Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim Acta A Mol Biomol Spectrosc 71:1901–1906
Chatterjee NS, Panda SK, Navitha M, Asha KK, Anandan R, Mathew S (2015) Vanillic acid and coumaric acid grafted chitosan derivatives: improved grafting ratio and potential application in functional food. J Food Sci Technol 52:7153–7162
Cirillo G, Puoci F, Iemma F, Curcio M, Parisi OI, Spizzirri UG, Altimari I, Picci N (2012) Starch-quercetin conjugate by radical grafting: synthesis and biological characterization. Pharm Dev Technol 17:466–476
Curcio M, Puoci F, Iemma F, Parisi OI, Cirillo G, Spizzirri UG, Picci N (2009) Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J Agric Food Chem 57:5933–5938
Guo P, Anderson JD, Bozell JJ, Zivanovic S (2016) The effect of solvent composition on grafting gallic acid onto chitosan via carbodiimide. Carbohydr Polym 140:171–180
Hu Q, Luo Y (2016) Polyphenol–chitosan conjugates: synthesis, characterization, and applications. Carbohydr Polym 151:624–639
Hu Q, Wang T, Zhou M, Xue J, Luo Y (2016) In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J Agric Food Chem 64:5893–5900
Khan I, Tango CN, Oh D-H (2017) Development and evaluation of chitosan and its derivative for the shelf life extension of beef meat under refrigeration storage. Int J Food Sci Tech 52:1111–1121
Lee DS, Woo JY, Ahn CB, Je JY (2014) Chitosan–hydroxycinnamic acid conjugates: preparation, antioxidant and antimicrobial activity. Food Chem 148:97–104
Lei F, Wang X, Liang C, Yuan F, Gao Y (2014) Preparation and functional evaluation of chitosan-EGCG conjugates. J Appl Polym Sci 131:39732
Li J, Zhang M, Zheng T (2009) The in vitro antioxidant activity of lotus germ oil from supercritical fluid carbon dioxide extraction. Food Chem 115:939–944
Liu J, Lu JF, Kan J, Jin CH (2013) Synthesis of chitosan–gallic acid conjugate: structure characterization and in vitro anti-diabetic potential. Int J Biol Macromol 62:321–329
Liu J, Wen XY, Lu JF, Kan J, Jin CH (2014) Free radical mediated grafting of chitosan with caffeic and ferulic acids: structures and antioxidant activity. Int J Biol Macromol 65:97–106
Moreno-Vasquez MJ, Valenzuela-Buitimea EL, Plascencia-Jatomea M, Encinas-Encinas JC, Rodriguez-Felix F, Sanchez-Valdes S, Rosas-Burgos EC, Ocano-Higuera VM, Graciano-Verdugo AZ (2017) Functionalization of chitosan by a free radical reaction: characterization, antioxidant and antibacterial potential. Carbohydr Polym 155:117–127
Ngo D-H, Vo T-S, Ngo D-N, Kang K-H, Je J-Y, Pham HN-D, Byun H-G, Kim S-K (2015) Biological effects of chitosan and its derivatives. Food Hydrocoll 51:200–216
Oliver S, Vittorio O, Cirillo G, Boyer C (2016) Enhancing the therapeutic effects of polyphenols with macromolecules. Polym Chem 7:1529–1544
Pralhad T, Rajendrakumar K (2004) Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal 34:333–339
Qin YY, Zhang ZH, Li L, Yuan ML, Fan J, Zhao TR (2015) Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J Food Sci Technol 52:1471–1479
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Sarbon NM, Sandanamsamy S, Kamaruzaman SF, Ahmad F (2015) Chitosan extracted from mud crab (Scylla olivicea) shells: physicochemical and antioxidant properties. J Food Sci Technol 52:4266–4275
Smith AJ, Kavuru P, Wojtas L, Zaworotko MJ, Shytle RD (2011) Cocrystals of quercetin with improved solubility and oral bioavailability. Mol Pharm 8:1867–1876
Song X, Li J, Wang J, Chen L (2009) Quercetin molecularly imprinted polymers: preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 80:694–702
Sousa F, Guebitz GM, Kokol V (2009) Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process Biochem 44:749–756
Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang B, Zhou S, Yang T, Mei Q (2013) Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS ONE 8:e54505
Torres E, Marín V, Aburto J, Beltrán HI, Shirai K, Villanueva S, Sandoval G (2012) Enzymatic modification of chitosan with quercetin and its application as antioxidant edible films. Appl Biochem Microbiol 48:151–158
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J (2014) Amphiphilic carboxymethyl chitosan–quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 35:7654–7665
Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, Gao Y (2016) The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol 56:21–38
Woo J-Y, Je J-Y (2013) Antioxidant and tyrosinase inhibitory activities of a novel chitosan–phloroglucinol conjugate. Int J Food Sci Tech 48:1172–1178
Woranuch S, Yoksan R (2013) Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr Polym 96:495–502
Xie M, Hu B, Wang Y, Zeng X (2014) Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J Agric Food Chem 62:9128–9136
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province (No. B2016202111).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diao, Y., Yu, X., Zhang, C. et al. Quercetin-grafted chitosan prepared by free radical grafting: characterization and evaluation of antioxidant and antibacterial properties. J Food Sci Technol 57, 2259–2268 (2020). https://doi.org/10.1007/s13197-020-04263-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04263-2