Abstract
Alzheimer’s disease, characterized by amyloid beta peptides and neurofibrillary tangles, is the most prevalent cause of dementia. Nowadays, some novel medicines being developed have displayed more illustrious therapeutic efficacies in Alzheimer’s disease. Recent studies have found andrographolide exhibiting therapeutic efficacy in a variety of Alzheimer’s disease models. Andrographolide is a traditional herbal medicine compound extracted from Andrographis paniculata. Evidence has shown that andrographolide reduces amyloid beta aggregation and suppresses neuroinflammatory response and synaptic dysfunction by reversing the microglia-mediated production of pro-inflammatory cytokines as well as Alzheimer’s disease-associated reduction in synaptic proteins. In the present review, the pharmacological effects of andrographolide are summarized and its mechanism of action against Alzheimer’s disease is discussed to discover the possibilities of andrographolide for Alzheimer’s disease prevention and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
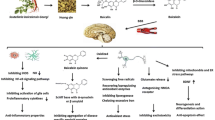
Andrographis paniculata (A. paniculata) is one of the traditional herbs (Fig. 1) used in China, South Asia, and East Asia (Okhuarobo et al., 2014; Tan et al., 2017). Recent reports have highlighted the anti-inflammatory effect of andrographolide (AGP), a diterpenoid lactone and a major component of A. paniculata (Kumar et al., 2014; Tan et al., 2017). Figure 2 shows the chemical structure of AGP.
This medicine has been used to treat various disorders, such as cancer, inflammation-related disorders, rheumatoid arthritis, fever, respiratory infection, and diarrhea (Tran et al., 2020). Table 1 shows the most anti-inflammatory mechanisms and pharmacological effects of andrographolide briefly in various diseases models. Multiple mechanisms of AGP, such as anti-oxidation (Tan et al., 2017), anti-inflammation (Islam, 2017), anti-apoptosis, and pro-apoptosis (Wang et al., 2019) develop into neuroprotective effects. In addition, other molecules, such as nuclear factor-кB (NF-кB) (Ding et al., 2017; Yang et al., 2017a, b; Yang et al., 2017a, b), protein kinase B (Akt) (Chen et al., 2014), c-Jun N-terminal kinase (JNK) (Yen et al., 2016), protein kinase C (PKC) (Lu et al., 2012), and p38 (Yen et al., 2016), may also lead to the neuroprotective effects of AGP.
In the latest research, the AGP in rat’s brain tissues was examined (Bera et al., 2014; Zhao et al., 2018), suggesting AGP’s ability to cross the blood–brain barrier (BBB) and distribute into several regions of the brain.
Following that, more research has discovered the neuroprotective effects of AGP in the central nervous system of Alzheimer’s disease (AD) and cognitive impairment of rodent models (Das et al., 2017; Sani et al., 2019; Seo et al., 2017; Serrano et al., 2014; Wang et al., 2004).
AD is one of the most prevalent forms of dementia, distinguished by progressive neuropathological changes in specific regions of the brain that preserve the memory and cognitive functions, so the damage to this area results in memory loss (Abedi et al., 2017; Selkoe, 2013; Tan et al., 2017). To date, there is no effective treatment for AD.
Researchers are trying to develop novel drugs with more excellent therapeutic efficacies. Recently, AGP has been shown to decrease multiple specific neuro pathological markers in several different models of AD.
Details investigations about AGP's pharmacological properties may accelerate the development and application of AGP in AD prevention and as a potential therapeutic agents. Therefore, this review summarizes and discusses the recent findings on AGP's pharmacological properties in alleviating the symptoms of AD.
AGP ameliorates the cognitive and spatial memory of different natural models of AD
AD is the most prevalent form of dementia. The disease is characterized by progressive memory loss and neuropathological changes in specific regions of the brain that eventually lead to death (Selkoe, 2013). Of the natural products available in the market, AGP seems to be the most suitable candidate for treating and preventing the progression of this neurodegenerative disease.
In recent years, several studies have examined the positive role of AGP in transgenic mouse AD models (Serrano et al., 2014; Tapia-Rojas et al., 2015; Varela-Nallar et al., 2015).
Octodon degus (degu) is recognized as a very valuable AD model because of its accumulation of senile plaques and neurofibrillary tangles. Besides, there is a 97.5% homology between degus and human amyloid beta (Aβ) peptide sequences (Rivera et al., 2016a, b). Moreover, the most significant neuropathological hallmarks of AD will develop in degus spontaneously after 3–4 years of age (Inestrosa et al., 2015; Rivera et al., 2016a, b). In addition to the above, some specific neuropathological hallmarks of AD, such as the aggregation of Aβ peptides and phosphorylated tau proteins, develop naturally within degus aged 12–36 months, indicating impairment in spatial and object recognition memory and a decline in synaptic function (Ardiles et al., 2012). Rivera et al. (2016a, b) investigated the effect of AGP (2 and 4 mg/kg) on the hippocampus cognitive function and spatial memory in 56- and 12-month-old degus. The authors conducted an open-field trial to investigate the generic behavior of degus. The results showed no significant differences between the young (12 months) and the old degus (56-month old) treated with the vehicle and the aged degus (56 months) treated with AGP, suggesting that the degus have a normal generic behavior.
The novel location recognition (NLR) and novel object recognition (NOR) are used to investigate cognitive and memory functions (Tarragon et al., 2014). Therefore, AGP was tested on NLR and NOR to assess its cognitive function specifically recognition memory. Their findings from NLR and NOR tasks showed a higher decline in spatial working memory in the aged control (56-month old) degus compared to the young control (12-month old) degus. Moreover, a significant increase was observed in the spatial working memory of the degus treated with AGP (both 2 and 4 mg/kg) (Rivera et al., 2016a, b).
The increase in the recognition index confirms the recovery in recognition memory in both the NLR and NOR experiments (Antunes and Biala, 2012); therefore, the NLR and NOR findings indicated a significant recovery in the recognition memory based on the increased recognition index in the degus treated with AGP (both 2 and 4 mg/kg). During the NLR sessions, the aged degus (control and both AGP groups) spent more time exploring objects compared to the young degus. Moreover, during the NOR session, no differences were observed in the exploration time of the groups, suggesting that the aged degus had a similar motivation to explore objects with the young degus. Hence, the differences observed were not affected by this factor (Rivera et al., 2016a, b).
The spatial learning and memory processes of the degus was also investigated via Barnes maze test, adapted from previous research (Kumazawa-Manita et al., 2013; Rosenfeld & Ferguson, 2014; Sunyer et al., 2007). The Barnes maze showed the time of the first visit to the escape hole, during training sessions progressively reduced in both the young and aged groups over consecutive days. Interestingly, the latency of the first visit significantly reduced in the aged degus treated with AGP (both 2 and 4 mg/kg) in comparison to the aged control degus. This finding shows that the test phase significantly increased the latency time for the degus treated with the vehicle to find the escape hole compared to the young group. This suggest degraded long-term memory retention in the older animals. In contrast, both AGP concentrations improved the latency time in the aged degus group. Both the AGP-treated groups were able to find the escape hole within a similar timeframe as that of the young degus treated with the vehicle. In addition, these results were confirmed from the percentage of time spent in the target quadrant, suggesting that the 2 and 4 mg/kg AGP concentration managed to recover the spatial learning and memory of the degus.
Rivera et al. (2016a, b)’s investigation into reference and working memory errors during the 10 days of training unfolded a considerable effect, that is, a decline in the number of errors made by all groups. Therefore, the AGP treatment helped the animals to learn and use spatial cues to find the escape hole. Moreover, the AGP treatment groups showed a significant reduction in errors made compared to the aged animal group.
The 56-month old degus treated with the vehicle failed to move to a spatial-oriented strategy during the test phase or by the end of training. This means that the aged animals had varied attractional or cognitive abilities. Moreover, the aged degus treated with AGP crossed using 3 strategies in the first day of training and continued with a sequential search throughout the last day of training and the test phases. The aged degus treated with 2 and 4 mg/kg AGP used a combination of spatial strategies (Rivera et al., 2016a, b). Some research has indicated that animals are inclined to change their navigation strategy from accidental search to an efficient and accurate spatial orientation search from beginning to the end of training (Harrison et al., 2006; Jašarević et al., 2011).
Rivera et al. (2016a, b)’s findings showed that the 56-month old degus treated with AGP (2 and 4 mg/kg), significantly saved time and had enhanced efficiency in reaching the escape hole. The aged degus group treated with AGP 2 mg/kg especially had the capacity to repair their attentional or cognitive abilities. In summary, this result supports the theory that the 56-month old animals had comparable memory functions compared to the young animals, with the same findings confirmed by Ming and Song (2005). Interestingly, cognitive function was restored in the aged degus group treated with AGP with results approximately similar to the young degus group (Rivera et al., 2016a, b).
Serrano et al. (2014) found that AGP (Intraperitoneal (IP) injection of 2 mg/kg of AGP) recovered the spatial memory of AβPPswe/PS-1 double transgenic mice of different ages.
Toledo and Inestrosa (2010) and Pérez and Quintanilla (2015) also noted a highly variable cognitive deficit associated with the spatial memory of young AβPPswe/PS-1 mice. The AβPPswe/PS-1 mice (7-month-old) represented by an amended spatial memory model related with episodic memory (memory flexibility) was more sensitive at detecting hippocampal dysfunctions, as examined by Serrano et al. (2014). The spatial memory performance of AβPPswe/PS-1 mice (12-month old) was assessed using a Morris water maze test (MWM). The finding on the behavioral tasks showed that fewer trials were required for the AGP-treated AβPPswe/PS-1 mice to attain the learning scale in comparison to the AβPPswe/PS-1 control mice (Serrano et al., 2014).
The highest latency, which is consistent with hippocampal dysfunction triggered by Aβ neurotoxic effects, was found in AβPPswe/PS-1 mice (Pérez and Quintanilla, 2015). The Serrano et al. (2014)’s results show that wild-type animals injected with the vehicle had normal escape latency during training. However, AGP-treated AβPPswe/PS-1 mice showed significantly lower escape latency, similar to the wild-type mice, proving that AGP can reduce the cognitive impairment and enhance spatial memory performance within two weeks’ training. The results illustrate the reduced cognitive impairment in young and mature AβPPswe/PS-1 transgenic mice treated with AGP.
Based on the consistent findings from the latest studies showing specific AD hallmarks and a recovery of cognitive functions and a decline in synaptic proteins, Serrano et al. (2014) examined the synaptic plasticity of two groups of 7- and 12-month-old AβPPswe/PS-1 mice with a long-term potentiation (LTP) value in hippocampal CA3- CA1 transmission, which is correlated to learning and memory function (Marsh and Alifragis, 2018; Palop and Mucke, 2010; Peña-Ortega, 2013).
The results did not confirm LTP infusion in untreated AβPPswe/PS-1 mice of different ages; however, AGP showed the capability to induce LTP in 7-month-old AβPPswe/ PS-1 mice, sustained for at least 1 h. Although there was no perceived LTP in the untreated 12-month-old AβPPswe/PS-1 group, the LTP infusion was reduced after the AGP treatment in comparison to the 12-month-old AβPPswe/PS-1 model. In addition, no LTP infusion was observed in the wild-type animals treated with AGP (for both age groups). The findings strongly suggest that the synaptic structure and function in the AβPPswe/PS-1 mice was protected by AGP and the induction of synaptic operation. Additionally, the synaptic construct and function were unscathed so AGP was not needed (Serrano et al., 2014).
Another study tested the effect of AGP (1 mg/kg AGP (i.p.), 3 times a week) on an AD model involving adult BALB/c male mice, induced with Lipopolysaccharides (LPS) (i.p. 250 μg/kg) for 1 week (Das et al., 2017). The study showed increased working memory error (WME) after LPS administration using a radial arm maze (ARM). The task was used to evaluate hippocampus function and memory (ARM) (Brown and Giumetti, 2006; Sharma et al., 2010). The WME significantly increased in LPS-injected mice on the 15th day of testing and onwards compared to the control mice. Overall, the working memory impairment, especially on days 10, 14, 18, 22, and 26 of the test significantly decreased after the AGP treatment (Das et al., 2017).
Overall, the neuroprotective actions of AGP and ameliorates the cognitive and spatial memory in different natural models of AD, may be attributed to its regulatory effects on pre- and postsynaptic proteins (Rivera et al., 2016a, b; Serrano et al., 2014), reduction of nuclear factor кB (NF‑кB) and nuclear factor erythroid 2-related factor (Nrf2) activity (Xu et al., 2019a, b; Yang et al., 2017a, b), and microglia inflammatory response (Gruber et al., 2011; Guo et al., 2012). It is worth pointing out that there is no a single mechanism that can be used to explain all of the pharmacological effects of AGP in improvement of cognitive and spatial memory. This property is in accordance with the basic characteristic of various therapeutic drugs, which target different molecules in different types of cells, thereby producing either therapeutic or detrimental effects. In future studies, researchers should focus on the common targets or signaling pathways for the therapeutic actions of AGP in hope of getting an in-depth insight into the neuroprotective effects of AGP.
AGP recovers the synaptic functions in the AD models
The primary trait of cognitive and memory impairment have revealed with disruption of protein and synaptic function in AD brain which are changed in transgenic AD models (Das et al., 2017; Marcello et al., 2012; Rivera et al., 2016a, b; Song et al., 2019). Recently, the combination of synapses and the examination of pre- and postsynaptic proteins were investigated across different experiments.
Rivera et al. (2016a, b) performed a western blot to investigate pre- and postsynaptic proteins in the hippocampus of degus mice. The data showed no consistent differences in presynaptic proteins. Moreover, no changes were observed for the synapsin (SYN) in young degus in comparison to old degus treated with vehicle or in old degus treated with AGP (only a slight change was observed with the dose of 2 mg/kg AGP). The results showed a slight decrease in synaptophysin (SYP) in the old animals, and AGP treatment did not show any recovery effect for this decline. The vesicular glutamate transporter 1 (VGluT1) protein decreased in the old degus and was partially recovered with 2 mg/kg and fully recovered with 4 mg/kg AGP.
A clear decrease in postsynaptic proteins in the aged degus was observed in comparison to young degus. Additionally, the N-methyl-D-aspartate (NMDA) receptor subunit (GluN2A) was partially improved with 2 mg/kg AGP and completely ameliorated with 4 mg/kg AGP, while an opposite effect was observed for postsynaptic density 95 (PSD-95) (Rivera et al., 2016a, b).
Serrano et al. (2014) analyzed the synaptic protein changes in the hippocampus and the cortex of AβPPswe/PS-1 mice using an immunoblot test. Although the post-synaptic proteins in the hippocampus of young AβPPswe/PS-1 mice were significantly recovered after the AGP treatment, the pre-synaptic protein level such as SYP and vesicle-associated integral membrane protein (VAMP) were unaltered in the young AβPPswe/PS-1 hippocampus. The effect of AGP on the synaptic protein level was also assessed in 12-month-old AβPPswe/PS-1 mice. The results showed that PSD-95 and GluA2 levels increased but the level of SYP and VAMP in the AβPPswe/PS-1 hippocampus was unaltered. This finding verifies that it is feasible to recover specific proteins associated with the transmission and postsynaptic structure in advanced neurodegeneration models (Sheng and Kim, 2011). Therefore, this result proves that AGP can prevent postsynaptic protein reduction in young mice (7-month-old) and further improve the synaptic protein level in adult transgenic mice (Serrano et al., 2014).
Furthermore, Das et al. (2017) evaluated the effect of AGP and LPS on the protein expression level of PSD-95 and SYN in the prefrontal cortex of old mature mice. The evidence indicated that the expression of PSD-95 and SYN was increased in the hippocampus of the mature mice in comparison to the control group. Thus suggesting AGP’s ability to compensate for the LPS effect.
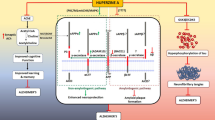
According to these findings, inhibition of neuroinflammatory response via AGP may be a potential strategy for AD treatment (Fig. 3). Moreover, it is unclear how AGP reduces NF-κB phosphorylation in Aβ1-42-triggered microglia. These issues should be resolved in future researches.
This suggested outline displays the potential mechanisms and protective effects of AGP in AD. A reduce in expression of SYP, vGluT1, GluN2A, GluN2B, PSD-95, shank and fEPSP, as well as increase in GSK3β activity, which is properly induced by Aβ aggregation, may mediate the pathogenesis of neuronal damage in AD, can be reversed by AGP treatment. The Aβ-induced NF-κB phosphorylation and microglial over activation can be also inhibited by AGP treatment
AGP reduces the activation of NF‑кB and Nrf2
The NF-кB is one of the most important transcription factors in proinflammatory cytokine expression, which intervenes in microglia-mediated Aβ toxicity (Jones and Kounatidis, 2017; Shih et al., 2015).
Yang et al. (2017a, b) discovered the effects of AGP on Aβ (1–42)-induced neuroinflammation. The results revealed that Aβ (1–42) significantly reduced the inhibitory kappa (IкB) level and the nuclear NF-кB p65 subunit in comparison to the control groups, suggesting NF-кB pathway activation. The findings also showed that pre-treatment with 5 μM AGP blocked the changes in IкB and the nuclear NF-кB p65 subunit considerably. Therefore, the NF-кB activation in Aβ (1–42)-induced BV-2 cells and the primary microglia was inhibited by the AGP treatment(Yang et al., 2017a, b).
An in vitro and in vivo study by Das et al. (2017) examined the anti-inflammatory effect of AGP on p-NFкB activation in LPS-induced mice in a western blot experiment. The results demonstrated that the nuclear translocation of p-NFкB-p65 increased significantly in the LPS-induced adult brain and mixed primary glial in comparison to control groups. Further findings indicate that AGP decreased the glial p-NFкB-p65 considerably, in comparison to the LPS control group (Das et al., 2017).
Furthermore, the effect of AGP (0.5, 5, 12.5, 25 μM) on the translocation of NF-кB in LPS (1 μg/ml) exposure BV2 microglia cells was investigated via confocal microscopy (Xu et al., 2019a, b).
In this case, LPS exposure led to a significant accumulation of NF-кB p65 in the nucleus; however, AGP (especially at 25 μM) significantly reduced the translocation of NF-кB in LPS-induced BV2 cells. The activation of Nrf2 was analyzed to further investigate the AGP anti-inflammatory mechanism via immunocytochemical and western blot experiments. The results demonstrated AGP (particularly 25 μM) significantly increasing the Nrf2 protein in comparison to the LPS control group. Additionally, the effect of AGP on anti-oxidant protein heme oxygenase-1 (HO-1) expression, which is directly connected to Nrf2-dependent activation, was investigated in an RT-PCR experiment. The results showed that AGP in concentrations of 12.5 μM and 25 μM increased the expression of HO-1 mRNA in comparison to the LPS control group (Xu et al., 2019a, b).
The effect of AGP treatment on Nrf2 protein was further investigated (Seo et al., 2017) in the hippocampus HT22 cell of mice. The expression model of nuclear Nrf2 was investigated via western blot. In this case, the AGP treatment (10 μM) significantly upregulated Nrf2 and nuclear Nrf2 by 2.6 times and 3.4 times, respectively, compared to the control cells. Additionally, the expression of Nrf2 in both the cytoplasm and the nucleus was upregulated after the AGP treatment, further enforcing the translocation of Nrf2 from the cytoplasm to the nucleus. The expression of Kelch-like ECH-associated protein 1 (Keap1, an cytoplasmic protein which is important regulatory factor of the oxidative stress signaling pathway) which is an inhibitory protein of Nrf2 (Motyl et al., 2018), was investigated in an immunocytochemistry test, and the results showed no difference after the AGP treatment. In brief, the Nrf2/Keap1-mediated HO-1 signaling pathway in mice hippocampal HT22 cells was activated after AGP treatment, as well as elevating the reduction of intracellular Aβ42 peptides in BV-2cells, and inhibiting the pNF-κB signaling pathway (Seo et al., 2017). Additionally, AGP exhibited neuro-inflammation inhibitory activity. Therefore, more investigations into the effect of AGP on AD are needed.
Since the pNF-κB signaling pathway activated by Aβ42 and enforced inflammatory responses in microglial cells, further investigation was done to determine the effects of AGP on pNF-κB accumulation on microglial BV-2 cells induced by 2.0 μg of Aβ42 and treated with 1 to 10 μM of AGP. According to the findings, pNF-κB was accumulated in the nucleus after BV-2 was induced by 2 μg of Aβ42. After AGP treatment, the nuclear level of pNF-κB (Ser536) was reduced significantly (Seo et al., 2017). Mostly, the pNF-κB RelA/p65-p50 heterodimer shifted to the nucleus after diffusion from IκBα and phosphorylation at Ser536 (Matoba et al., 2014; Takeda et al., 2019). Seo et al. (2017) showed that AGP treatment suppressed the pNF-κB accumulation in the nucleus. However, the specific mechanism for this case is not yet clear. Hence, further research and details consideration of how AGP therapy modulates the pNF-κB signaling pathway are required. Further, it is unclear how andrographolide reduces NF-κB phosphorylation in Aβ1-42-triggered microglia. Future studies can solve these issues.
AGP decreases the production of inflammatory mediators
Pro-inflammatory mediators normally lead to neuronal loss. Pro-inflammatory mediators, such as Nitric oxides (NO), Tumor Necrosis Factor-α (TNF-α), Interleukin 1 beta (IL-1β), and Prostaglandin E2 (PGE2), could induce neurotoxicity by increasing neuronal apoptosis, reducing synaptic function, and prohibiting neurogenesis (Soong and Liu, 2019). Meanwhile, NO is released from glial cells and plays a crucial role in the CNS inflammatory process. NO production is enforced by the over activation of the nitric oxide synthase (iNos) enzyme (Reis et al., 2017).
The immunofluorescence results in Das et al. (2017) indicated that LPS significantly increased the iNos and cyclooxygenase-2 (COX-2) level in the prefrontal cortex of mice, in comparison to the control groups. Moreover, the level of NO was considerably upregulated in the LPS-induced cortex primary glial cells.
Interestingly, the iNos and COX-2 expression in the prefrontal cortex significantly decreased with AGP post-treatment. The immunoblotting result showed that the iNos level in primary cells downregulated significantly in comparison to the LPS control group. The LPS-induced iNos mRNA expression was suppressed in the prefrontal cortex of the adult animal. The immunofluorescence results indicate that the rest of the microglia phenotype markers, such as arginase 1 (Arg-1), were considerably upregulated in the primary microglial culture. Another glia pro-inflammatory mediator is TNF-α, which has a critical role in neurodegeneration, where the transforming growth factor beta (TGF-β) and Interleukin 10 (IL-10) have shown anti-inflammatory effects besides protecting neurons from inflammation and degeneration (Subedi et al., 2020; Tobore, 2019). (Das et al., 2017) and Lively and Schlichter (2018) demonstrated significant upregulation of TNF-α, while TGF-β and IL-10 levels were downregulated in response to LPS exposure. Moreover, Das et al. (2017) also showed that AGP treatment after LPS administration reduced the TNF-α in both the translational (24 and 48 h) and transcriptional level. On the other hand, AGP treatment increased the TGF-β expression considerably at both the translational and transcriptional levels, per the ELISA and RT-PCR report. Additionally, AGP treatment inhibited the IL-1β LPS deponent at 24-h time points for both the translational and transcriptional levels. The IL-10 expression level in the mixed glial cells also increased after AGP treatment compared to the LPS-induced group. Moreover, the effect of AGP on macrophage inflammatory protein 1(MIP1), which is known as chemokinetic, a proinflammatory cytokine, and another central marker of neuroinflammation, was evaluated. The result from the western blot test demonstrated significant downregulation of the MIP1 expression level (Das et al., 2017).
Yang et al. (2017a, b) found that TNF-α, IL-1β, PGE2, and NO proinflammatory mediators were generated with microglia cells’ exposure to Aβ (1–42). The production of TNF-α, IL-1β, PGE2, and nitrite (an indicator of NO production) increased significantly with BV-2 cells’ exposure to 1 μM of Aβ (1–42) for 24 h. The significant inhibition of TNF-α, IL-1β, PGE2, and nitrite production was revealed at 5 μM of AGP. These changes were the same in the primary microglia. Therefore, the neuroinflammation induced by Aβ (1–42) was inhibited with 5 μM of AGP, which proves the neuroprotective effect of AGP (Yang et al., 2017a, b).
Recent studies have shown that iNOS and COX-2 produced NO and PGE2, respectively (Rubach et al., 2019; Salvemini et al., 2013). The western blot result by Yang et al. (2017a, b) demonstrated a few iNOS and COX-2 levels derived from microglial cells without stimulation. The expressions of iNOS and COX-2 protein were increased significantly after administration of 1 μM of Aβ (1–42) for 24 h. The up-regulations of iNOS and COX-2 induced by Aβ (1–42) were dramatically inhibited after AGP treatment in both BV-2 cells and primary microglia. The expression of TNF-a, IL-1β, COX-2, and iNOS mRNA levels, which were induced by Aβ (1–42), was examined via real-time PCR to further explore the responsible mechanism for the inhibitory effect of AGP on pro-inflammatory production. The results demonstrated that the pro-inflammatory production at the transcriptional level was regulated negatively after AGP treatment on Aβ (1–42)-induced microglial cells (Yang et al., 2017a, b).
Xu et al. (2019a, b) also showed reduced NO production with AGP treatment in LPS-induced BV-2 cells. In the in vitro study, MTT assay was used to exclude the cytotoxicity of andrographolide on NO inhibition. The result showed that AGP treatment did not change the cell viability of the BV-2 cells; however, the treatment improved the morphological variation of the BV-2 cells in a dose-dependent manner. With increased AGP concentration, the LPS-induced iNOS expression level gradually decreased. Additionally, the iNOS gene expression was also downregulated after AGP treatment. The TNF-α and Interleukin 6 (IL-6) production level was considerably inhibited. The BV-2 cells exposed to the highest concentration of andrographolide (25 μM) had reduced secretion of IL-6. A destructive cascade event in the neuro-inflammatory process is triggered by intracellular ROS. Intracellular ROS can trigger a cascade of deleterious events in the inflammatory process. The deleterious effects of LPS were reduced with AGP pre-treatment(Xu et al., 2019a, b).
In another study, a conditional medium was used to analyze the PGE2 and NO production level, revealing the effect of AGP on inflammatory cytokines in microglial BV-2 cells (Seo et al., 2017). As of the results, the IL-1β or IL-6 level increased significantly and dramatically in the Aβ42-induced BV-2 conditional medium. The AGP treatment (10 μM) on the BV-2 cells significantly reduced the level of IL-1β or IL-6.
Recent studies have shown IL-1 as the main regulator of neuroinflammation (Salmeron et al., 2019). Recent studies also showed IL-1 as the main regulator of neuroinflammation (Salmeron et al., 2019). IL-1β is known as a pyrogenic cytokine (Sun et al., 2019). It is also known to increase IL-1β production in different brain regions of AD patients (Italiani et al., 2018). Since, intracellular Aβ synthesis was regulated with increased IL-1β (Gray et al., 2020; Pšemeneckienė et al., 2019), it is crucial to decrease the levels of IL-1β secretion in Aβ42-induced BV-2 cells.
Overall, these findings are in line with a previous findings that AGP can destabilize iNOS protein, and illustrated that the anti-AD mechanism of AGP may be different from that of traditional anti-inflammatory agents. Since both NO and PGE2 play Key roles in AD [87], researchers should further appraise whether the anti-AD effect of AGP is dependent on NO and/or PGE2 inhibition in future studies.
AGP decreases Tau phosphorylation in AD models
One of the most critical and earliest hallmarks of AD is the tau protein, particularly in its phosphorylated form (Al-Hilaly et al., 2017). The tau protein plays a crucial role in elevating microtubule assembly, and stabilizing and protecting neuron morphology. Additionally, a critical factor of memory impairment in the prefrontal cortex is the hyper-phosphorylation of tau protein (Chong et al., 2018). Rivera et al. (2016a, b) examined the effect of AGP on the tau phosphorylation level in the hippocampus of degus mice. Therefore, the level of Thr205-Ser202 (AT8), Thr231 and Ser235 phosphorylation was investigated. The results for the aged degus showed that the phosphorylation of these residues was increased. In fact, the tau epitopes phosphorylation, meaning that Ser235 and Thr231 decreased completely after AGP treatment (2 mg/kg), but the residues significantly reduced with 4 mg/kg of AGP treatment. The total molecular weight of tau protein shifted, possibly due to several presented phosphorylation at the tau protein that caused a slight enhancement in its molecular weight (Rivera et al., 2016a, b).
Recent study has shown the paired helical filaments (PHF) accumulation and neurofibrillary tangle formation (NFTs) generated by over phosphorylation of the tau protein (Alonso et al., 2018). Serrano et al. (2014) investigated the phosphorylation of tau protein in AD epitopes in adult animals. Moreover, the phospho-epitope revelation in tau protein, which is associated with neuronal damage, was induced by Aβ (Brandt et al., 2019; Serrano et al., 2014). The phosphorylation of Ser-202 (AT8) and Ser-396-Ser-404 (PHF-1), which are associated with AD, was investigated by Serrano et al. (2014) through an immunoblotting test. The PHF-1- and AT8-positive tau levels were reduced in the hippocampus of young AβPPswe/PS-1 mice (7-month-old) treated with AGP in comparison to untreated AβPPswe/PS-1 mice.
Brandt et al. (2019) demonstrated that enhancement of tau phosphorylation, in particular phosphorylation epitopes, surrounding the plaque is a mark of Aβ-induced neuronal damage. Accordingly, the level of AT8 tau epitope appearance near Aβ plaques was investigated by Serrano et al. (2014). However, as different parts of the cortex and hippocampus were affected by the tau phosphorylation, Serrano 2014 evaluated AT8-positive cells in a circular region surrounding amyloid plaques (r ≈ 100 mm)(Serrano et al., 2014; Vargas et al., 2018). The findings showed an obvious reduction in the number of AT8-positive neurons near to amyloid deposits in young AβPPswe/PS- 1 mice (7-month-old). In addition, the total number of AT8-positive cells which were found in circular regions near to the Aβ plaques slowly decreased after the AGP treatment.
Moreover, the AGP treatment had decreased the levels of PHF-1- and AT8-positive tau significantly in the hippocampus of aged AβPPswe/PS-1 mice (12-month-old). The total number of AT8-positive cells that are found outside of circular regions near plaques was reduced after AGP treatment. The findings of Serrano et al. (2014) also revealed that AGP treatment was able to inhibit and inverse tau phosphorylation, both in young and aged AβPPswe/ PS-1 mice.
Das et al. (2017) also investigated the effect of AGP on the expression level of phosphorylated tau in LPS-administrated mice. The western blot results indicated that the protein expression of phosphorylated tau enzyme had increased considerably in systemic LPS administration, in comparison to control groups. Interestingly, LPS-induced tau hyperphosphorylation was reduced dramatically after AGP treatment. These recent findings demonstrated that tau phosphorylation, which is a key marker in AD pathology, was inhibited by AGP. However the relation between inhibition of tau phosphorylation and reduction of Aβ plaque by AGP treatment is still unclear. Future studies can get in-depth insight into the particular signaling pathway and find if there is any associate with the regulation of Aβ plaques and tau phosphorylation by AGP treatment.
AGP decreased Aβ40 and Aβ42 peptides and Aβ aggregates in AD models
Cognitive impairment in AD patients and AD animal models are related to amyloid levels (Wang et al., 2018). Rivera et al. (2016a, b) examined the Aβ40 and Aβ42 peptides in the degus’ hippocampus, to investigate AGP treatment by using the ELISA experiment. The results illustrated that the level of Aβ42 had been increasing in aged degus, but was reduced by the AGP treatment. This effect was significant at 4 mg/kg concentration of AGP. Rivera et al. (2016a, b) also performed the western blot task, using the 4G8 antibody, to determine the level of soluble Aβ oligomers and other types of Aβ in degus hippocampus. The findings showed the level of low-molecular-weight Aβ oligomers (36 and 42 kDa) in both hippocampi of old and young degus vehicles treated increased; the level of 34-kDa Aβ oligomers, particularly, had increased in aged degus. The level of all low-molecular-weight Aβ oligomers had reduced significantly after AGP treatment (Rivera et al., 2016a, b).
Overall, these findings illustrate that the level of Aβ oligomers and Aβ42 peptide decreased significantly with AGP treatment.
Rivera et al. (2016a, b) also examined how the AGP effects the Aβ burden in young and aged degus hippocampus, using Thioflavin-S staining (Th-s). The senile plaque formations, which are insoluble forms of Aβ, are not seen in young degus; however, multiple plaques were seen in old degus. The total number of senile plaques in the hippocampus were dramatically reduced in a concentration-dependent manner with AGP.
The 6E10 antibody, which is respondent to a specific amino acid sequence of Aβ peptide (1e16), was used to investigate the Aβ aggregation (Rivera et al., 2016a, b; Song et al., 2017; Yokokawa et al., 2019). Rivera et al. (2016a, b) illustrated that the 6E10 level had increased significantly in aged degus; however, it was not seen in young degus.
The 4G8 antibody is specific to amino acid sequence of Aβ peptide (17–24). It is used as a secondary antibody. Its appearance is similar to the 6E10 antibody in both young and old degus. In fact, the 4G8 antibody was not observed in young animals, while appearing in old animals instead. The results also indicated that the Aβ aggregation was reduced considerably in the hippocampus of old degus after AGP treatment (Rivera et al., 2016a, b).
Since the impairment of the synaptic function in the postsynaptic area is caused by Aβ oligomers (Li et al., 2018; Stakos et al., 2020), therefore, Serrano et al. (2014) investigated the effect of AGP (treated for 4 weeks) on Aβ aggregation in both 7 and 12-month-old AβPPswe/ PS-1 mice. The hippocampus and layers of cortex were examined to evaluate amyloid plaques, in young AβPPswe/PS-1 mice. The total number of amyloid plaques was significantly reduced in the I-IV level of cortex after AGP treatment; however, there was no difference in amyloid plaques numbers in the V layers. The Th-S burden was also reduced in the hippocampus. Also, the levels of Aβ oligomers changes in hippocampi of young AβPPswe/PS-1 mice treated with AGP was evaluated by slot blot using the A11 antibody, and it did not show any significant changes. Although AGP treatment had decreased the total number of Aβ plaques in young AβPPswe/PS-1 mice, the Aβ oligomers level was not changed. Serrano et al. (2014) also analyzed plaque size diffusion. The AβPPswe/PS-1 mice treated with AGP had changed their plaque size into a smaller plaque size distribution from the cortex. In contrast, the levels of amyloid plaques had increased significantly in the cortex and hippocampus of aged AβPPswe/PS-1 mice (12-month-old). The results demonstrated that the amyloid deposits levels had not changed in the hippocampus and different layers of cortex (I-IV, V, VI). Similarly, no significant difference was observed in Aβ oligomers level. In fact, these findings illustrated that the level of Aβ burden in aged AβPPswe/PS-1 mice was not affected after AGP treatment; however, the Aβ aggregation was reduced significantly in young AβPPswe/PS-1 mice treated with AGP. Therefore, the Aβ aggregation was prevented in the primary stages during disease development in this animal model (Serrano et al., 2014).
Das et al. (2017) also illustrated that systemic LPS administration increased the level of Aβ in the hippocampus and prefrontal cortex in comparison to control groups, using immunohistochemistry, immunofluorescence and ELISA experiments. In this case, AGP treatment reduced the level of the amyloid precursor protein (APP) significantly in the prefrontal cortex area. The LPS-induced increases of Aβ had rebounded considerably in the prefrontal cortex after AGP treatment. The variation of Aβ in the dentate gyrus (DG) region of the hippocampus was also investigated in this study, but no significant changes were observed (Das et al., 2017).
Since a possible strategy for AD treatment is neuroinflammatory response inhibition, therefore, AGP pre-treatment has been shown to inhibit Aβ1-42-induced production of pro-inflammatory mediators, including TNF-α, IL-6, IL-1β, PGE2, iNOS, COX-2, and NO, in microglia, which are mediated at least by mechanisms that obliterate intracellular Aβ and prevent p-NF-κB accumulation in the nucleus (Fig. 3). However, whether the anti-Aβ effect of AGP mediates the anti-neuronal damage and anti-AD effect of AGP remains unclear.
AGP inhibited GSK-3β, preventing LTD induction
Glycogen synthase kinase 3 beta (GSK-3β) is a key enzyme that was investigated in the processes of plasticity, memory, and neurodegenerative diseases (Jaworski et al., 2019; Liu et al., 2017). GSK-3β especially has interfered in the internalization of AMPA receptors from the synaptic spine (Lee et al., 2019). GSK-3β also plays a role in PSD-95 modulation (Delgado, 2020; Nelson et al., 2013), and controls some of LTD occurrences (Collingridge et al., 2010; Liu et al., 2017; Xing et al., 2016).
Recent studies demonstrated that GSK-3β activity had increased in transgenic models of AD (Tapia-Rojas & Inestrosa, 2018a, b). Accordingly, Serrano et al. (2014) evaluated the effect of AGP on GSK-3β activity in the hippocampus and cortex of aged AβPPswe/PS-1 mice (12-old-month) by immunoblotting. The findings illustrated that the phosphorylation of serine 9 (Ser-9), which is an inactive form of GSK-3β, had increased but the phosphorylation of tyrosine 216 (Tyr-216), the active form of GSK-3β, had reduced. These findings illustrated that AGP protected the synaptic protein and specific enzyme in the neuropathology of AD.
The level of β-catenin, a downstream GSK-3β protein, which is phosphorylated by GSK-3β (Patel et al., 2019) was also evaluated (Serrano et al., 2014). The β-catenin level had increased in the hippocampus slice incubated with AGP, suggesting that the GSK-3β activity has decreased. The previous studies indicated an increase of GSK-3β activation and a decrease of the β-catenin level in the presence of Aβ (Palomer et al., 2019; Patel et al., 2019). However, the active form of GSK-3β and level of β-catenin had decreased and increased, respectively, due to AGP treatment.
Serrano et al. (2014) also examined the effect of AGP treatment in synaptic transmission through an in-vitro study; therefore, the slices of hippocampus from 2-month-old WT mice were prepared and treated with 10 μM of AGP for 30 min. The slope of fEPSP increased in this procedure. The paired-pulse facilitation (PPF) index was evaluated to determine if this efficacy corresponded to a pre- or post-synaptic effect or not. The findings illustrated that the facilitation index did not change after AGP treatment which confirms that the reason was not dependent on pre-synaptic modulation and was mostly due to the post-synaptic mediator effect. To evaluate synaptic strength, Serrano et al. (2014) investigated input–output experiments but no effects were observed on basal transmission after AGP treatment. Additionally, The effect of AGP on synaptic plasticity was also evaluated with an investigation of long-term potentiation (LTP) and longterm depression (LTD) which are correlated to learning and memory functions (Guimaraes Marques et al., 2018). Although, Serrano et al. (2014) did not observe any induced changes in the LTP after AGP treatment (1-h incubation), incubation of Aβ oligomers, which are linked to inhibiting the LTP induction (Li et al., 2018; Marsh & Alifragis, 2018) in the proximity of AGP induced LTP, illustrating synaptic plasticity protection in hippocampus slices (Serrano et al., 2014). The hippocampus slices exposed to Aβ oligomers (for 1 h), to further investigation of the neuroprotective effect of AGP on the damage induced for Aβ. The levels of the postsynaptic proteins such as GluN2B, GluA2, and PSD-95 were reduced compared to the slice of hippocampus ACSF treated, and no changes were observed in presynaptic components. Therefore, the vital role of AGP on neuroprotective synaptic protein and LTP in the exposure of Aβ oligomers (in vitro) suggests that AGP was able to improve the neuronal function without modification of the amyloid level in the AD model. The LTD interfered by reducing synaptic strength and it is relevant to the signaling of Aβ peptide (Kou et al., 2019). Serrano et al. (2014) found that induction of LTD was inhibited after AGP treatment in a concentration-dependent manner (0.1, 5, 10 μM).
These findings revealed that AGP has a hand in synaptic plasticity modulation, correlated with LTD and that this role is in all probability related to GSK-3β inhibition.
Other studies indicated that the activity of GSK-3β, which is a component of the Wnt/β-catenin signaling pathway that caused over-activation of the pathway, was inhibited due to the AGP treatment (Tapia-Rojas et al., 2015; Varela-Nallar et al., 2015). Therefore, the phosphorylation of its target, β-catenin, which is translocated into the nucleus to activate the transcription of Wnt target genes, was inhibited (Silva-García et al., 2019).
Varela-Nallar et al. (2015) has evaluated the effect of AGP treatment (i.p. 2 mg/kg 3 times a week for 4 weeks) on the Wnt signaling pathway in the hippocampus of 2-month-old mice. The level of β-catenin had increased significantly in the hippocampus of AGP treated mice in comparison to the control group. The inactive form of GSK-3β phosphorylated in serine-9 residue had also increased.
Since NeuroD1 was identified as a transcription agent which is involved in neurogenesis in both fetal and mature brains and a known Wnt target gene (Richetin et al., 2015; Xu et al., 2019a, b); thereby the level of NeuroD1 was evaluated by Varela-Nallar et al. (2015). The results have illustrated that the level of NeuroD1 had increased significantly in the hippocampus of AGP treated animals. Overall, these findings illustrate that the activation of the Wnt/β-catenin signaling pathway was inhibited with AGP treatment in the hippocampus of adult mice.
Overall, with these findings that AGP can improve chronic stress-induced depression-like behaviors in rats, it is reasonable to speculate that the AGP could be developed as an anti-depression medication. This supposition should be evaluate in future studies.
Conclusion
This review summarizes the pharmacological effects of andrographolide in the different model of AD. The lack of effective medicine or available treatments for AD has opened a new perspective to find a new and proper medicine from natural products. The subsequent recovery with AGP in several neurotoxicity studies and different models of AD support the neuroprotective effect of AGP. The loss of cognitive function affected by aging and toxicities is correlated with attenuation of synaptic functions and elevate of main AD hallmarks. Most importantly the AGP neuroprotective effects list as follows: (1) protection of postsynaptic proteins; (2) recovery of synaptic strength and plasticity; (3) recovery of spatial memory and learning proficiency; (4) decrease of Aβ aggregate maturation and phosphorylated tau protein. In fact, selective neuroprotection effect of AGP in different AD models might allow an alternative strategy to achieve new therapeutics to remedy and prevent the sequence of neurodegeneration diseases, like AD.
Thus, researchers should focus on more pharmacological effects of andrographolide as well as its in-depth mechanisms in prevention and/or treatment of disorders afflicting the CNS. For example, on the basis of its promotion effects on neurogenesis as well as its reversal effects on chronic stress-induced pathological changes in rodent mood-associated behaviors, researchers should further evaluate the antidepressant-like activity of andrographolide, thereby promoting its development in depression and dementia treatment.
References
Abedi Z, Khaza’ai H, Vidyadaran S, Mutalib MSA (2017) The modulation of NMDA and AMPA/kainate receptors by tocotrienol-rich fraction and α-tocopherol in glutamate-induced injury of primary astrocytes. Biomedicines 5(4):68
Al-Hilaly YK, Pollack SJ, Vadukul DM, Citossi F, Rickard JE, Simpson M, Serpell LC (2017) Alzheimer’s disease-like paired helical filament assembly from truncated tau protein is independent of disulfide crosslinking. J Mol Biol 429(23):3650–3665
Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, Kleiman FE (2018) Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci 12:338
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Ardiles ÁO, Tapia-Rojas CC, Mandal M, Alexandre F, Kirkwood A, Inestrosa NC, Palacios AG (2012) Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc Natl Acad Sci 109(34):13835–13840
Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, Wong WF (2009) A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am J Respir Crit Care Med 179(8):657–665
Bera R, Ahmed SM, Sarkar L, Sen T, Karmakar S (2014) Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm Biol 52(3):321–329
Brandt R, Trushina NI, Bakota L, Mulkidjanian AY (2019) The evolution of tau phosphorylation and interactions. Front Aging Neurosci 11:256
Brown MF, Giumetti GW (2006) Spatial pattern learning in the radial arm maze. Learn Behav 34(1):102–108
Chen Y-Y, Hsu M-J, Hsieh C-Y, Lee L-W, Chen Z-C, Sheu J-R (2014) Andrographolide inhibits nuclear factor-B activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-Stimulated vascular smooth muscle cells. Sci World J 2014:1–14
Chong FP, Ng KY, Koh RY, Chye SM (2018) Tau proteins and tauopathies in Alzheimer’s disease. Cell Mol Neurobiol 38(5):965–980
Collingridge GL, Peineau S, Howland JG, Wang YT (2010) Long-term depression in the CNS. Nat Rev Neurosci 11(7):459–473
Das S, Mishra K, Ganju L, Singh S (2017) Andrographolide-A promising therapeutic agent, negatively regulates glial cell derived neurodegeneration of prefrontal cortex, hippocampus and working memory impairment. J Neuroimmunol 313:161–175
Delgado JY (2020). An Alternative Pin1 Binding and Isomerization Site in the N-Terminus Domain of PSD-95. Frontiers in Molecular Neuroscience, 13.
Ding Y, Shi C, Chen L, Ma P, Li K, Jin J, Li A (2017) Effects of andrographolide on postoperative cognitive dysfunction and the association with NF-κB/MAPK pathway. Oncol Lett 14(6):7367–7373
Gray SC, Kinghorn KJ, Woodling NS (2020) Shifting equilibriums in Alzheimer’s disease: the complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis. Neural Regen Res 15(7):1208–1219
Gruber J, Ng LF, Fong S, Wong YT, Koh SA, Chen C-B, Wenk MR (2011) Mitochondrial changes in ageing Caenorhabditis elegans–what do we learn from superoxide dismutase knockouts? PLoS ONE 6(5):e19444
Guimaraes Marques MJ, Reyes-Garcia SZ, Marques-Carneiro JE, Lopes-Silva LB, Andersen ML, Cavalheiro EA, Scorza CA (2018) Long-term potentiation decay and poor long-lasting memory process in the wild rodents Proechimys from brazil’s amazon rainforest. Front Behav Neurosci 12:2
Guo W, Liu W, Chen G, Hong S, Qian C, Xie N, Xu Q (2012) Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int Immunopharmacol 14(4):613–619
Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP (2006) Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem 13(6):809–819
Inestrosa NC, Ríos JA, Cisternas P, Tapia-Rojas C, Rivera DS, Braidy N, Ardiles AO (2015) Age progression of neuropathological markers in the brain of the chilean rodent octodon degus, a natural model of Alzheimer’s disease. Brain Pathol 25(6):679–691
Iruretagoyena MI, Sepúlveda SE, Lezana JP, Hermoso M, Bronfman M, Gutiérrez MA, Kalergis AM (2006) Inhibition of nuclear factor-κB enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther 318(1):59–67
Islam MT (2017) Andrographolide, a new hope in the prevention and treatment of metabolic syndrome. Front Pharmacol 8:571
Italiani P, Puxeddu I, Napoletano S, Scala E, Melillo D, Manocchio S, Vitale E (2018) Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation 15(1):1–12
Jašarević E, Sieli PT, Twellman EE, Welsh TH, Schachtman TR, Roberts RM, Rosenfeld CS (2011) Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci 108(28):11715–11720
Jaworski T, Banach-Kasper E, Gralec K (2019) GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural plasticity 2019:1–14
Jones SV, Kounatidis I (2017) Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol 8:1805
Kalergis AM, Iruretagoyena MI, Barrientos MJ, González PA, Herrada AA, Leiva ED, Jacobelli SH (2009) Modulation of nuclear factor-κB activity can influence the susceptibility to systemic lupus erythematosus. Immunology 128(12):e306–e314
Kou X, Chen D, Chen N (2019) Physical activity alleviates cognitive dysfunction of Alzheimer’s disease through regulating the mtor signaling pathway. Int J Mol Sci 20(7):1591
Kumar V, Thakur A, Chatterjee S (2014) Perspective of Andrographis paniculata in neurological disorders. Clinic Pharmacol Biopharmaceut S 2:2
Kumazawa-Manita N, Hama H, Miyawaki A, Iriki A (2013). Tool use specific adult neurogenesis and synaptogenesis in rodent Octodon degus hippocampus. PLoS One, 8(3)
Lee JC, Tseng CK, Young KC, Sun HY, Wang SW, Chen WC, Wu YH (2014a) Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/N rf2 pathway in human hepatoma cells. Br J Pharmacol 171(1):237–252
Lee T-Y, Chang H-H, Wen C-K, Huang T-H, Chang Y-S (2014b) Modulation of thioacetamide-induced hepatic inflammations, angiogenesis and fibrosis by andrographolide in mice. J Ethnopharmacol 158:423–430
Lee H, Shin W, Kim K, Lee S, Lee EJ, Kim J, Kang M (2019) NGL-3 in the regulation of brain development, Akt/GSK3b signaling, long-term depression, and locomotive and cognitive behaviors. PLoS biology, 17(6).
Li J, Luo L, Wang X, Liao B, Li G (2009) Inhibition of NF-κB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell Mol Immunol 6(5):381–385
Li Y-J, Yu C-H, Li J-B, Wu X-Y (2013) Andrographolide antagonizes cigarette smoke extract-induced inflammatory response and oxidative stress in human alveolar epithelial A549 cells through induction of microRNA-218. Exp Lung Res 39(10):463–471
Li G-F, Qin Y-H, Du P-Q (2015) Andrographolide inhibits the migration, invasion and matrix metalloproteinase expression of rheumatoid arthritis fibroblast-like synoviocytes via inhibition of HIF-1α signaling. Life Sci 136:67–72
Li C-X, Li H-G, Zhang H, Cheng R-H, Li M, Liang J-Y, Yu H (2016) Andrographolide suppresses thymic stromal lymphopoietin in phorbol myristate acetate/calcium ionophore A23187-activated mast cells and 2, 4-dinitrofluorobenzene-induced atopic dermatitis-like mice model. Drug Des Dev Ther 10:781
Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ (2018) Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer’s disease. Acta Neuropathol Commun 6(1):1–16
Liao W, Tan WD, Wong WF (2016) Andrographolide restores steroid sensitivity to block lipopolysaccharide/IFN-γ–induced IL-27 and airway hyperresponsiveness in mice. J Immunol 196(11):4706–4712
Liu W, Guo W, Guo L, Gu Y, Cai P, Xie N, Sun Y (2014) Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol 20(2):337–345
Liu E, Xie A-J, Zhou Q, Li M, Zhang S, Li S, Wang J-Z (2017) GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Sci Rep 7(1):1–11
Lively S, Schlichter LC (2018) Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+ TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front Cell Neurosci 12:215
Lu C-Y, Li C-C, Lii C-K, Yao H-T, Liu K-L, Tsai C-W, Chen H-W (2011) Andrographolide-induced pi class of glutathione S-transferase gene expression via PI3K/Akt pathway in rat primary hepatocytes. Food Chem Toxicol 49(1):281–289
Lu WJ, Lin KH, Hsu MJ, Chou DS, Hsiao G, Sheu JR (2012) Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol 84(7):914–924
Marcello E, Epis R, Saraceno C, Di Luca M (2012). Synaptic dysfunction in Alzheimer’s disease Synaptic Plasticity (pp. 573–601): Springer.
Marsh J, Alifragis P (2018) Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen Res 13(4):616
Matoba K, Kawanami D, Tsukamoto M, Kinoshita J, Ito T, Ishizawa S, Matsufuji S (2014) Rho-kinase regulation of TNF-α-induced nuclear translocation of NF-κB RelA/p65 and M-CSF expression via p38 MAPK in mesangial cells. Am J Physiol Ren Physiol 307(5):F571–F580
Ming G-L, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250
Mittal SP, Khole S, Jagadish N, Ghosh D, Gadgil V, Sinkar V, Ghaskadbi SS (2016). Andrographolide protects liver cells from H2O2 induced cell death by upregulation of Nrf-2/HO-1 mediated via adenosine A2a receptor signalling. Biochimica et Biophysica Acta (BBA)-General Subjects, 1860(11), 2377–2390.
Motyl J, Wencel P, Cieślik M, Strosznajder R, Strosznajder J (2018) Alpha-synuclein alters differently gene expression of Sirts, PARPs and other stress response proteins: implications for neurodegenerative disorders. Mol Neurobiol 55(1):727–740
Nelson CD, Kim MJ, Hsin H, Chen Y, Sheng M (2013) Phosphorylation of threonine-19 of PSD-95 by GSK-3β is required for PSD-95 mobilization and long-term depression. J Neurosci 33(29):12122–12135
Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P (2014) Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pac J Trop Dis 4(3):213
Palomer E, Buechler J, Salinas PC (2019) Wnt signalling deregulation in the ageing and Alzheimer´ s brain. Front Cell Neurosci 13:227
Palop JJ, Mucke L (2010) Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13(7):812–818
Patel S, Alam A, Pant R, Chattopadhyay S (2019). Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Frontiers in Immunology, 10.
Peña-Ortega F (2013). Amyloid beta-protein and neural network dysfunction. Journal of Neurodegenerative Diseases, 2013.
Peng S, Gao J, Liu W, Jiang C, Yang X, Sun Y, Xu Q (2016) Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget 7(49):80262
Pérez MJ, Quintanilla RA (2015) 2015. Therapeutic actions of the thiazolidinediones in Alzheimer’s disease, PPAR research
Pšemeneckienė G, Petrikonis K, Rastenytė D (2019) Polymorphisms of proinflammatory cytokines in relation to APOE epsilon 4 and risk of Alzheimer’s disease in the lithuanian population. Medicina 55(10):689
Reis PA, de Albuquerque CFG, Gutierrez T, Silva AR, de Castro Faria Neto, H. (2017) Role of nitric oxide synthase in the function of the central nervous system under normal and infectious conditions. Nitric oxide synthase-simple enzyme-complex roles. London, InTech, pp 55–70
Richetin K, Leclerc C, Toni N, Gallopin T, Pech S, Roybon L, Rampon C (2015) Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer’s disease. Brain 138(2):440–455
Rivera DS, Inestrosa NC, Bozinovic F (2016a) On cognitive ecology and the environmental factors that promote Alzheimer disease: lessons from octodon degus (Rodentia: Octodontidae). Biol Res 49(1):10
Rivera DS, Lindsay C, Codocedo JF, Morel I, Pinto C, Cisternas P, Inestrosa NC (2016b) Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol Aging 46:204–220
Rosenfeld CS, Ferguson SA (2014). Barnes maze testing strategies with small and large rodent models. JoVE (Journal of Visualized Experiments)(84), e51194.
Roy DN, Sen G, Chowdhury KD, Biswas T (2011) Combination therapy with andrographolide and d-penicillamine enhanced therapeutic advantage over monotherapy with d-penicillamine in attenuating fibrogenic response and cell death in the periportal zone of liver in rats during copper toxicosis. Toxicol Appl Pharmacol 250(1):54–68
Rubach MP, Zhang H, Florence SM, Mukemba JP, Kalingonji AR, Anstey NM, Mwaikambo ED (2019) Kinetic and cross-sectional studies on the genesis of hypoargininemia in severe pediatric Plasmodium falciparum malaria. Infect Immun 87(4):e00655-e618
Salmeron KE, Maniskas ME, Edwards DN, Wong R, Rajkovic I, Trout A, Pinteaux E (2019) Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J Neuroinflammation 16(1):222
Salvemini D, Kim SF, Mollace V (2013) Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol 304(7):R473–R487
Sani D, Khatab NI, Kirby BP, Yong A, Hasan S, Basri H, Stanslas J (2019) A standardised andrographis paniculata Burm. Nees aqueous extract prevents Lipopolysaccharide-induced cognitive deficits through suppression of inflammatory cytokines and oxidative stress mediators. J Adv Res 16:87–97
Selkoe DJ (2013) The therapeutics of Alzheimer’s disease: where we stand and where we are heading. Ann Neurol 74(3):328–336
Seo JY, Pyo E, An JP, Kim J, Sung SH, Oh WK (2017). Andrographolide activates Keap1/Nrf2/ARE/HO-1 pathway in HT22 cells and suppresses microglial activation by Aβ42 through Nrf2-related inflammatory response. Mediators of inflammation, 2017.
Serrano FG, Tapia-Rojas C, Carvajal FJ, Hancke J, Cerpa W, Inestrosa NC (2014) Andrographolide reduces cognitive impairment in young and mature AβPPswe/PS-1 mice. Mol Neurodegener 9(1):61
Shao F, Tan T, Tan Y, Sun Y, Wu X, Xu Q (2016) Andrographolide alleviates imiquimod-induced psoriasis in mice via inducing autophagic proteolysis of MyD88. Biochem Pharmacol 115:94–103
Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87(17–18):521–536
Sheng M, Kim E (2011) The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 3(12):a005678
Shih R-H, Wang C-Y, Yang C-M (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77
Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM (2019) Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front Immunol 10:2135
Singha PK, Roy S, Dey S (2007). Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in mice. Journal of Ethnopharmacology, 111(1):13–21.
Song Y, Kim H-D, Lee M-K, Hong I-H, Won C-K, Bai H-W, Cho J-H (2017). Maysin and its flavonoid derivative from Centipedegrass attenuates amyloid plaques by inducting humoral immune response with Th2 skewed cytokine response in the tg (APPswe, PS1dE9) Alzheimer’s mouse model. PloS one, 12(1).
Song Y, Hu M, Zhang J, Teng Z-Q, Chen C (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine 39:409–421
Soong TW, Liu C (2019) Nitric oxide, iron and neurodegeneration. Front Neurosci 13:114
Stakos DA, Stamatelopoulos K, Bampatsias D, Sachse M, Zormpas E, Vlachogiannis NI, Stellos K (2020) The Alzheimer’s disease amyloid-beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J Am Coll Cardiol 75(8):952–967
Subedi L, Lee SE, Madiha S, Gaire BP, Jin M, Yumnam S, Kim SY (2020) Phytochemicals against TNFα-mediated neuroinflammatory diseases. Int J Mol Sci 21(3):764
Sun Y, Ma J, Li D, Li P, Zhou X, Li Y, Luo X (2019) Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J Neuroinflammation 16(1):66
Sunyer B, Patil S, Höger H, Luber G (2007). Barnes maze, a useful task to assess spatial reference memory in the mice. Protocol Exchange.
Takeda Y, Matoba K, Kawanami D, Nagai Y, Akamine T, Ishizawa S, Utsunomiya K (2019) ROCK2 regulates monocyte migration and cell to cell adhesion in vascular endothelial cells. Int J Mol Sci 20(6):1331
Tan WD, Liao W, Zhou S, Wong WF (2017) Is there a future for andrographolide to be an anti-inflammatory drug? deciphering its major mechanisms of action. Biochem Pharmacol 139:71–81
Tapia-Rojas C, Inestrosa NC (2018a) Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen Res 13(10):1705
Tapia-Rojas C, Inestrosa NC (2018b) Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J Neurochem 144(4):443–465
Tapia-Rojas C, Schüller A, Lindsay CB, Ureta RC, Mejías-Reyes C, Hancke J, Inestrosa NC (2015) Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: autoregulation of GSK-3β in vivo. Biochem J 466(2):415–430
Tarragon E, Lopez D, Estrada C, Gonzalez-Cuello A, Ros CM, Lamberty Y, Guiso G (2014) Memantine prevents reference and working memory impairment caused by sleep deprivation in both young and aged Octodon degus. Neuropharmacology 85:206–214
Tobore TO (2019). On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurological Sciences, 1–14.
Toledo E, Inestrosa N (2010) Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Mol Psychiatry 15(3):272–285
Tran QT, Tan WD, Wong WF, Chai CL (2020) Polypharmacology of andrographolide: beyond one molecule one target. Natural Product Reports.
Varela-Nallar L, Arredondo SB, Tapia-Rojas C, Hancke J, Inestrosa NC (2015) 2015. Andrographolide stimulates neurogenesis in the adult hippocampus, Neural plasticity
Vargas L, Cerpa W, Muñoz F, Zanlungo S, Alvarez A (2018). Amyloid-β oligomers synaptotoxicity: The emerging role of EphA4/c-Abl signaling in Alzheimer's disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1864(4):1148–1159.
Visen P, Shukia B, Patnaik G, Dhawan B (1993) Andrographolide protects rat hepatocytes against paracetamol-induced damage. J Ethnopharmacol 40(2):131–136
Wang T, Liu B, Zhang W, Wilson B, Hong J-S (2004) Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J Pharmacol Exp Ther 308(3):975–983
Wang X, Kastanenka KV, Arbel-Ornath M, Commins C, Kuzuya A, Lariviere AJ, Bacskai BJ (2018) An acute functional screen identifies an effective antibody targeting amyloid-β oligomers based on calcium imaging. Sci Rep 8(1):1–15
Wang L, Cao F, Zhu L-L, Liu P, Shang Y-R, Liu W-H, Wang Z-Y (2019) Andrographolide impairs alpha-naphthylisothiocyanate-induced cholestatic liver injury in vivo. J Nat Med 73(2):388–396
Xia Y-F, Ye B-Q, Li Y-D, Wang J-G, He X-J, Lin X, Hebbel RP (2004) Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173(6):4207–4217
Xing B, Li Y-C, Gao W-J (2016) GSK3 β hyperactivity during an early critical period impairs prefrontal synaptic plasticity and induces lasting deficits in spine morphology and working memory. Neuropsychopharmacology 41(13):3003–3015
Xu D, Hou K, Li F, Chen S, Fang W, Li Y (2019a) XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/β-catenin signaling dependent way. Life Sci 235:116844
Xu Y, Tang D, Wang J, Wei H, Gao J (2019b) Neuroprotection of andrographolide against microglia-mediated inflammatory injury and oxidative damage in PC12 neurons. Neurochem Res 44(11):2619–2630
Yan J, Chen Y, He C, Yang Z-Z, Lü C, Chen X-S (2012) Andrographolide induces cell cycle arrest and apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Cell Biol Toxicol 28(1):47–56
Yang D, Zhang W, Song L, Guo F (2013) Andrographolide protects against cigarette smoke-induced lung inflammation through activation of Heme Oxygenase-1. J Biochem Mol Toxicol 27(5):259–265
Yang C-H, Yen T-L, Hsu C-Y, Thomas P-A, Sheu J-R, Jayakumar T (2017a) Multi-targeting andrographolide, a novel NF-κB inhibitor, as a potential therapeutic agent for stroke. Int J Mol Sci 18(8):1638
Yang R, Liu S, Zhou J, Bu S, Zhang J (2017b) Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK MAPK signaling pathway. Immunopharmacol Immunotoxicol 39(5):276–284
Yen T-L, Chen R-J, Jayakumar T, Lu W-J, Hsieh C-Y, Hsu M-J, Lin K-H (2016) Andrographolide stimulates p38 mitogen-activated protein kinase–nuclear factor erythroid-2-related factor 2–heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Transl Res 170:57–72
Yin J-N, Li Y-N, Gao Y, Li S-B, Li J-D (2015) Andrographolide plays an important role in bleomycin-induced pulmonary fibrosis treatment. Int J Clin Exp Med 8(8):12374
Yokokawa K, Iwahara N, Hisahara S, Emoto MC, Saito T, Suzuki H, Suzuki S (2019). Transplantation of mesenchymal stem cells improves Amyloid-β pathology by modifying microglial function and suppressing oxidative stress. Journal of Alzheimer's Disease(Preprint), 72(3):1–18.
Zhang C, Gui L, Xu Y, Wu T, Liu D (2013) Preventive effects of andrographolide on the development of diabetes in autoimmune diabetic NOD mice by inducing immune tolerance. Int Immunopharmacol 16(4):451–456
Zhang Z, Lai D, Wang L, Yu P, Zhu L, Guo B, Lee SMY (2014) Neuroprotective effects of the andrographolide analogue AL-1 in the MPP+/MPTP-induced Parkinson’s disease model in vitro and in mice. Pharmacol Biochem Behav 122:191–202
Zhao Y, Wang M, Li Y, Dong W (2018) Andrographolide attenuates viral myocarditis through interactions with the IL-10/STAT3 and P13K/AKT/NF-κβ signaling pathways. Exp Ther Med 16(3):2138–2143
Zhu T, Wang D-X, Zhang W, Liao X-Q, Guan X, Bo H, Zhang Y-K (2013a) Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-κB. PLoS ONE 8(2):e56407
Zhu T, Zhang W, Xiao M, Chen H, Jin H (2013b) Protective role of andrographolide in bleomycin-induced pulmonary fibrosis in mice. Int J Mol Sci 14(12):23581–23596
Acknowledgements
The authors declare that this study has no funding.
Author information
Authors and Affiliations
Contributions
ZA wrote the manuscript. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies involving animals performed by any of the authors. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
Zahra Abedi has no conflict of interest. Hamidon Basri has no conflict of interest. Zurina Hassan has no conflict of intrest. Liyana Najwa Inche Mat has no conflict of ineterest. Huzwah Khaza’ai has no conflict of interest. Nur Afiqah Mohamad has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedi, Z., Basri, H., Hassan, Z. et al. A review of the neuroprotective effects of andrographolide in Alzheimer's disease. ADV TRADIT MED (ADTM) 21, 253–266 (2021). https://doi.org/10.1007/s13596-021-00573-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-021-00573-8