Abstract
Alzheimer’s disease (AD) is characterized by progressive memory loss and cognitive function deficits. There are two major pathological hallmarks that contribute to the pathogenesis of AD which are the presence of extracellular amyloid plaques composed of amyloid-β (Aβ) and intracellular neurofibrillary tangles composed of hyperphosphorylated tau. Despite extensive research that has been done on Aβ in the last two decades, therapies targeting Aβ were not very fruitful at treating AD as the efficacy of Aβ therapies observed in animal models is not reflected in human clinical trials. Hence, tau-directed therapies have received tremendous attention as the potential treatments for AD. Tauopathies are closely correlated with dementia and immunotherapy has been effective at reducing tau pathology and improving cognitive deficits in animal models. Thus, in this review article, we discussed the pathological mechanism of tau proteins, the key factors contributing to tauopathies, and therapeutic approaches for tauopathies in AD based on the recent progress in tau-based research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia with no effective treatment available. It is the sixth leading cause of death in United States which mostly affected the people of age 65 and above. The number of patients is estimated to be over 46 million worldwide and it is predicted that the number will reach 131.5 million by 2050 (Prince et al. 2015). It is estimated to have cost the world $818 billion and become a trillion dollar disease by 2018 (Prince et al. 2015). The pathological hallmarks of AD are the presence of extracellular amyloid-β (Aβ) deposition and the formation of intracellular neurofibrillary tangles (NFTs) in the brain, along with the typical degeneration and loss of neurons characterized by memory loss and learning difficulty (Bloom 2014; Wang et al. 2017). Several studies have focused on Aβ targeting pathology as a potential treatment for AD; however, clinical evidence has shown that anti-amyloid treatment for AD has not been effective at slowing down the progression of disease. Since then, attention has been shifted towards the research on the pathology of tau proteins in AD (Giacobini and Gold 2013; Desikan et al. 2012).
Tau is a normal, unfolded, highly soluble protein which plays a critical role in tubulin assembly and stabilization of microtubules, thereby promoting normal function of neurons (Duan et al. 2017). In AD, several post-translational modifications especially tau hyperphosphorylation were believed to be a key factor that affects microtubule assembly and induces tau aggregation. In tau hyperphosphorylation, tau undergoes conformational changes in which the conversion of tau monomer to tau oligomer induces the aggregation of tau into pair helical filament, leading to the formation of NFTs (Chirita et al. 2005; Sahara et al. 2007; Mondragón-Rodríguez et al. 2008; Lasagna-Reeves et al. 2010; Patterson et al. 2011). Scientific evidence shows that NFT itself may not be involved in inducing neurotoxicity; however, the intermediate tau oligomer has been demonstrated to be the most toxic form of tau that results in synaptic impairment in AD (Fá et al. 2016). Besides, in vivo and in vitro studies have also shown that tau pathology may spread to different brain regions (Clavaguera et al. 2009; Holmes and Diamond 2014; Fá et al. 2016). As tauopathies are indicated in dementia and AD, hereby we review the physiological role of tau proteins in brains, the key factors contributing to tauopathies, and the mechanisms involved, as well as potential tau-targeted therapies for AD.
Tau and Tauopathies
Tau Physiology and Function
In the past decade, research in AD has been focused on Aβ. Recently, the research target began to shift from Aβ to tau when studies revealed that tau proteins were the main component that made up NFTs. Tau is a microtubule-associated protein which plays a crucial role in stabilizing microtubules by promoting tubulin assembly to regulate the normal functions of neurons (Giacobini and Gold 2013). The location of tau gene, MAPT, was found on the long arm of human chromosome 17. In human brain, tau proteins encode six isoforms which vary in size, ranging from 352 to 441 amino acids. These isoforms are different from each other by the presence of three repeats (3R) or four repeats (4R) in the C terminal and the presence or absence of one (29 amino acids) or two inserts (58 amino acids) in the N-terminal region. Studies have suggested that the repeat regions (244–268 amino acids) in the C terminal are the domain that is responsible for the binding of tau to microtubule (Goedert et al. 1989a, b; Himmler et al. 1989). The six isoforms have their specific physiological role as they are differently expressed during brain development. The shortest isoforms of tau with the presence of 3R and without the presence of N-terminal inserts have been found to express during the foetal stages. However, the rest of the isoforms with either 3R or 4R and one or two inserts in C terminal and N terminal, respectively, have been revealed to be expressed during adulthood (Kosik et al. 1989; Stanford et al. 2003). Studies have discovered that tau isoforms that are expressed during adulthood are about 40-fold more efficient than the foetal isoform in promoting microtubule assembly (Lindwall and Cole 1984).
Tau protein has been demonstrated to play a pivotal role in binding to the microtubule for its stabilization (Daun et al. 2017). Microtubule is a cellular important structure that transports organelle for the plus-end-directed motor kinesin and the minus-end-directed motor dynein. The plus-end-directed motor kinesin is responsible for the delivery of their cargoes such as mitochondria, endocytic or endocytic vesicles, and lysosomes towards the peripheral cells, whereas the minus-end-directed motor dynein is responsible for delivering the cargoes backwards to the microtubule organizing centre. The role of these motors helps in regulating the axonal transport in the brain. Study has shown that tau proteins which bind to the microtubule tightly regulate the transport of cargoes which in turn affect the axonal transport (Callahan et al. 2002). Due to the strong tau’s affinity to the microtubule, it could detach the cargoes from the kinesin and hence inhibit the axonal transport and result in the symptoms of AD. This indicates that tau phosphorylation plays a crucial role because it regulates the tau’s affinity to microtubule (Brady and Sperry 1995; Lippincott-Schwartz et al. 1995).
Role of Tau Protein in Tauopathies
Conformational Changes of Tau
In AD patients, the misfoldings of tau would affect the microtubule binding, and hence the tau is neither bound to tubulin nor promotes the microtubule assembly, thus disrupting the organization of microtubule. Tau in its abnormal phosphorylated form would form tau dimer, followed by the formation of tau oligomer. A number of studies have provided evidence that the dimerization of tau monomers is an essential step for the formation of tau oligomers (Chirita et al. 2005; Sahara et al. 2007; Mondragón-Rodríguez et al. 2008; Lasagna-Reeves et al. 2010; Patterson et al. 2011). The tau oligomer then aggregates and forms the paired helical filaments (PHF), which eventually leads to the formation of NFTs that are abundant in the brain of AD patient (Grundke-Iqbal et al. 1986).
Spreading of Tau Pathology Extracellularly
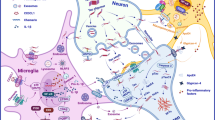
Tau protein is mainly localized in the axonal and somatodendritic compartment (Binder et al. 1985). However, previous studies have shown a wider distribution of tau in other compartments including the nucleus (Sultan et al. 2011) and dendrites (Hoover et al. 2010). The aggregates were first seen in the entorhinal cortex, then the pathology would propagate to the hippocampus and spread to other brain regions including the limbic cortex and association cortices (DeKosky and Scheff 1990; Terry et al. 1991). In an in vivo study, the trans-synaptic propagation of tau pathology through anatomically connected neuronal networks was demonstrated in the transgenic mouse model that expressed human tau in the entorhinal cortex (Liu et al. 2012). Several studies have proposed that tau oligomers induce neurotoxicity more than the aggregated form of tau and also play a more prominent role in spreading the pathology. In vitro studies have shown that in the frontal cortex of the AD brain, T22 and AT8 antibodies have been detected in different brain regions apart from the regions that were exclusively stained for tau oligomers. These data suggested that tau oligomers can transmit from one region to another in the AD brain. The presence of oligomeric tau in the extracellular space has been found to be associated with memory deficits in AD. In this case, several in vitro and in vivo studies have revealed that tau oligomerization can induce the aggregation of tau prior to the formation of NFTs (Clavaguera et al. 2009; Fá et al. 2016).
Synaptic Impairment
The symptoms of AD include progressive memory loss, cognitive decline, and learning difficulty. These symptoms are strongly associated with the dysfunction of synapses which eventually lead to irreparable synaptic loss in AD. Previous research mainly focused on the toxicity of Aβ plaques which were thought to be involved in the synaptic damage in AD; however, a later study has found an emerging role of tau in synapses (Delacourte et al. 2002). In the healthy brain, the presence of tau plays a prominent role in regulating normal synaptic function. Previous study has discovered that the presence of tau oligomers in synapses are toxic to the neurons and can cause synaptic impairment prior to the formation of NFTs, an event that would trigger the neurodegeneration such as those in AD (Tai et al. 2014). Studies on drosophila model have demonstrated that overexpressing tau results in neuronal loss with the absence of NFTs. In agreement with that, other studies have also shown that when reducing the levels of overexpressing tau in mutant tau transgenic mice neuronal loss is also decreased, despite the continuous formation of NFTs (Williams et al. 2000, 2001; Mershin et al. 2004). Although various studies have claimed that tau oligomer is responsible for the neurotoxicity in tauopathies, its underlying mechanism still awaits further elaboration. Past studies also show that NFT formation rather than the deposition of Aβ plaques correlates well with a cognitive decline in AD. Besides that, cognitive decline has been shown to correlate well with the synaptic loss in AD in parallel with the severity of AD. This is supported by the study that shows the extensive synaptic loss in the AD brain (Masliah et al. 1989). Tau affects the synaptic function in several ways. Axonal transport of mitochondria can be disrupted by the detachment of tau from the microtubule, synaptophysin mRNA was found to be reduced by 35–37% in the AD brain with the presence of NFTs (Callahan et al. 2002), and, most importantly, deregulation of synapses was observed in the AD brain which is independent from the Aβ pathology (Masliah et al. 1993). In transgenic mouse models, results such as loss of synaptophysin, dendritic targeting of Fyn kinase, and impairment of synaptic transmission were reported (Alldred et al. 2012; Harris et al. 2012). Previous evidence has evidenced the role of Aβ, tau, and Fyn in dysregulating synaptic function, which causes neuronal synchrony. In an in vivo study carried out in hAPP mice, the results have shown that by reducing tau, synaptic impairment, cognitive deficits, and behavioural abnormalities induced by the synergistic effects of Aβ and Fyn can be prevented. The involvement of Aβ, tau, and Fyn in synaptic impairment is strongly supported by the previous studies; however, the mechanism by which these components cooperate in AD-related pathogenesis still remains to be elucidated (Roberson et al. 2011).
Key Factors Contribute to Tauopathies
It is evident that, in many neurodegenerative disorders including AD, microtubule-associated tau has undergone several post-translational modifications. These modifications are highly associated with the aggregation of tau in AD. The post-translational modifications that are identified in tauopathies include tau hyperphosphorylation, truncation (Wischik et al. 1988; Novak et al. 1993; Gamblin et al. 2003), glycosylation (Wang et al. 1996; Liu et al. 2004), glycation (Yan et al. 1994, 1995), nitration (Zhang et al. 2005), and ubiquitination (Iqbal and Grundke-Iqbal 1991). We will also discuss other post-translational modifications associated with tau aggregation in this review.
Hyperphosphorylation of Tau
Tau phosphorylation is essential for the neurons to maintain its normal physiological function, including the binding of normal tau to the microtubule and the regulation of microtubule assembly and its stabilization. In the studies performed by Ksiezak-Reding et al., tau was found to contain 2–3 mol of phosphates per molecule of tau in the normal brain; however, in the autopsied AD brain, the level of tau phosphorylation is at least 3–4 times higher than that in the normal brain, which is around 6–8 mol of phosphates per molecule of tau (Ksiezak-Reding et al. 1992). Hence, hyperphosphorylation of tau plays a critical role in tau aggregation and contributes to the pathogenesis of AD (Ihara et al. 1986). It should be noted that the actual causes that lead to hyperphosphorylation are still in debate. Studies have reported that the largest brain tau isoform, tau 441, contains 80 serine or threonine and five tyrosine residue phosphorylation sites which are potentially phosphorylated in AD (Hasegawa et al. 1992). With the use of phosphorylation-dependent monoclonal antibodies against tau and mass spectrometric analysis, the results have shown that at least 39 serine or threonine (Hanger et al. 2007) and two tyrosine residues are phosphorylated in PHF (Lee et al. 2004).
The normal tau phosphorylation is regulated by the activities of both cellular enzymes, protein kinases, and protein phosphatases. Both enzymes are crucial in maintaining microtubule assembly and normal neuron function in the human brain. Past studies have shown that the imbalance between kinases and phosphatases is the potential cause of the formation of hyperphosphorylated tau in the AD brain (Wang et al. 2007). The major protein kinases involved in tau phosphorylation are part of the proline-directed protein kinases, including cyclin-dependent kinase 5 (Cdk5) (Baumann et al. 1993) and mitogen-activated protein kinase (MAP). However, kinases that phosphorylate at non-Ser/Thr-Pro sites include cyclic AMP-dependent protein kinase (PKA) (Jicha et al. 1999), microtubule-associated regulatory kinase (MARK) (Drewes et al. 1997), casein kinase I and II, and calmodulin-dependent protein kinase II (CaMKII) (Baudier and Cole 1987). Other than that, glycogen synthase kinase 3β (GSK3β) is able to phosphorylate at both proline-directed sites and non-proline-directed sites (Hanger et al. 1992). Tau phosphorylation occurred in different sites can be regulated by different kinases. Having different tau phosphorylation sites can have various impacts on neuronal function. In the in vitro studies demonstrated by Liu et al., Thr231, Ser262, and Ser356 phosphorylation sites have been shown to inhibit the binding of normal tau to microtubule, leading to the disruption of microtubule assembly. The results of the study show that tau phosphorylation that occurred at proline-rich region inhibits microtubule assembly and promotes self-aggregation into tangles of filaments. At the C-terminal tail region, tau phosphorylation was shown to increase its microtubule-stimulating activity and promote self-aggregation. The results, taken together, revealed that tau phosphorylation occurred at all the proline-rich region, C-terminal tail region, and microtubule-binding region has diminished the normal function of microtubules (Liu et al. 2007). Luna-Monoz et al. have reported that normal tau appeared to undergo conformational changes at the phosphorylation site Thr231. The structurally changed tau was first detected by monoclonal antibody TG-3 and Alz-50 subsequently (Luna-Muñoz et al. 2005, 2007).
Besides, tau has also shown its involvement in neurotransmission via the Fyn pathway (Lee et al. 1998). A previous study has also suggested that the src family kinase Fyn is upregulated in the AD brain (Shirazi and Wood 1993). In the study, Fyn is involved in the tau phosphorylation at tyr18 with the use of antibody probes specific for phosphor-tyr18. Results of immunocytochemical studies also indicated the presence of tyrosine-phosphorylated tau in NFTs in the AD brain. Taken together, all these findings supported the role of Fyn in the neurodegenerative diseases (Lee et al. 2004). Wood and Zinsmeister have reported the involvement of the tyrosine phosphorylation system in AD pathology. Elevated levels of phosphotyrosine were observed in the somatodendritic compartment of neuritic plaque and dystrophic neurites, which reflects the role of the tyrosine kinase system in neurodegeneration process (Wood and Zinsmeister 1991).
Alonso et al. suggested that the products of mutant tau gene with alterations in the tau conformation are more prone to hyperphosphorylation. Hence, hyperphosphorylation of mutant tau would result in further conformational changes, enables them to sequester normal microtubule-associated tau proteins, and aggregates to form PHFs. However, the actual mechanism by which the mutations lead to tau hyperphosphorylation is not fully understood (Alonso et al. 2004).
Besides kinases, a decrease in the levels of phosphatases has also been shown in the autopsied AD brain. A previous study has revealed that the activity of phosphatases in the isolated AD brain is reduced by approximately 20% in grey matter and 40% in white matter (Gong et al. 1993). Phosphatases play an opposite role of kinases and hence promote tau dephosphorylation instead. In the AD brain, the phosphate levels were found to be 3- to 4-fold higher than those in the normal brain (Ksiezak-Reding et al. 1992). The underlying cause of the elevated levels of phosphates is the downregulation of phosphatases, and thus the efficiency of the neurons to remove the phosphate group is dampened, which eventually leads to the accumulation of phosphates in the AD brain. The major phosphatase involved in tau phosphorylation is PP2A. I1PP2A and I2PP2A are the endogenous PP2A inhibitors that play a crucial role in the regulation of PP2A activity. However, both inhibitors have been shown to be downregulated in the AD brain, leading to increased levels of inhibitors and decreased PP2A activity (Sontag et al. 2004; Chen et al. 2008). A previous study has reported that brain acidosis activates and induces the translocation of asparaginyl endopeptidase from the neuronal lysosomes to the cytoplasm, inhibiting PP2A through the cleavage of I2PP2A, causing hyperphosphorylation of tau, and likely contributes to the pathogenesis of AD (Basurto-Islas et al. 2013).
Truncation
Other than tau hyperphosphorylation, truncation of tau is also one of the important modifications that have been associated with tau aggregation. It is defined as the proteolytic cleavage of tau which leads to aberrant aggregation. In the AD brain, caspase 3, a member of the caspase family of cysteine proteases, has been proved to be involved in the proteolytic cleavage of tau at aspartic acid 421 residue (D421) in vitro (Gamblin et al. 2003; Rissman et al. 2004). The truncated tau at D421 aggregates more readily than the full-length tau (Derisbourg et al. 2015). A previous study also reported that the occurrence of tau truncation at D421 has increased cell death via apoptosis significantly (Stadelmann et al. 1999). Interestingly, the caspase 3 cleavage site can be recognized by the monoclonal antibody Tau-C3. There is also in vitro evidence suggesting that phosphorylated tau at serine 422 can inhibit caspase cleavage at D421 (Guillozet-Bongaarts et al. 2006). Besides, a previous study has shown that the truncation of tau also occurred at glutamic acid 391 residue (E391) and the cleavage site can be recognized by antibody MN243 due to the formation of MN243 epitope (Novak et al. 1993). Other than that, the N terminus of tau is also cleaved at D13 and D402 by caspase 6 in the AD brain (Horowitz et al. 2004). Studies also suggested that caspase 6 is implicated in the apoptosis and neuronal degeneration in AD (Guo et al. 2004; Albrecht et al. 2007). Garcia-Sierra et al. reported that the truncation of the C terminus of tau is correlated with the cognitive impairment in AD (García-Sierra et al. 2001). When all these were taken together, cumulative evidence has suggested that the truncation of tau plays a critical role in the tau aggregation, as it can result in tau polymerization to form insoluble PHF.
Glycosylation
In the AD brain, tau is abnormally modified by glycosylation which is associated with tau aggregation. Study has shown that hyperphosphorylated tau was glycosylated mainly through N-glycosylation. The role of N-glycosylation in the AD brain is to maintain and stabilize the structure of PHF, which also helps in facilitating kinases to promote tau hyperphosphorylation and suppressing the tau dephosphorylation by phosphatases (Liu et al. 2004). Non-hyperphosphorylated tau isolated from the AD brain was found to be glycosylated, as opposed to the absence of glycan in the normal brain. Thus, this suggests that tau glycosylation precedes tau hyperphosphorylation (Wang et al. 1996). In vitro studies have shown that glycosylated tau acts as a better substrate for cAMP-dependent protein kinase than the non-glycosylated tau. This suggests that glycosylated tau facilitates the subsequent hyperphosphorylation in the AD brain, a process probably catalysed by cyclin-dependent kinase 5 (cdk5) and glycogen synthase kinase-3 beta (GSK3β) at site-specific phosphorylation sites (Liu et al. 2002a, b). In contrast to N-glycosylation, O-GlcNAcylation, a type of O-glycosylation helps in regulating tau phosphorylation in a site-specific manner. In the AD brain, the level of O-GlcNAcylation was found to be reduced, which probably explains the presence of tau hyperphosphorylation and tau aggregation. Using starved mice as the model, impaired glucose uptake or metabolism is shown to decrease the level of O-GlcNAcylation and promote tau hyperphosphorylation (Liu et al. 2004). This suggests that patients with diabetes mellitus, which characterized a similar impairment of glucose metabolism, are likely to have a higher risk of developing AD (Ott et al. 1999). In vivo studies have shown that the reduction of O-GlcNAcylation suppressed PKA–CREB signalling, which in turn impairs the ability of learning and memory in AD (Xie et al. 2016). This is because PKA catalytic subunits play a pivotal role in learning and memory and were post-translationally modified by O-GlcNAc.
Glycation
Glycation is a process of non-enzymatic post-translational modification which may facilitate the aggregation of tau with the formation of advanced glycation end products (AGEs). Previous studies have shown that the accumulation of AGEs in PHF induces oxidative stress which promotes neuronal dysfunction in AD patients (Yan et al. 1994, 1995). Li et al. have reported that the level of methylglyoxal (MG), a reactive carbonyl compound, is increased in the AD brain. The data have suggested that MG is involved in inducing tau hyperphosphorylation through AGE formation, upregulation of receptors of AGEs (RAGE), and activation of GSK-3β and p38 MAPK (Li et al. 2012). Other studies have also shown that MG could enhance tangle formation in vivo and this process could be slightly accelerated by tau hyperphosphorylation (Kuhla et al. 2007). Recent studies have also revealed that the level of 4R2N tau glycation is increased with ageing and/or development of diabetes mellitus. This would result in the promotion of tau phosphorylation and aggregation, which in turn contribute to the pathogenesis of AD (Liu et al. 2016).
Nitration
Tau nitration is also a possible modification which may be involved in tau aggregation. Abnormal nitration of tau was found in NFTs in the AD brain. Tyrosine residues that are involved in tau nitration are Tyr18, Tyr29, Tyr197, and Tyr394. Studies showed that nitrated tau Tyr197 can be found in the normal healthy brain and may play a crucial physiological role in human body. However, the rest of the Tyr residues are only detected in the AD brain. However, the involvement of tau nitration in AD pathology remains unclear. Zhang et al. have reported that peroxynitrite treatment can result in tau nitration and oligomerization in a dose-dependent manner. The study revealed that the nitrated tau undergoes conformational change, which inhibits its binding to microtubule and promotes tau aggregation (Zhang et al. 2005). With the use of quantitative electron microscopy, Reynolds et al. have demonstrated that nitration of residues Tyr29 and Tyr197 increases the average length of tau filaments without alteration in the steady-state polymer mass. However, nitration of residues Tyr18 and Tyr394 has been shown to decrease the average length of tau filaments, causing filamentous mass reduction. Taken together, in vitro evidence suggested that nitration affects microtubule assembly differently, based on the site of nitration (Reynolds et al. 2005). In another study, the monoclonal antibody Tau-nY29 was used to detect soluble tau and PHF isolated from the severely affected AD brain that has nitration at Tyr29. It was shown that nitration at Tyr29 is a disease-related event which may cause alteration on tau, inducing self-polymerization in neurodegenerative tauopathies (Reynolds et al. 2006).
Ubiquitination
Tau is also known to be ubiquitylated and involved in tau aggregation into tangles of filaments, leading to neuronal death (Iqbal and Grundke-Iqbal 1991). The impairment of ubiquitin ligases, carboxyl terminus of the Hsc70-interacting protein (CHIP), has been implicated in neurodegenerative tauopathies. The studies have reported that the interaction of CHIP with Hsp70/90 can induce ubiquitination of tau, resulting in tau aggregation. Conversely, the mouse model that overexpressed inducible Hsp70 has been shown to have a significant reduction in tau levels. Taken together, these data suggest that the Hsp70/CHIP chaperone system plays a critical role in regulating tau ubiquitination and degradation as well as preventing tau aggregation (Petrucelli et al. 2004). Besides, the CHIP–Hsc70 complex could regulate the ubiquitination of phosphorylated tau species, enhancing neuronal cell survival (Petrucelli et al. 2004; Shimura et al. 2004).
Other Post-translational Modifications Associated with Tau Aggregation
Other post-translational modifications including lysine methylation (Funk et al. 2014) and sumoylation (Dorval and Fraser 2006; Luo et al. 2014) are also indicated in the mechanism of tau aggregation. According to that study, lysine methylation retains tau’s ability to promote microtubule assembly which in turn attenuates tau aggregation propensity. This methylation could help in suppressing tau aggregation in vitro which might confer neuroprotection in AD (Funk et al. 2014). Besides, sumoylation is also another important post-translational modification that has been indicated in AD pathology. It is known that tau contains two small ubiquitin-like modifier (SUMO) consensus sequences and Lys(340) being the major sumoylation site (Dorval and Fraser 2006). A previous study has reported that tau sumoylation induces tau hyperphosphorylation at multiple AD-associated sites. This would in turn promote further sumoylation and inhibit tau ubiquitination and degradation. These data suggested that sumoylation may play a pathogenic role in the neurodegenerative tauopathies (Luo et al. 2014).
Therapeutic Approaches for Tauopathies in AD
In the past 40 years, studies have been performed to investigate the feasibility of targeting Aβ pathology as a potential treatment for AD. Positive results such as cognition improvement and reduction of tau hyperphosphorylation with low adverse effects have been observed in the AD transgenic mice treated with anti-amyloid therapies. However, the results reported in animal studies were not parallel with the results obtained in the clinical trials (Giannakopoulos et al. 2003; Desikan et al. 2012). On the hand, more and more emerging evidence suggests that tauopathies might play a key role in the pathogenesis of AD, and hence tau protein has now received substantial attention to be the next treatment target for AD. Many studies have been conducted to find ways to reduce tau hyperphosphorylation by inhibiting the activity of kinases and restoring the activity of phosphatases. On top of that, neuroscientists are also exploring other potential approaches such as inhibiting tau aggregation, restoring tau GlcNAcylation, and deploying tau immunotherapy to target tauopathies in AD.
Tau Aggregation Inhibitors
Previous studies have shown that methylene blue (MB) can block tau–tau binding interaction through the repeat domain. This has been shown to effectively reduce the stability of the protease-resistant PHF and inhibit tau aggregation in vitro (Wischik et al. 1996). Previous studies have demonstrated that MB treatment preserves cognition in transgenic mice expressing full-length pro-aggregant human tau before the onset of cognition impairment. Therefore, MB that serves as a tau aggregation inhibitor can be used as a potential drug for AD treatment before the onset of cognitive impairments (Hochgräfe et al. 2015). TRx0237 (LMTX™), methylthioninium chloride, is a derivative of MB which is also capable of inhibiting tau aggregation (Wischik et al. 2015).
Microtubule Stabilizers
In AD, microtubule is destabilized due to the inhibition of tubulin assembly caused by the conformational changes of tau, which would contribute to the neuronal dysfunction. Therefore, treatment with microtubule stabilizer is a potential therapeutic approach for AD. Microtubule stabilizers such as paclitaxel and other taxanes are not suitable for the treatment of tauopathies due to their poor blood–brain barrier permeability. In contrast to paclitaxel, BMS-241027, which is also known as Epothilone D (EpoD), is a microtubule-stabilizing agent with better blood–brain barrier permeability. Brunden et al. have reported that treatment with BMS-241027 improved microtubule density and axonal integrity, and reduced cognitive deficits in the transgenic mouse model of tauopathies without inducing any side-effects (Brunden et al. 2010; Zhang et al. 2012). Due to its positive effects on animal studies, the therapeutic effect of BMS-241027 is recommended for clinical testing; however, the clinical trial was discontinued in 2011 and the results have been inconclusive. NAP or AL-108, which is also known as davunetide, is another microtubule-stabilizing peptide derived from activity-dependent neuroprotective protein. It helps in promoting microtubule stability and has shown a reduction effect on tau phosphorylation in preclinical studies. These findings were supported by Shiryaev et al.’s study in which memory improvement and tau hyperphosphorylation reduction have been observed in NAP-treated transgenic mice (Shiryaev et al. 2009). In phase II clinical study of NAP, the results suggest that intranasal administration of NAP was safe, well tolerated and showed improvement in cognitive function in subjects with mild cognitive impairment (Morimoto et al. 2013).
Kinase Inhibitors and Phosphatase Activators
Kinases and phosphatases have been targeted for the novel therapeutic approaches in AD due to their involvement in tau hyperphosphorylation (Wang et al. 2007). Therefore, these two enzymes have been targeted for the novel therapeutic approaches in AD. Lee et al. suggested that the Src family tyrosine kinase, Fyn, has shown its involvement in AD by inducing synaptic loss and memory impairments in the transgenic mouse model (Lee et al. 2004; Chin et al. 2005). Elevated Fyn in AD patients has been found to be responsible for tau phosphorylation and the mediated neurodegeneration process in AD. Hence, treatment targeting Fyn kinase can be a novel therapeutic approach for AD. Saracatinib (AZD0530), a Fyn kinase inhibitor, which is a potential candidate drug for treating AD, has now entered clinical trial. It has passed phase I clinical trial and now currently enters into phase II (Nygaard et al. 2015). Le Corre et al. have reported that the kinase inhibitor named K252a is effective at clearing hyperphosphorylated tau effects against ERK2 in the tau transgenic mouse model. The in vivo findings have also shown that a reduction of tau hyperphosphorylation directly helped in preventing motor impairment in the transgenic mice. However, NFTs are not reduced in the treated mice (Le Corre et al. 2006). A kinase inhibitor, diaminothiazole, which targets Cdk5/p25 and GSK3β, has been shown to improve memories and reduce the amount of PHFs in the AD mouse models (Zhang et al. 2013). A previous study has reported that microglial activation promotes tau hyperphosphorylation via activation of the p38α MAPK signalling pathway in the mouse model of tauopathies (Roy et al. 2015). Microglial activation also promotes MAPT hyperphosphorylation in the neurons of non-transgenic mice lacking microglia-specific fractalkine receptor (CX3CR1). In addition, in the humanized MAPT transgenic mice lacking CX3CR1 also showed increased MAPT hyperphosphorylation and behavioural impairments which correlated with increased levels of active p38 MAPK. The same results were obtained in other in vitro studies (Bhaskar et al. 2010; Maphis et al. 2015). Besides, studies have also suggested that synaptic dysfunction in AD is associated with the activation of p38α MAPK-mediated signalling pathway (Watterson et al. 2013). Because of that, MW181, a p38α MAPK inhibitor, has been developed. A recent study reported that the treatment with MW181 has multiple advantages: (1) it induces selectively the suppression of α-isoform of p38 MAPK, (2) it reduces tau hyperphosphorylation, (3) it reduces proinflammatory cytokine production, and (4) it prevents cognitive deficits in aged hTau mice. Thus, MW181 is a potential drug for further evaluation for treatment of AD (Maphis et al. 2016). In another study, Branca et al. reported that the Dyrk1A inhibitor reduces amyloid precursor protein (APP) and tau phosphorylation, thus making it another ideal candidate drug for the treatment of AD (Coutadeur et al. 2015; Branca et al. 2017). Another therapeutic approach is to induce the upregulation of protein phosphatases. Previous study has shown that the eicosanoyl-5-hydroxytryptamide (EHT), a minor component of coffee, has therapeutic benefits in the rat model of AD. Dietary supplementation with EHT inhibits tau hyperphosphorylation, reduces Aβ pathology, and also enhances cognition in the AD rat model (Basurto-Islas et al. 2014). The antidiabetic drug biguanide such as metformin has also been shown to promote the activity of PP2A and decrease tau hyperphosphorylation at PP2A-dependent epitope in vitro and in vivo, suggesting its potential therapeutic role for AD (Kickstein et al. 2010). VEL015 (sodium selenate) is another drug candidate that reduces the level of hyperphosphorylated tau and NFTs. In the VEL015-treated transgenic mice, it has been shown that VEL015 not only reduces the levels of hyperphosphorylated tau and NFTs, but also improves memory and motor functions. On top of that, VEL015 also stabilizes the PP2A activity (van Eersel et al. 2010). VEL015 is currently in phase III clinical trial for its evaluation as a potential disease-modifying therapy for AD (Malpas et al. 2016).
Insulin Sensitizer
In AD, brain glucose metabolism is impaired, implying that diabetes mellitus increases the risk of AD. However, the underlying mechanism by which the impairment leads to AD is not well understood. Previous studies have reported that hyperphosphorylated tau contains fourfold less levels of O-GlcNAc in comparison to non-hyperphosphorylated tau, suggesting that the impairment of brain glucose metabolism causes downregulation of O-GlcNacylation, resulting in abnormal hyperphosphorylation of tau, eventually leading to neurofibrillary degeneration in AD. Hence, restoration of brain tau O-GlcNacylation activity could help reverse tau hyperphosphorylation, offering a promising therapeutic target for AD treatment (Liu et al. 2009a, b). Previous studies have reported that thiazolidinediones including troglitazone and rosiglitazone have been shown to reduce tau phosphorylation in in vitro model (d’Abramo et al. 2006). Pedersen et al. have reported that rosiglitazone, which is an insulin sensitizer, attenuates learning and memory deficits in Tg2576 mice with AD. Based on the findings, the rosiglitazone-treated mice exhibited better spatial learning and memory abilities than the untreated mice (Pedersen et al. 2006). Due to the positive effects of rosiglitazone in attenuating learning and memory abilities in mice, the drug serves as a potential therapy for AD and has now been further tested in clinical trial. Another insulin sensitizer, pioglitazone, was also tested in clinical trial due to its anti-inflammatory effects. Both rosiglitazone and pioglitazone were well tolerated; however, oedema was observed in patients who were receiving these two drugs. Pioglitazone has produced conflicting results regarding its efficacy in AD due to numerous trial limitations. Thus, the current findings from the clinical trials suggested that rosiglitazone and pioglitazone are not recommended to be used for AD treatment (Miller et al. 2011).
O-GlcNAcase Inhibitors
Tau O-GlcNAcylation activity is regulated by both O-GlcNAc transferase (an enzyme which catalyse the addition of GlcNAc to proteins) and β-N-acetylglucosaminidase (OGA, an enzyme which catalyse the removal of GlcNAc from proteins). Studies have reported that the administration of the selective OGA inhibitor Thiamet G to JNPL3 transgenic mice exhibited an increase in tau O-GlcNAcylation and reduced NFT formation, eventually leading to reduced neuronal loss in mice. Hence, OGA inhibitors could serve as a potential therapeutic drug for AD treatment (Graham et al. 2014; Yuzwa et al. 2012). Recent studies have investigated the effects of Thiamet G in inhibiting OGA in rTg4510 mice. Evidence has shown that OGA inhibition leads to the activation of O-ClcNAc tau, which in turn reduces pathological tau in the cerebrospinal fluid of mice. Due to the positive effects of OGA inhibitors in mice, it has provided support for further clinical development of OGA inhibitors for treatment of tauopathies (Hastings et al. 2017).
Tau Immunotherapy
Immunotherapy has emerged to be a promising therapeutic approach for AD treatment. Many studies have been conducted using active and passive immunotherapy targeting Aβ peptide. Studies have revealed that Aβ immunotherapy has shown efficacy not only in the Aβ clearance but also in the clearance of early tau pathology. However, the Aβ immunization cannot clear the hyperphosphorylated tau (Oddo et al. 2004; Serrano-Pozo et al. 2010). In phase III clinical trial, the first Aβ vaccination trial has failed to show efficacy on clearing tau pathology as well as halting and slowing down disease progression in subjects with mild to moderate AD (Boche et al. 2010). Hence, recent studies have shifted the focus to tau pathology. Both active and passive immunization have been shown to reduce tau pathology with brain function improvement in the tauopathy mouse model. Studies have shown that neuronal uptake of tau antibodies occurs via clathrin-dependent Fcγ II/III receptor endocytosis. The intracellular interaction of antibodies and tau aggregates results in the clearance of tau pathology (Congdon et al. 2013). Other than that, an in vivo study has revealed that peripheral administration of anti-tau/pS422 antibody formed a complex with tau/pS422 which is present in membrane microdomains on neuronal cell surface, inducing the clearance of tau pathology intracellularly (Collin et al. 2014). Yanamandra et al. have demonstrated that anti-tau antibodies have reduced hyperphosphorylated and aggregated tau in P301S mice effectively. The antibodies are also capable of blocking the development of tau seeding activity and transcellular propagation of tau pathology in mice (Yanamandra et al. 2013). A previous study showed that neuronal uptake of cis tau-specific antibody via Fcγ receptors helps prevent tauopathy development and propagation of tau pathology following traumatic brain injury (Kondo et al. 2015).
Asuni et al. have reported that active immunization against phospho-tau epitope is capable of reducing tau aggregates and improving motor performance in P301L tangle model mice (Asuni et al. 2007). In addition, a study has demonstrated the effects of peripheral administration of antibody against pathological tau in both JNPL3 and P301S mouse models. The findings have shown that passive immunization reduces tau pathology and delays disease progression in both mouse models (Chai et al. 2011). Another study has shown that immunotherapy targeting tau pathology is capable of reducing aggregated tau which can help in preventing cognitive impairments in htau/PS1, a new mouse model with accelerated tangle development. Taken together, the findings of the studies have paved the ways for immunotherapy to be assessed in clinical trials (Boutajangout et al. 2010). Besides, Rasool et al. also reported that systemic vaccination using anti-oligomeric monoclonal antibodies showed great potential in reducing amyloid load and hyperphosphorylated tau, which in turn improved the cognitive functions of 3xTg-AD mice (Rasool et al. 2013). Recent studies have also demonstrated that the 3xTg-AD mice treated with tau antibodies 43D and 77E9 showed successfully reduced total tau levels and tau hyperphosphorylation at different phosphorylation sites. This finding suggested that the passive immunotherapy could possibly reduce Aβ pathology in the AD brain. The passive immunization targeting the N-terminal projection domain of tau has shown memory and cognition improvements in 3xTg-AD mice (Dai et al. 2015, 2017).
The monoclonal antibody DC8E8 is able to recognize pretangles, intracellular and extracellular tangles in AD. DC8E8 can effectively reduce the amount of tau oligomers and NFTs in the transgenic mice. The binding of DC8E8 to the determinants inhibits tau–tau interaction in vitro and in vivo. Besides, DC8E8 also selectively targets the diseased tau but not the healthy tau (Kontsekova et al. 2014b). A tau peptide which encompasses the epitope targeted by DC8E8 was selected to develop an active vaccine, AADvac1. It is a vaccine candidate that targets the structural determinant that is essential for pathological tau–tau interaction on tau. Based on the results from preclinical study, the vaccine effectively reduced tau oligomers and decreased neurofibrillary pathology in transgenic rat model. The findings also showed that active immunotherapy reduced the level of hyperphosphorylated tau by approximately 95%. AADvac1 has entered phase I clinical trial in which its safety and tolerability have been assessed in patients with mild to moderate AD (Kontsekova et al. 2014a). Patients involved in the study have received 3–6 immunization doses of AADvac1. AADvac1 showed an excellent safety and tolerability profile in the first-in-man study (Novak et al. 2017). An 18-month safety follow-up study of AADvac1 in patients who have completed the phase 1 clinical trial of AADvac1 was completed in March 2017. AADvac1 is currently entering phase II clinical trial (Novak et al. 2017). A 24-month safety and efficacy study of AADvac1 in patients with mild AD is ongoing and the primary completion date is expected to be June 2019. In this study, 60% of the patients will receive AADvac1, while the rest of the patients will receive placebo (ClinicalTrials.gov. 2016).
Tauopathies in Other Diseases
Tau deposition in NFTs is the hallmark of AD, and therefore numerous therapeutic approaches are aimed at reducing tau levels in the AD brain. It is known that cardiovascular diseases (CVD) including stroke, high blood pressure, and high cholesterol level are the risk factors of AD (Eriksson et al. 2010; de Bruijn and Ikram 2014; Hersi et al. 2017). In a recent study making use of western blot and immunohistochemistry staining, tau proteins have been shown to be present in heart tissue and play a crucial functional role in the cardiovascular system (Betrie et al. 2017). Previously, tau proteins have been proved to play functional roles in the formation of endothelial cell lumen and induce post-translational modifications, microtubule assembly, and asymmetric cytoskeletal polarization (Kim et al. 2013). Hence, the reduction of tau proteins will likely lead to the suppression of these functions. Regulation of cardiac function, cytoskeleton organization, and myocardium survival by endothelial cell–cardiac myocyte interactions could also be affected by loss of tau proteins (Narmoneva et al. 2004). Betrie et al. conducted the study on wild-type (WT) and tau-deficient (KO) mice of two different age groups (aged 13 and 23 months) for cardiovascular phenotype assessment. Both the right and left atria and small mesenteric arteries were isolated from both mice. According to the results reported, 13-month-old tau KO mice showed evidence of increased systolic blood pressure and cardiac hypertrophy, in addition to decreased right atrial rate and decreased left atrial contractility. In ageing 23-month-old tau KO mice, they exhibited similar results to 13-month-old tau KO mice; however, the condition was more critical compared to young tau KO mice. In concordance with this, a significant reduction of left atrial contractility could be seen in the ageing tau KO mice. In addition, a significant reduction of contractile response to calcium, isoprenaline, and electrical nerve stimulation leading to left atrial decompensation had been observed in the ageing tau KO mice. However, cardiac hypertrophy in 13-month-old tau KO mice did not result in decompensation stage. In ageing WT mice, cardiac hypertrophy has been shown in different chambers of the heart, in which the heart-to-body ratio has increased. Based on the results reported from the contractile response assessment, ageing WT mice also showed a decrease in inotropic responses to calcium and isoprenaline. The sensitivity of mesenteric arteries to contraction by methoxamine has also decreased (Betrie et al. 2017). To sum up, this study showed the functional role of tau in cardiac tissue and the loss of tau induced by AD treatment would lead to damage of cardiovascular performance which worsens with age.
Previous research has suggested that increased tau aggregates were often accompanied by a decrease in the levels of soluble tau in the affected brain regions of patients with AD and PD (Lei et al. 2012). Soluble tau plays an important role in the brain as it helps in promoting the trafficking of APP to the neuronal surface in order to lower the iron levels present in the brain by promoting iron efflux (Brady and Sperry 1995; Lippincott-Schwartz et al. 1995). This prevents iron from accumulating in the brain over time. However, tau-directed therapeutic approaches for AD reduce tau aggregates and soluble tau in the brain. The loss of soluble tau would attenuate the efflux of iron, causing iron to accumulate in the brain, which in turn results in iron neurotoxicity (Lei et al. 2012, 2017). Other than that, neuronal iron retention was also seen in AD patients due to the inhibition of APP ferroxidase activity that helps in iron efflux by the accumulating zinc within the amyloid plaques found in AD (Duce et al. 2010; Lei et al. 2012).
Supporting evidence for the above statement was shown in the investigations conducted by Lei et al. The team had examined the presence of tau in the post-mortem brain samples collected from patients with Parkinson’s disease (PD) and healthy controls. With the use of blot densitometry, the results have shown lower soluble tau levels in the substantia nigra (SN) of PD patients when compared with healthy controls. In this study, results also demonstrated that iron levels in SN were 39% higher than those in the healthy controls, indicating the abnormal accumulation of iron in the SN region. The data also demonstrated that the deficiency of soluble tau could result in the accumulation of iron due to the impairment of APP trafficking to the neuronal surface. When comparing tau KO mice with age-matched WT mice of different ages (6, 12, 15, 18, 24 months old), brain atrophy was seen in tau KO mice with the conditions worsening with age. Age-dependent neuronal loss of SN, cognitive decline, and parkinsonism were seen in the mice. Iron levels in different regions of the brain have been assessed. It showed that the iron levels were significantly higher in the SN, hippocampus, and cortex (around 20% higher in each case) in 12-month-old tau KO mice compared to the WT mice of the same age. Both Fe(II) and Fe(III) were found to be abundant in the regions of the hippocampus and SN of tau KO mice, suggesting that the accumulation of iron causes age-dependent neurodegeneration in tau KO mice. Supporting this, there were no significant changes observed in 6-month-old young mice. This study has emphasized the role of tau in AD, PD, and other tauopathies whereby tau is not only a neurotoxic aggregate in the brain, but it also plays an important role in preventing age-related damage by regulating APP trafficking (Lei et al. 2012).
In a recent study published by Lei et al., lithium can suppress tau levels but can induce iron accumulation in the brain, resulting in neurodegeneration (Lei et al. 2017). By knocking down tau levels using lithium, iron-induced parkinsonism with cognitive impairment can be observed among mice. Considering both studies conducted by Lei et al., it is likely that decreased soluble tau could lead to neurotoxic iron accumulation in AD, PD, and other tauopathies, and hence more caution is needed when providing tau-directed therapies for AD due to tau’s unexpected physiological role in the central nervous system (Lei et al. 2012).
In September 2017, two papers published by Bi et al. and Tuo et al. have confirmed that young tau KO mice (3 months old) were protected against ischaemia–reperfusion injury after stroke, compared to WT mice where extensive cell death had been observed after stroke. The authors have demonstrated that it is likely that tau KO mice were protected against excitotoxicity due to the accumulation of a protective protein called synaptic RAS GTP-activating protein (SynGAP1). This protein exhibits silencing activity towards the excitotoxic signalling pathway in the post synapses of mice. The silencing of the excitotoxic signalling pathway enhanced the recovery of 3-month-old tau KO mice after stroke. However, the neuroprotection effect was not observed in older tau KO mice probably due to the accumulation of iron in the brain. When looking at the effect of excitotoxicity-induced brain damage in mouse stroke model created by blockage of middle cerebral arteries, data have shown that both groups of mice experienced local cell death at the blockage site and exhibited similar minor movement deficit after 1 h of injury. No further cell loss was observed over time in tau KO mice. In comparison to tau KO mice, the changes occurred in the brain of WT mice were more critical as the condition continued to worsen over time. Supporting this, massive cell death was observed. There is also evidence of a pattern of cell loss spreading throughout the affected hemisphere, causing severe motor deficits. The differences in the conditions observed between the tau KO mice and WT mice may be explained by the differential gene expressions in both mice after stroke. After administration of pentylenetetrazole (PTZ), the findings showed that ERK signalling was activated, which in turn results in the activation of Ras GTPase, causing excitotoxic genes in WT mice to be expressed. This mechanism explained why the condition worsens in WT mice over time. In contrast to tau KO mice, the presence of SynGAP1 leads to the inactivation of Ras GTPase, causing Ras deactivation through binding to PSD-95 in the post synapses. As the in vitro study showed that tau would compete with SynGAP1 for the binding site of PSD-95 in the post synapses, the increased levels of SynGAP1 would result in the silencing of the ERK signalling pathway. Therefore, the excitotoxic pathway was inactivated, which helps prevent further brain damage in tau KO mice. However, in the case of AD, further investigations are required to identify whether tau would compete with SynGAP1 binding to PSD-95 in the post synapses which resulting unable silencing excitotoxic signalling pathway and prevent further brain damage as what happening in stroke. Other than that, further investigation is also needed to determine whether SynGAP1 could be a good therapeutic target to prevent excitotoxicity (Bi et al. 2017).
Tuo et al.’s study has reported that there was no evidence of increased iron levels in the affected hemisphere in tau KO mice after blockage of middle cerebral arteries. Enhanced recovery with only little neurodegeneration was observed after ischaemic stroke. Hence, the authors hypothesized that the suppression of tau may prevent ferroptosis damage, which is a form of non-apoptotic cell death programme dependent upon intracellular iron level. However, differences have been observed in WT mice whereby the iron levels in the affected hemisphere had been shown to increase 50% several hours after the blockage, meanwhile tau levels in the particular area had decreased. Unlike tau KO mice, extensive neuronal cell death and motor deficits were observed in WT mice. In this case, the authors proposed a hypothesized statement whereby the presence of compensatory mechanisms in young tau KO mice may compensate for the loss of tau and help in iron export, reducing the accumulation of iron in the brain. However, neurotoxic iron accumulation increased with age. Supporting this, the findings reported that ageing tau KO mice accumulated 50% iron more than the WT mice. This has negated the neuroprotective effect of tau suppression against hemispheric reperfusion injury following middle cerebral artery occlusion (Tuo et al. 2017).
Findings also showed that the inhibition of ferroptosis exerts a neuroprotective effect on organisms (Dixon et al. 2012). Tuo et al. reported that 3-month-old WT mice treated with ferroptosis inhibitors, liproxstatin-1 and ferrostatin-1, after blockage showed better performance compared to untreated mice within 6 h. Taken together, further investigations are needed to assess the effects of the combination therapy to lower both iron and tau in AD patients (Tuo et al. 2017).
Conclusion Remarks
This article has discussed the pivotal roles of tau pathology in causing AD. Tau has played an important role in regulating the normal function of neurons, but it can also be involved in the pathogenesis of AD under certain conditions. The balance of the activities of both kinase and phosphatase is crucial to regulate normal tau phosphorylation level in the human brain. In AD, the imbalance of their activities leads to tau hyperphosphorylation. Tau hyperphosphorylation induced tau conformational changes, leading to synaptic impairment in AD. The propagation of tau pathology extracellularly was also discussed in this review. Hence, tau-directed therapies might be useful to slow down the progression of AD and other tauopathies. Accumulating evidence has shown that many drugs have been developed as inhibitors for therapeutic targets of AD; however, most of the drugs have only shown some symptomatic benefits. Some of the drugs such as TRx0237 and VEL015 have entered clinical trials for further evaluation as a potential disease-modifying therapy for AD. As a future perspective, drugs targeting tauopathies and drugs targeting amyloid deposition can be administered together to serve as a combination therapy that would be a potential therapeutic approach for AD. It is anticipated that the combination therapy of these drugs could be more efficacious than the anti-amyloid or anti-tau monotherapy. Most importantly, further understanding of the exact mechanism of AD is still of great importance in order to develop an effective treatment for AD. Only then, a more effective treatment regimen can be designed to cure the incurable disease.
References
Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC (2007) Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol 170(4):1200–1209. https://doi.org/10.2353/ajpath.2007.060974
Alldred MJ, Duff KE, Ginsberg SD (2012) Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction. Neurobiol Dis 45(2):751–762. https://doi.org/10.1016/j.nbd.2011.10.022
Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K (2004) Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279(33):34873–34881. https://doi.org/10.1074/jbc.M405131200
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27(34):9115–9129. https://doi.org/10.1523/JNEUROSCI.2361-07.2007
Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K (2013) Activation of asparaginyl endopeptidase leads to tau hyperphosphorylation in Alzheimer disease. J Biol Chem 288(24):17495–17507. https://doi.org/10.1074/jbc.M112.446070
Basurto-Islas G, Blanchard J, Tung YC, Fernandez JR, Voronkov M, Stock M, Zhang S, Stock JB, Iqbal K (2014) Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol Aging 35(12):2701–2712. https://doi.org/10.1016/j.neurobiolaging.2014.06.012
Baudier J, Cole RD (1987) Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem 262(36):17577–17583
Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E (1993) Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 336(3):417–424
Betrie AH, Ayton S, Bush AI, Angus JA, Lei P, Wright CE (2017) Evidence of a cardiovascular function for microtubule-associated protein tau. J Alzheimers Dis 56(2):849–860. https://doi.org/10.3233/JAD-161093
Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT (2010) Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68(1):19–31. https://doi.org/10.1016/j.neuron.2010.08.023
Bi M, Gladbach A, van Eersel J, Ittner A, Przybyla M, van Hummel A, Chua SW, van der Hoven J, Lee WS, Müller J, Parmar J, Jonquieres GV, Stefen H, Guccione E, Fath T, Housley GD, Klugmann M, Ke YD, Ittner LM (2017) Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun 8(1):473. https://doi.org/10.1038/s41467-017-00618-0
Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101(4):1371–1378
Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71(4):505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, Nicoll JAR (2010) Reduction of aggregated tau in neuronal processes but not in the cell bodies after Aβ42 immunisation in Alzheimer’s disease. Acta Neuropathol 120(1):13–20. https://doi.org/10.1007/s00401-010-0705-y
Boutajangout A, Quartermain D, Sigurdsson EM (2010) Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci 30(49):16559–16566. https://doi.org/10.1523/JNEUROSCI.4363-10.2010
Brady ST, Sperry AO (1995) Biochemical and functional diversity of microtubule motors in the nervous system. Curr Opin Neurobiol 5(5):551–558
Branca C, Shaw DM, Belfiore R, Gokhale V, Shaw AY, Foley C, Smith B, Hulme C, Dunckley T, Meechoovet B, Caccamo A, Oddo S (2017) Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell 16(5):1146–1154. https://doi.org/10.1111/acel.12648
Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan A-ML, Iba M, James MJ, Xie SX, Ballatore C, Smith AB, Lee VM, Trojanowski JQ (2010) Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci 30(41):13861–13866. https://doi.org/10.1523/jneurosci.3059-10.2010
Callahan LM, Vaules WA, Coleman PD (2002) Progressive reduction of synaptophysin message in single neurons in Alzheimer disease. J Neuropathol Exp Neurol 61(5):384–395
Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M (2011) Passive immunization with anti-tau antibodies in two transgenic models. J Biol Chem 286(39):34457–34467. https://doi.org/10.1074/jbc.M111.229633
Chen S, Li B, Grundke-Iqbal I, Iqbal K (2008) I PP2A 1 affects tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. J Biol Chem 283(16):10513–10521. https://doi.org/10.1074/jbc.M709852200
Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L (2005) Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci 25(42):9694–9703. https://doi.org/10.1523/JNEUROSCI.2980-05.2005
Chirita CN, Congdon EE, Yin H, Kuret J (2005) Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry 44(15):5862–5872. https://doi.org/10.1021/bi0500123
Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11(7):909–913. https://doi.org/10.1038/ncb1901
ClinicalTrials.gov (2016) 24 months safety and efficacy study of AADvac1 in patients with mild Alzheimer’s Disease. https://clinicaltrials.gov/ct2/show/results/NCT02579252
Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F (2014) Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer‘s disease. Brain 137(10):2834–2846. https://doi.org/10.1093/brain/awu213
Congdon EE, Gu J, Sait HB, Sigurdsson EM (2013) Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem 288(49):35452–35465. https://doi.org/10.1074/jbc.M113.491001
Coutadeur S, Benyamine H, Delalonde L, de Oliveira C, Leblond B, Foucourt A, Besson T, Casagrande AS, Taverne T, Girard A, Pando MP, Désiré L (2015) A novel DYRK1A (dual specificity tyrosine phosphorylation-regulated kinase 1A) inhibitor for the treatment of Alzheimer’s disease: effect on Tau and amyloid pathologies in vitro. J Neurochem 133(3):440–451. https://doi.org/10.1111/jnc.13018
d’Abramo C, Ricciarelli R, Pronzato MA, Davies P (2006) Troglitazone, a peroxisome proliferator-activated receptor-γ agonist, decreases tau phosphorylation in CHOtau4R cells. J Neurochem 98(4):1068–1077. https://doi.org/10.1111/j.1471-4159.2006.03931.x
Dai C, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, Iqbal K (2015) Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J Neural Transm 122(4):607–617. https://doi.org/10.1007/s00702-014-1315-y
Dai C, Tung YC, Liu F, Gong CX, Iqbal K (2017) Tau passive immunization inhibits not only tau but also Aβ pathology. Alzheimers Res Ther 9(1):1. https://doi.org/10.1186/s13195-016-0227-5
de Bruijn R, Ikram MA (2014) Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med 12(1):130. https://doi.org/10.1186/s12916-014-0130-5
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27(5):457–464. https://doi.org/10.1002/ana.410270502
Delacourte A, Sergeant N, Champain D, Wattez A, Maurage CA, Lebert F, Pasquier F, David J-P (2002) Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer’s disease. Neurology 59(3):398–407
Derisbourg M, Leghay C, Chiappetta G, Fernandez-Gomez F-J, Laurent C, Demeyer D, Carrier S, Buée-Scherrer V, Blum D, Vinh J, Sergeant N, Verdier Y, Buée L, Hamdane M (2015) Role of the tau N-terminal region in microtubule stabilization revealed by new endogenous truncated forms. Sci Rep 5(1):9659. https://doi.org/10.1038/srep09659
Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM (2012) Alzheimer’s disease neuroimaging initiative the ADN. Amyloid-β–associated clinical decline occurs only in the presence of elevated P-tau. Arch Neurol 69(6):709–713. https://doi.org/10.1001/archneurol.2011.3354
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Dorval V, Fraser PE (2006) Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and α-synuclein. J Biol Chem 281(15):9919–9924. https://doi.org/10.1074/jbc.M510127200
Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89(2):297–308
Duan AR, Jonasson EM, Alberico EO, Li C, Scripture JP, Miller RA, Alber MS, Goodson HV (2017) Interactions between tau and different conformations of tubulin: implications for tau function and mechanism. J Mol Biol 429(9):1424–1438. https://doi.org/10.1016/j.jmb.2017.03.018
Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI (2010) Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142(6):857–867. https://doi.org/10.1016/j.cell.2010.08.014
Eriksson UK, Bennet AM, Gatz M, Dickman PW, Pedersen NL (2010) Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Dis Assoc Disord 24(3):1. https://doi.org/10.1097/WAD.0b013e3181d1b99b
Fá M, Puzzo D, Piacentini R, Staniszewski A, Zhang H, Baltrons MA, Lipuma DD, Chatterjee I, Li J, Saeed F, Berman HL, Ripoli C, Gulisano W, Gonzalez J, Tian H, Costa JA, Lopez P, Davidowitz E, Yu WH, Haroutunian V, Brown LM, Palmeri A, Sigurdsson EM, Duff KE, Teich AF, Honig LS, Sierks M, Moe JG, D’Adamio L, Grassi C, Kanaan NM, Fraser PE, Arancio O (2016) Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci Rep 6(1):19393. https://doi.org/10.1038/srep19393
Funk KE, Thomas SN, Schafer KN, Cooper GL, Liao Z, Clark DJ, Yang AJ, Kuret J (2014) Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem J 462(1):77–88. https://doi.org/10.1042/BJ20140372
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci 100(17):10032–10037. https://doi.org/10.1073/pnas.1630428100
García-Sierra F, Wischik CM, Harrington CR, Luna-Muñoz J, Mena R (2001) Accumulation of C-terminally truncated tau protein associated with vulnerability of the perforant pathway in early stages of neurofibrillary pathology in Alzheimer’s disease. J Chem Neuroanat 22(1–2):65–77
Giacobini E, Gold G (2013) Alzheimer disease therapy—moving from amyloid-β to tau. Nat Rev Neurol 9(12):677–686. https://doi.org/10.1038/nrneurol.2013.223
Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60(9):1495–1500
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989a) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3(4):519–526
Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989b) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8(2):393–399
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61(3):921–927
Graham DL, Gray AJ, Joyce JA, Yu D, O’Moore J, Carlson GA, Shearman MS, Dellovade TL, Hering H (2014) Increased O-GlcNAcylation reduces pathological tau without affecting its normal phosphorylation in a mouse model of tauopathy. Neuropharmacology 79:307–313. https://doi.org/10.1016/j.neuropharm.2013.11.025
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83(13):4913–4917
Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI (2006) Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 97(4):1005–1014. https://doi.org/10.1111/j.1471-4159.2006.03784.x
Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165(2):523–531. https://doi.org/10.1016/S0002-9440(10)63317-2
Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH (1992) Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett 147(1):58–62
Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH (2007) Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem 282(32):23645–23654. https://doi.org/10.1074/jbc.M703269200
Harris JA, Koyama A, Maeda S, Ho K, Devidze N, Dubal DB, Yu GQ, Masliah E, Mucke L (2012) Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS ONE 7(9):e45881. https://doi.org/10.1371/journal.pone.0045881
Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, Ihara Y (1992) Protein sequence and mass spectrometric analyses of tau in the Alzheimer’s disease brain. J Biol Chem 267(24):17047–17054
Hastings NB, Wang X, Song L, Butts BD, Grotz D, Hargreaves R, Fred Hess J, Hong KK, Huang CR, Hyde L, Laverty M, Lee J, Levitan D, Lu SX, Maguire M, Mahadomrongkul V, McEachern EJ, Ouyang X, Rosahl TW, Selnick H, Stanton M, Terracina G, Vocadlo DJ, Wang G, Duffy JL, Parker EM, Zhang L (2017) Inhibition of O-GlcNAcase leads to elevation of O-GlcNAc tau and reduction of tauopathy and cerebrospinal fluid tau in rTg4510 mice. Mol Neurodegener 12(1):39. https://doi.org/10.1186/s13024-017-0181-0
Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D (2017) Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology 61:143–187. https://doi.org/10.1016/j.neuro.2017.03.006
Himmler A, Drechsel D, Kirschner MW, Martin DW Jr (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol 9(4):1381–1388
Hochgräfe K, Sydow A, Matenia D, Cadinu D, Könen S, Petrova O, Pickhardt M, Goll P, Morellini F, Mandelkow E, Mandelkow EM (2015) Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human tau. Acta Neuropathol Commun 3(1):25. https://doi.org/10.1186/s40478-015-0204-4
Holmes BB, Diamond MI (2014) Prion-like properties of tau protein: the importance of extracellular tau as a therapeutic target. J Biol Chem 289(29):19855–19861. https://doi.org/10.1074/jbc.R114.549295
Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan L-L, Ashe KH, Liao D (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68(6):1067–1081. https://doi.org/10.1016/j.neuron.2010.11.030
Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24(36):7895–7902. https://doi.org/10.1523/JNEUROSCI.1988-04.2004
Ihara Y, Nukina N, Miura R, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem 99(6):1807–1810
Iqbal K, Grundke-Iqbal I (1991) Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer’s disease. Mol Neurobiol 5(2–4):399–410
Jicha GA, O’Donnell A, Weaver C, Angeletti R, Davies P (1999) Hierarchical phosphorylation of recombinant tau by the paired-helical filament-associated protein kinase is dependent on cyclic AMP-dependent protein kinase. J Neurochem 72(1):214–224
Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Kohler A, Glossmann H, Schneider R, Sutherland C, Schweiger S (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci 107(50):21830–21835. https://doi.org/10.1073/pnas.0912793107
Kim DJ, Martinez-Lemus LA, Davis GE (2013) EB1, p150Glued, and Clasp1 control endothelial tubulogenesis through microtubule assembly, acetylation, and apical polarization. Blood 121(17):3521–3530. https://doi.org/10.1182/blood-2012-11-470179
Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP (2015) Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523(7561):431–436. https://doi.org/10.1038/nature14658
Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M (2014a) First-in-man tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther 6(4):44. https://doi.org/10.1186/alzrt278
Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M (2014b) Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimers Res Ther 6(4):45. https://doi.org/10.1186/alzrt277
Kosik KS, Orecchio LD, Bakalis S, Neve RL (1989) Developmentally regulated expression of specific tau sequences. Neuron 2(4):1389–1397
Ksiezak-Reding H, Liu WK, Yen SH (1992) Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res 597(2):209–219
Kuhla B, Haase C, Flach K, Lüth HJ, Arendt T, Münch G (2007) Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem 282(10):6984–6991. https://doi.org/10.1074/jbc.M609521200
Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muñoz MJ, Jackson GR, Kayed R (2010) Preparation and characterization of neurotoxic tau oligomers. Biochemistry 49(47):10039–10041. https://doi.org/10.1021/bi1016233
Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM (2006) An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci 103(25):9673–9678. https://doi.org/10.1073/pnas.0602913103
Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G (1998) Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci 111(21):3167–3177
Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H (2004) Phosphorylation of tau by Fyn: implications for Alzheimer’s disease. J Neurosci 24(9):2304–2312. https://doi.org/10.1523/JNEUROSCI.4162-03.2004
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BXW, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18(2):291–295. https://doi.org/10.1038/nm.2613
Lei P, Ayton S, Appukuttan AT, Moon S, Duce JA, Volitakis I, Cherny R, Wood SJ, Greenough M, Berger G, Pantelis C, McGorry P, Yung A, Finkelstein DI, Bush AI (2017) Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol Psychiatry 22(3):396–406. https://doi.org/10.1038/mp.2016.96
Li XH, Xie JZ, Jiang X, Lv BL, Cheng XS, Du LL, Zhang JY, Wang JZ, Zhou XW (2012) Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. NeuroMolecular Med 14(4):338–348. https://doi.org/10.1007/s12017-012-8191-0
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259(8):5301–5305
Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS (1995) Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol 128(3):293–306
Liu F, Iqbal K, Grundke-Iqbal I, Gong CX (2002a) Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett 530(1–3):209–214
Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Merkle RK, Gong CX (2002b) Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett 512(1–3):101–106
Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX (2004) O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci 101(29):10804–10809. https://doi.org/10.1073/pnas.0400348101
Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci 26(12):3429. https://doi.org/10.1111/j.1460-9568.2007.05955.x
Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX (2009a) Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 132(7):1820–1832. https://doi.org/10.1093/brain/awp099
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2009b) Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem 111(1):242–249. https://doi.org/10.1111/j.1471-4159.2009.06320.x
Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7(2):e31302. https://doi.org/10.1371/journal.pone.0031302
Liu K, Liu Y, Li L, Qin P, Iqbal J, Deng Y, Qing H (2016) Glycation alter the process of tau phosphorylation to change tau isoforms aggregation property. Biochim Biophys Acta 1862(2):192–201. https://doi.org/10.1016/j.bbadis.2015.12.002
Luna-Muñoz J, García-Sierra F, Falcón V, Menéndez I, Chávez-Macías L, Mena R (2005) Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis 8(1):29–41
Luna-Muñoz J, Chávez-Macías L, García-Sierra F, Mena R (2007) Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J Alzheimers Dis 12(4):365–375
Luo HB, Xia YY, Shu XJ, Liu ZC, Feng Y, Liu XH, Yu G, Yin G, Xiong YS, Zeng K, Jiang J, Ye K, Wang XC, Wang JZ (2014) SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc Natl Acad Sci 111(46):16586–16591. https://doi.org/10.1073/pnas.1417548111
Malpas CB, Vivash L, Genc S, Saling MM, Desmond P, Steward C, Hicks RJ, Callahan J, Brodtmann A, Collins S, Macfarlane S, Corcoran NM, Hovens CM, Velakoulis D, O’Brien TJ (2016) A phase IIa randomized control trial of VEL015 (sodium selenate) in mild-moderate Alzheimer’s disease. J Alzheimers Dis 54(1):223–232. https://doi.org/10.3233/JAD-160544
Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, Lamb BT, Bhaskar K (2015) Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138(6):1738–1755. https://doi.org/10.1093/brain/awv081
Maphis N, Jiang S, Xu G, Kokiko-Cochran ON, Roy SM, Van Eldik LJ, Watterson DM, Lamb BT, Bhaskar K (2016) Selective suppression of the α isoform of p38 MAPK rescues late-stage tau pathology. Alzheimers Res Ther 8(1):54. https://doi.org/10.1186/s13195-016-0221-y
Masliah E, Terry RD, DeTeresa RM, Hansen LA (1989) Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett 103(2):234–239
Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD (1993) Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43(1):192–197
Mershin A, Pavlopoulos E, Fitch O, Braden BC, Nanopoulos DV, Skoulakis EM (2004) Learning and memory deficits upon tau accumulation in Drosophila mushroom body neurons. Learn Mem 11(3):277–287. https://doi.org/10.1101/lm.70804
Miller BW, Willett KC, Desilets AR (2011) Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann Pharmacother 45(11):1416–1424. https://doi.org/10.1345/aph.1Q238
Mondragón-Rodríguez S, Basurto-Islas G, Santa-Maria I, Mena R, Binder LI, Avila J, Smith MA, Perry G, García-Sierra F (2008) Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer’s disease. Int J Exp Pathol 89(2):81–90. https://doi.org/10.1111/j.1365-2613.2007.00568.x
Morimoto BH, Schmechel D, Hirman J, Blackwell A, Keith J, Gold M (2013) AL-108-211 study. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement Geriatr Cogn Disord 35(5–6):325–339. https://doi.org/10.1159/000348347
Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT (2004) Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 110(8):962–968. https://doi.org/10.1161/01.CIR.0000140667.37070.07
Novak M, Kabat J, Wischik CM (1993) Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J 12(1):365–370
Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, Vince-Kazmerova Z, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Winblad B, Novak M (2017) Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16(2):123–134. https://doi.org/10.1016/S1474-4422(16)30331-3
Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, Kaufman AC, Rosenberg BJ, Sekine-Konno T, Varma P, Chen K, Koleske AJ, Reiman EM, Strittmatter SM, van Dyck CH (2015) A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther 7(1):35. https://doi.org/10.1186/s13195-015-0119-0
Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM (2004) Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43(3):321–332. https://doi.org/10.1016/j.neuron.2004.07.003
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53(9):1937–1942
Patterson KR, Remmers C, Fu Y, Brooker S, Kanaan NM, Vana L, Ward S, Reyes JF, Philibert K, Glucksman MJ, Binder LI (2011) Characterization of prefibrillar tau oligomers in vitro and in Alzheimer disease. J Biol Chem 286(26):23063–23076. https://doi.org/10.1074/jbc.M111.237974
Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR (2006) Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol 199(2):265–273. https://doi.org/10.1016/j.expneurol.2006.01.018
Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13(7):703–714. https://doi.org/10.1093/hmg/ddh083
Prince M, Wimo A, Guerchet M, Ali G-C, Wu YT, Prina M, Yee CK, Xia Z (2015) World Alzheimer report 2015 the global impact of dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London
Rasool S, Martinez-Coria H, Wu JW, LaFerla F, Glabe CG (2013) Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Aβ deposition and tau pathology in 3xTg-AD mice. J Neurochem 126(4):473–482. https://doi.org/10.1111/jnc.12305
Reynolds MR, Berry RW, Binder LI (2005) Site-specific nitration differentially influences τ assembly in vitro. Biochemistry 44(42):13997–13999. https://doi.org/10.1021/bi051028w
Reynolds MR, Reyes JF, Fu Y, Bigio EH, Guillozet-Bongaarts AL, Berry RW, Binder LI (2006) Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tauopathies. J Neurosci 26(42):10636–10645. https://doi.org/10.1523/JNEUROSCI.2143-06.2006
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114(1):121–130. https://doi.org/10.1172/JCI20640
Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu G-Q, Palop JJ, Noebels JL, Mucke L (2011) Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci 31(2):700–711. https://doi.org/10.1523/JNEUROSCI.4152-10.2011
Roy SM, Grum-Tokars VL, Schavocky JP, Saeed F, Staniszewski A, Teich AF, Arancio O, Bachstetter AD, Webster SJ, Van Eldik LJ, Minasov G, Anderson WF, Pelletier JC, Watterson DM (2015) Targeting human central nervous system protein kinases: an isoform selective p38αMAPK inhibitor that attenuates disease progression in Alzheimer’s disease mouse models. ACS Chem Neurosci 6(4):666–680. https://doi.org/10.1021/acschemneuro.5b00002
Sahara N, Maeda S, Murayama M, Suzuki T, Dohmae N, Yen SH, Takashima A (2007) Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur J Neurosci 25(10):3020–3029. https://doi.org/10.1111/j.1460-9568.2007.05555.x
Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP, Hyman BT (2010) Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain 133(5):1312–1327. https://doi.org/10.1093/brain/awq056
Shimura H, Schwartz D, Gygi SP, Kosik KS (2004) CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 279(6):4869–4876. https://doi.org/10.1074/jbc.M305838200
Shirazi SK, Wood JG (1993) The protein tyrosine kinase, fyn, in Alzheimer’s disease pathology. Neuroreport 4(4):435–437
Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H, Gozes I (2009) NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis 34(2):381–388. https://doi.org/10.1016/j.nbd.2009.02.011
Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL (2004) Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol 63(10):1080–1091
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Am J Pathol 155(5):1459–1466. https://doi.org/10.1016/S0002-9440(10)65460-0
Stanford PM, Shepherd CE, Halliday GM, Brooks WS, Schofield PW, Brodaty H, Martins RN, Kwok JBJ, Schofield PR (2003) Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain 126(4):814–826
Sultan A, Nesslany F, Violet M, Bégard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buée L, Galas MC (2011) Nuclear tau, a key player in neuronal DNA protection. J Biol Chem 286(6):4566–4575. https://doi.org/10.1074/jbc.M110.199976
Tai HC, Wang BY, Serrano-Pozo A, Frosch MP, Spires-Jones TL, Hyman BT (2014) Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol Commun 2(1):146. https://doi.org/10.1186/s40478-014-0146-2
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580. https://doi.org/10.1002/ana.410300410
Tuo Q, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, Wang Q, Crouch PJ, Ganio K, Wang XC, Pei L, Adlard PA, Lu YM, Cappai R, Wang JZ, Liu R, Bush AI (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Gotz J, Ittner LM (2010) Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci 107(31):13888–13893. https://doi.org/10.1073/pnas.1009038107
Wang JZ, Grundke-Iqbal I, Iqbal K (1996) Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat Med 2(8):871–875
Wang JZ, Grundke-Iqbal I, Iqbal K (2007) Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 25(1):59–68. https://doi.org/10.1111/j.1460-9568.2006.05226.x
Wang J, Gu BJ, Masters CL, Wang YJ (2017) A systemic view of Alzheimer disease—insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13(11):703. https://doi.org/10.1038/nrneurol.2017.147
Watterson DM, Grum-Tokars VL, Roy SM, Schavocky JP, Bradaric BD, Bachstetter AD, Xing B, Dimayuga E, Saeed F, Zhang H, Staniszewski A, Pelletier JC, Minasov G, Anderson WF, Arancio O, Van Eldik LJ (2013) Development of novel in vivo chemical probes to address CNS protein kinase involvement in synaptic dysfunction. PLoS ONE 8(6):e66226. https://doi.org/10.1371/journal.pone.0066226
Williams DW, Tyrer M, Shepherd D (2000) Tau and tau reporters disrupt central projections of sensory neurons in Drosophila. J Comp Neurol 428(4):630–640
Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA (1988) Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85(13):4884–4888
Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR (1996) Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci USA 93(20):11213–11218
Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JMD, Kook KA, Harrington CR (2015) Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis 44(2):705–720. https://doi.org/10.3233/JAD-142874
Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293(5530):711–714. https://doi.org/10.1126/science.1062382
Wood JG, Zinsmeister P (1991) Tyrosine phosphorylation systems in Alzheimer’s disease pathology. Neurosci Lett 121(1–2):12–16
Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, Zhang L, Dai C, Gu J, Gong CX, Iqbal K, Liu F (2016) O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell 15(3):455–464. https://doi.org/10.1111/acel.12449
Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA (1994) Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA 91(16):7787–7791
Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P (1995) Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med 1(7):693–699
Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM (2013) Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80(2):402–414. https://doi.org/10.1016/j.neuron.2013.07.046
Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, Vocadlo DJ (2012) Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol 8(4):393–399. https://doi.org/10.1038/nchembio.797
Zhang YJ, Xu YF, Chen XQ, Wang XC, Wang JZ (2005) Nitration and oligomerization of tau induced by peroxynitrite inhibit its microtubule-binding activity. FEBS Lett 579(11):2421–2427. https://doi.org/10.1016/j.febslet.2005.03.041
Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, Lee VM, Brunden KR (2012) The microtubule-stabilizing agent, Epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 32(11):3601–3611. https://doi.org/10.1523/JNEUROSCI.4922-11.2012
Zhang X, Hernandez I, Rei D, Mair W, Laha JK, Cornwell ME, Cuny GD, Tsai LH, Steen JAJ, Kosik KS (2013) Diaminothiazoles modify tau phosphorylation and improve the tauopathy in mouse models. J Biol Chem 288(30):22042–22056. https://doi.org/10.1074/jbc.M112.436402
Author information
Authors and Affiliations
Contributions
FPC wrote the manuscript. KYN and RYK critically reviewed the manuscript. SMC formulated the entire concept and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Chong, F.P., Ng, K.Y., Koh, R.Y. et al. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell Mol Neurobiol 38, 965–980 (2018). https://doi.org/10.1007/s10571-017-0574-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0574-1