Abstract
Prunus domestica L. cv. Ximei fruit perishes quickly due to intense metabolic activity after being harvested. To prolong shelf life and maintain fruit quality, the effects of 1-methylcyclopropene (1-MCP) treatment on P. domestica fruit during storage at 4 ± 1 °C were investigated. The results showed that the soluble solid content (SSC), respiratory rate (29.8%), ethylene production (27.2%), anthocyanin content, malonaldehyde content (MDA), hydrogen peroxide content (H2O2), and superoxide anion activity (O2·−) of P. domestica fruit were all significantly reduced by 1-MCP treatment (1.0 µL L−1), while the content of ascorbic acid and total phenol, and the activity of SUPEROXIDE DISMUTASE (SOD, 61.3%), CATALASE (CAT, 39.0%), ASCORBATE PEROXIDASE (APX, 23.7%), and PEROXIDASE (POD, 38.0%) increased compared to untreated fruit after 35 days of cold storage. Overall, 1-MCP treatment could maintain high postharvest quality and antioxidant activity in P. domestica fruit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Prunus domestica L. cv. Ximei (Rosaceae), a plant native to Southwest France, is widely cultivated in Western China. P. domestica fruit does not contain fat or cholesterol, but is rich in bioactive compounds such as phenols, anthocyanins, vitamins, minerals, and trace elements. The bioactive compounds in P. domestica fruit possess pharmacological effects that benefit human health such as immunity enhancement, antioxidation, anti-aging, vascular sclerosis prevention, and constipation relief (Smith et al. 2014). For these reasons, P. domestica fruit has been gaining commercial popularity in recent years. However, P. domestica fruits deteriorate quickly after harvest and are susceptible to mechanical damage and microbial infection due to their fragile structure and high sugar content. Therefore, the abundant supply of P. domestica fruit during its short harvest season can lead to significant economic losses due to spoilage. In order to extend shelf life and abate these losses, semi-mature P. domestica fruit are harvested and held in cold storage before being sent to market. However, once removed from cold storage and brought to room temperature, the fruit softens rapidly and becomes inedible, which can also lead to economic losses (Fan et al. 2018). Therefore, exploring more effective storage methods to prolong the shelf life of P. domestica fruit has attracted attention from researchers worldwide.
1-Methylcyclopropene (1-MCP), as an ethylene receptor inhibitor that suppresses the expression of ethylene biosynthesis-related genes (such as ACO and ETR) in some respiratory climacteric fruit by competitively binding to ethylene receptors, delaying fruit ripening and senescence (Cheemaa et al. 2013). In addition, studies have revealed that 1-MCP not only suppresses the ethylene production and respiration rates, it also delays softening to improve postharvest quality in kiwifruit (Xu et al. 2019), winter jujube (Cheng et al. 2020), durian (Thongkum et al. 2018), plum (Lin et al. 2018), and pear (Escribano et al. 2017). Lien et al. (2016) reported that apricots treated with 1-MCP could be stored for 6 weeks at 1 °C. However, the effect of 1-MCP on suppressing ethylene synthesis in postharvest fruit depends on several factors, including fruit variety (Pan et al. 2016), harvest maturity (Rupavatharam et al. 2015), 1-MCP concentration (Cheng et al. 2019), fumigation time, and storage conditions (Xu et al. 2020). 1-MCP is generally applied in the form of tablets or powder, but these application methods make it difficult to accurately control concentrations (Chen et al. 2015, 2016; Lin et al. 2018). A recent study found that 1-MCP infused paper, one of several upgraded 1-MCP products (Lytone Enterprise, Inc., Taipei, China), can enhance 1-MCP stability thanks to a special embedding method (Chen et al. 2015). Chen et al. (2016) showed that 1-MCP paper treatment delayed the softening process of “Huanghua” pear. Cheng et al. (2020) applied 1-MCP paper to the storage of winter jujube, finding that it could effectively maintain the firmness of winter jujube, reduce the rate of weight loss, and extend shelf life.
Although several studies have investigated the role of 1-MCP during postharvest storage, several questions remain regarding how paper containing 1-MCP maintains the content of non-enzymatic antioxidants and the activity of antioxidant enzymes in P. domestica fruit at low temperatures. Therefore, considering the importance of P. domestica fruit in commercial agriculture and its perishability, the objectives of this study were (1) to evaluate changes in non-enzymatic antioxidants (ascorbic acid, total phenols, and anthocyanin) in P. domestica fruit during postharvest storage; (2) to investigate changes in the content of reactive oxygen species (H2O2 and O2·−) and the activity of antioxidant enzymes (SUPEROXIDE DISMUTASE [SOD], CATALASE [CAT], ASCORBATE PEROXIDASE [APX], and PEROXIDASE [POD]) during postharvest storage; and (3) to clarify the correlations between non-enzymatic antioxidants, reactive oxygen species, and antioxidant enzyme activities.
2 Materials and methods
2.1 Plant materials and storage conditions
P. domestica fruit was harvested from an orchard in Qiemo County, Korla City, Xinjiang Province, China (38°13'N, 85°53'E) on September 5, 2020 and immediately taken to the lab in Shihezi University. Fruit with similar maturity (firmness: about 3.5 N, soluble solid content [SSC]: about 24.2%), size, uniform color, and without mechanical damage or surface defects were selected for experiments. The selected fruit was randomly divided into 4 groups (7 baskets per group, 2.8 kg per basket), including (1) control group: fruit were put directly into breathable microporous fresh-keeping bags without any treatment; (2) 0.5 µL L−1 1-MCP treatment; (3) 1.0 µL L−1 1-MCP treatment; and (4) 1.5 µL L−1 1-MCP treatment. The four groups were pre-cooled for 24 h at 4 °C. All fruit was stored in breathable microporous fresh-keeping bags at 4 °C with relative humidity of 85–90% for 35 d. During storage, the physiological quality and antioxidant activity were measured every 7 d. One hundred and fifty fruits were selected from each group and used for measurement. One half of each fruit sample was used immediately for the determination of physiological indices, and the other half was frozen in liquid nitrogen at − 80 °C for the determination of enzymatic activity. All experiments were done in triplicate, and the results were averaged.
2.2 1-MCP paper treatment
1-MCP paper (AnsiP-S) was purchased from Taiwan Litong Co., Ltd., China, being 25 × 20 cm in size. The concentrations of 1-MCP including 0.5 µL L−1, 1.0 µL L−1, and 1.5 µL L−1 were determined according to the size of the paper, and a small amount of distilled water was sprayed on the paper to release 1-MCP gas into a 1 m3 airtight container. The P. domestica fruit was fumigated for 24 h in the airtight chamber with an ambient temperature of 4 °C, and then stored at 4 °C for further analysis.
2.3 Determination of weight loss rate, firmness, SSC, TA, respiration rate, and ethylene production
Fresh P. domestica fruit (1.0 kg) was taken from each group to determine the rate of weight loss. The fresh samples were weighed before cold storage (initial weight) and at each sampling time (final weight). The calculation formula was as follows: Weight loss rate (%) = [(initial weight − final weight)/initial weight] × 100%.
Firmness (N) was determined by using a durometer (GY-B, Yueqing Aidebao Instrument Co., Ltd., China) with a 3.5-mm probe. Six fruits were selected from each group, and two spots on opposite sides of the fruit equator were peeled off to measure the firmness of each fruit.
SSC (%) was determined by using a portable refractometer (LB90T, Guangzhou Suwei Electronic Technology Co., Ltd., China). Five fruits were measured for each group.
Titratable acidity (TA) content was determined by titration with 0.1 mol L−1 of sodium hydroxide. Six fruits were selected from each group for analysis. The results were expressed as% malic acid.
Respiratory rate was measured with a respiration tester (FS-3080A, Shijiazhuang Fanseng Technology Co., Ltd., China) according to the instructions. Fruit (1.0 kg) was put into a response breathing chamber with a volume of 1 L and sealed at 25 °C. The measurement was performed every 15 min. The results were expressed as ng kg−1 s−1.
P. domestica fruit (600 g) was sealed in a 1 L glass container and placed at 25 °C for 1 h. Then, 1 mL of headspace gas was collected from the glass container and injected into a gas chromatograph equipped with a hydrogen flame ionization detector (FID) and a stainless-steel column (30 m × 0.25 mm × 0.5 µm) (7890B, Agilent, USA). The carrier gas was N2, the fuel gas was H2, the combustion-supporting gas was air, the column temperature was 200 °C, and the detector temperature was 280 °C. Ethylene production was identified by standard peak time and quantified by standard curve. The results were expressed as μL kg−1 s−1 (Cai et al. 2019).
2.4 Determination of color difference, ascorbic acid, total phenol, and anthocyanin
A color difference meter (YS3060, Shenzhen Sanenshi Technology Co., Ltd., China) was used to measure the color parameters including L* (brightness), a* (− green, + red), and b* (− blue, + yellow) of flesh pulp at each sampling time, where L0, a0, and b0 are the values obtained before cold storage. Six fruits were taken from each group for color analysis. After peeling (1 mm), three spots one each fruit were randomly selected for measurement and the color difference was expressed as ΔE.
The content of ascorbic acid was determined according to Yang et al. (2021). Oxalic acid solution (10 mL of 2%) was mixed with 10 g of fruit pulp, ground into a homogenate in an ice bath, and transferred to a volumetric flask with 2% oxalic acid solution to make the volume 100 mL. After 10 min, it was filtered and the filtrate was collected. Then, 10 mL of filtrate was dripped with 2, 6-dichlorophenol indophenol solution. When the solution became reddish and did not fade within 15 s, the amount of dye was recorded. The results were expressed as g kg−1.
To assess total phenol and anthocyanin content, fruit pulp was ground into powder with liquid nitrogen. About 2.0 g of pulp powder was extracted with 1% HCl methanol solution for 20 min under dark conditions at 4 °C. After that, the filtrate was taken and the absorbance value was determined using a spectrophotometer (UV-2600, Shimazu Instruments Co., Ltd., China). The total phenol content was calculated based on the absorbance value at 280 nm combined with the standard curve of gallic acid, and the result was expressed as g kg−1. The anthocyanin content was expressed as A(530–600) kg−1 (Khaleghnezhad et al. 2019).
2.5 Determination of MDA, O 2 ·− , and H 2 O 2 content
Pulp tissues (0.5 g) were mixed with 2 mL of phosphate buffer solution (0.1 mol L−1; pH = 7.4), homogenized in an ice bath, and centrifuged at 4000 × g for 10 min. The supernatant was collected and the content of malonaldehyde (MDA), O2·−, and H2O2 were determined.
The content of MDA in homogenized pulp was determined according to the reaction characteristics of thiobarbituric acid (TBA) using the MDA test kit (A003, Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). The red product of the reaction was quantified at 532 nm with a spectrophotometer (UV-2600, Shimadzu Instruments Co., Ltd., China). Other operations were carried out in accordance with the instructions (Zhu et al. 2014). The results were expressed as mmol kg−1 pro.
The content of O2·− was determined using the O2·− test kit (A052; Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). O2·− is produced from the reaction of xanthine and xanthine oxidase in fruit, and a color reagent was added to make it become purplish red. The absorption value at 550 nm was determined using a spectrophotometer (UV-2600, Shimadzu Instruments Co., Ltd., China) with vitamin C as the standard, and the O2·− activity was calculated. The results were expressed as U kg−1 pro (Zhu et al. 2014).
The content of H2O2 in fruit pulp was determined using the H2O2 test kit (A064, Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). The H2O2 reacted with molybdic acid and then the amount of product was measured by spectrophotometer (UV-2600, Shimadzu Instruments Co., Ltd., China) at 405 nm, after which the H2O2 content was calculated. The results were expressed as mol kg−1 pro (Tiryaki et al. 2019).
The content of MDA, O2·− and H2O2 were expressed in protein units. Protein content was determined by using a protein test kit (A045, Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China) based on the Coomassie Brilliant Blue method.
2.6 Determination of antioxidant enzyme activities
The SOD activity in P. domestica fruit was determined by using a superoxide dismutase assay kit (Beijing Solarbio Science and Technology co., Ltd., China) based on the xanthine oxidase assay (Zhang et al. 2014). O2·− was produced from the xanthine-xanthine oxidase reaction system and reacted with a chromogenic agent to make it blue. The absorbance was measured at 560 nm. This determination was performed three times to obtain the average value.
The CAT activity was determined following the method of Zhang et al. (2015). The absorbance of the reaction mixture (20 mmol L−1, 2.9 mL H2O2, and 0.1 mL enzyme extract) was measured at 240 nm. The amount of enzyme required to reduce the absorbance value by 0.01 per minute was defined as a unit of catalase activity.
The APX activity was determined following the method of Chu et al. (2018). Fruit pulp (5 g) was transferred into a precooled mortar, and then 5 mL of extraction buffer (containing 0.1 mmol L−1 EDTA, 1 mmol L−1 ascorbic acid, and 2% PVPP) was added. The APX activity was determined after an incubation at 4 °C and centrifugation at 8000 × g for 30 min.
The POD activity was determined following the method of Zhang et al. (2015). The data were recorded every one min.
2.7 Statistical analysis
Data were analyzed by one-way ANOVA at the significance level of P < 0.05 using SPSS software (version 25.0, SPSS Inc., Chicago, IL, USA). OriginPro software (version 2020b, Origin Lab Co., Massachusetts, USA) was used for plotting. Pearson correlation tests were performed to analyze the correlations between physicochemical indexes and antioxidant enzyme activity using SPSS software (version 25.0, SPSS Inc., Chicago, IL, USA).
3 Results
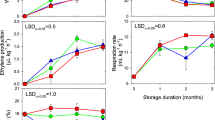
3.1 1-MCP treatments positively affected firmness, SSC, TA, and negatively affected weight loss rate, respiration rate, and ethylene production of P. domestica fruit
The weight loss rate of P. domestica fruit increased continuously during cold storage. However, the weight loss rate of the 1-MCP groups were lower than that of the control group (Fig. 1A). At the end of storage, the weight loss rate of fruit of 0.5 and 1.5 µL L−1 1-MCP groups were 15.1% and 14.0% lower than that of the control group, respectively, but there was no difference between the two groups (P > 0.05). The 1.0 µL L−1 1-MCP group had the lowest weight loss rate (37.6% lower than control).
Fruit firmness decreased slowly at the early stage of storage and then the rate accelerated. The fruit firmness of the 1.0 µL L−1 1-MCP group was 22.4%, 9.9%, and 7.2% higher than that of the control on day 21, 28, and 35 (P < 0.05), respectively. However, a difference between the 1.5 µL L−1 1-MCP group and the control was only found on day 21. The fruit firmness of 0.5 and 1.5 µL L−1 1-MCP groups were 2.6% and 0.7% higher than that of the control, respectively, at the end of storage (Fig. 1B).
The SSC first increased, and then decreased after reaching a peak on day 14 (Fig. 1C). The changes in SSC of the 1-MCP groups showed the same trend, but the peak value occurred later in the control. At the end of storage, the SSC of 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 5.5%, 10.3%, and 7.1% higher than that of the control (P > 0.05), respectively. Among them, the SSC of the 1.0 µL L−1 1-MCP group was the highest.
The change in TA content of P. domestica fruit during storage is shown in Fig. 1D. There were no differences in the content of TA between the 1-MCP groups and the control (P > 0.05).
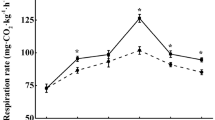
At the early stage of storage, the respiratory rate of P. domestica fruit in the control group increased rapidly and peaked on day 14 (4277.8 ng kg−1 s−1) (Fig. 1E). The peak respiration rate of the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 26.1%, 29.8%, and 17.6% lower than that of the control, respectively (P < 0.05).
Ethylene production in postharvest P. domestica fruit in all groups increased rapidly until day 21 and then decreased (Fig. 1F). The three 1-MCP treatments suppressed ethylene production in P. domestica fruit during storage. On day 21, the peak values of 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 16.3%, 27.0%, and 22.1% lower than that of the control, respectively (P < 0.05). The 1.0 µL L−1 1-MCP group had the lowest ethylene production.
3.2 1-MCP treatments positively affected total phenol, and negatively affected color difference, ascorbic acid, and anthocyanin content of P. domestica fruit
During storage, the peel color of P. domestica fruit changed from light purple to dark purple, and the flesh color also changed. The ΔE of flesh tissue increased with prolonged storage time (Fig. 2A). At the end of storage, the ΔE value of the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 26.9%, 33.7%, and 25.6% lower than that of the control, respectively (P < 0.05).
During the whole storage process, the content of ascorbic acid continued to decrease. At the end of storage, the content of ascorbic acid of the 0.5, 1.0 and 1.5 µL L−1 1-MCP groups were 3.9%, 13.3% (P < 0.05), and 1.8% higher than that of the control group, respectively (Fig. 2B).
The total phenol content increased rapidly at the early stage of storage, and then decreased slowly. From day 21 to day 35, the total phenol content of 1-MCP groups were higher than that of the control. At the end of storage, the total phenol content of the 1.0 and 1.5 µL L−1 1-MCP treatment groups were 2.7% and 2.5% higher than that of the control, respectively (Fig. 2C).
Anthocyanin content showed the same trend as ΔE. At the end of storage, the anthocyanin content of the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 11.9%, 37.9%, and 27.0% lower than that of the control group (P < 0.05), respectively (Fig. 2D).
3.3 1-MCP treatments negatively affected MDA content, H 2 O 2 content, and O 2 ·− production rate of P. domestica fruit
The content of MDA in fruit from the control group increased continuously during cold storage, especially on day 7 and 35. At the end of storage, the MDA content of 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 46.9%, 51.2%, and 48.6% lower than that of the control group (P < 0.05), respectively (Fig. 3A).
The trends of H2O2 and O2·− content were similar to that of MDA content (Fig. 3B and C). The content of H2O2 and O2·− of the 1-MCP groups were always lower than that of the control group during storage. The trend of H2O2 content in the 0.5 and 1.5 µL L−1 1-MCP groups were similar, and the trend of O2·− production in the 1.0 and 1.5 µL L−1 1-MCP groups were similar. At the end of storage, the content of H2O2 and O2·− in the 1.0 µL L−1 1-MCP group was the lowest, being 41.2% and 39.7% lower than that of the control group (H2O2 = 535.4 mol kg−1 pro, O2·− = 1.6 U kg−1 pro), respectively (P < 0.05).
3.4 1-MCP treatments positively affected SOD, CAT, APX, and POD activity in P. domestica fruit
1-MCP treatments significantly altered the activities of several active oxygen scavenging enzymes in P. domestica fruit during storage at 4 °C, stimulating a rapid increase in SOD activity until day 14. On day 14, the SOD activity of the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 35.8%, 61.3%, and 39.0% higher than that of the control group, respectively (P < 0.05) (Fig. 4A).
The CAT activity increased sharply until day 14, and then decreased (Fig. 4B). The changes in CAT activity for the 1-MCP groups and the control group were similar. The CAT activity in the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 13.0%, 39.0%, and 29.9% higher than that of the control group on day 14, respectively (P < 0.05). At the end of storage, CAT activity in the 1.0 µL L−1 1-MCP group was 2.5 times higher than that of the control group (P < 0.05).
The trend of APX activity was similar to that of SOD activity (Fig. 4C). On day 14, the peak APX activity of 0.5 and 1.0 µL L−1 1-MCP groups were 22.3% and 23.7% higher than that of the control group, respectively (P < 0.05).
The POD activity increased slowly until day 21, and then stabilized. The POD activity of the 1-MCP groups, especially 1.0 µL L−1 1-MCP group, were higher than that of the control group. On day 21, the POD activity of the 0.5, 1.0, and 1.5 µL L−1 1-MCP groups were 25.7%, 38.0%, and 24.9% higher than that of the control group (P < 0.05), respectively (Fig. 4D).
3.5 Correlation analysis
Person correlation analysis showed that fruit firmness was negatively correlated with weight loss rate (r = − 0.961, P < 0.01), and positively correlated with respiration rate (r = 0.853, P < 0.05). ΔE was positively correlated with anthocyanin content (r = 0.962, P < 0.01). MDA content was positively correlated with H2O2 (r = 0.970, P < 0.01) and O2·− (r = 0.960, P < 0.01). Respiration rate was negatively correlated with H2O2 (r = − 0.921, P < 0.01) and O2·− (r = − 0.851, P < 0.05). The MDA, H2O2, and O2− contents were negatively correlated with SOD, CAT, and APX. The content of ascorbic acid was negatively correlated with H2O2 (r = − 0.995, P < 0.01) and O2·− (r = − 0.885, P < 0.05), while SOD activity was positively correlated with CAT activity (r = 0.961, P < 0.01) and APX activity (r = 0.945, P < 0.01) (Fig. 5).
Correlation analysis of antioxidant enzyme activity and physicochemical parameters of P. domestica fruit treated with 1.0 µL L−1 1-MCP paper under low temperature storage. The correlation coefficients are proportional to numerical size and color intensity. Positive correlations are displayed in red and negative correlations in blue
4 Discussion
Water loss during fruit storage is primarily caused by metabolic activities such as respiration. In this study, fruit firmness was negatively correlated with weight loss rate, and positively correlated with respiration rate. Therefore, it could be speculated that moisture loss from the fruit surface increases along with storage time, resulting in a continuous loss of weight. Therefore, the difference in weight loss rate of fruit in the 1-MCP groups may be due to the 1-MCP treatment reducing oxidative damage to the fruit and slowing down oxidation of the cell membrane (Habibi and Ramezanian 2017), which could inhibit water loss and maintain freshness during long-term storage. This is consistent with the results of a previous study on pears (Escribano et al. 2017). The firmness of fruit is one of the important indexes for evaluating fruit quality. It affects not only the texture but also the storage time and shelf life of fruit. In this study, the 1-MCP treatments maintained a higher firmness of P. domestica fruit (P < 0.05). Chen et al. (2015) found that 1-MCP treatment (0.9 µL L−1) could prolong the storage period of “Huanghua” pears and improve fruit firmness. Thongkum et al. (2018) found that1-MCP treatment (500 µL L−1) could slow down the decreasing firmness of durian pulp. The differences in 1-MCP concentrations for delaying postharvest fruit ripening between our study and others’ is possibly due to differences in fruit variety. For instance, it has been reported that the most effective concentration of 1-MCP was 1.2 µL L−1 in “Younai” plum (Lin et al. 2018) but 0.9 µL L−1 in kiwifruit (Xu et al. 2019).
In this study, 1-MCP treatments effectively delayed the reduction of SSC and TA during cold storage. This may be because 1-MCP treatments suppress the catabolism and respiration of fruit (Cheng et al. 2020). A previous study showed that the increase in SSC at the early stage of storage was related to starch hydrolysis, while the later decrease in SSC was due to a decreased respiration rate and increased metabolic rate (Petriccione et al. 2015). The gradual decrease of TA content in postharvest fruit could be attributed to the respiration and metabolic activities of organic acids in fruit (Habibi and Ramezanian 2017). However, in this study, the 1-MCP treatments significantly delayed the decrease in TA content during storage (Fig. 1D). Agehara et al. (2018) optimized the concentration of 1-MCP and soaking time, finding that 10 mg L−1 1-MCP soaking for 0.5 min improved the SSC of melon. Cheng et al. (2019) treated “Yali” pears with 0.25, 0.50, and 1.0 µL L−1 1-MCP, and found that the effect of 1.0 µL L−1 1-MCP treatment on maintaining the firmness, SSC, and TA content of fruit was greatest. The poor effect of low-concentration 1-MCP treatment in our study may be due to the low concentration not being enough to allow full binding with ethylene receptors. Previous studies showed that storage life of postharvest fruit was related to high respiratory rate (Ozturk et al. 2021). Xu et al. (2019) found that 1-MCP treatments could suppress the respiration rate of fruit during long-term cold storage. Ethylene is a hormone that is necessary for the ripening of climacteric fruit through the conversion of starch into monosaccharides (Thongkum et al. 2018). However, 1-MCP can inhibit ethylene production to delay fruit ripening and senescence (Thongkum et al. 2018). A previous study showed that 1000 nL L−1 1-MCP treatment was the most effective in suppressing ethylene production in green tomato (Sabir and Agar 2011).
Fan et al. (2018) found that 1.0 µL L−1 1-MCP treatment could suppress respiration rate, ethylene production, firmness decrease, and SSC increase while extending the shelf life of apricots. Our study obtained similar results. In a certain concentration range, the effects of 1-MCP treatment could be enhanced by increasing the concentration. However, high concentration of 1-MCP treatment may accelerate senescence due to interference with the defense system of plant tissues, either inhibiting some favorable metabolic responses or stimulating some unfavorable metabolic responses (Ma et al. 2019). Ku et al. (1999) found that high concentration of 1-MCP treatment could promote the ripening and decay of strawberry while increasing the occurrence of diseases and insect pests. Therefore, appropriate 1-MCP concentrations should be adopted for different fruit types to delay ripening and senescence. Another previous study found that ethylene production reached its peak when climacteric fruit was transferred from cold storage to room temperature (Fan et al. 2018). The increase in ethylene production accelerates fruit softening. However, Hanxu et al. (2016) found that 1-MCP treatment could inhibit this softening in plum fruits during storage. The effect of 1-MCP treatment on the shelf life and softening of P. domestica fruit transferred to room temperature after cold storage will be further analyzed in subsequent experiments.
ΔE reflects the change of fruit color. The positive correlation between ΔE and anthocyanin content indicates that the change in fruit color may be related to the accumulation of anthocyanins (Giménez et al. 2017). As important antioxidants, ascorbic acid, total phenols, and anthocyanins reflect the antioxidant capacity of P. domestica fruit. In our study, the 1-MCP treatments, especially the 1.0 µL L−1 1-MCP treatment, delayed the color change in P. domestica fruit flesh and the oxidation of anthocyanins, also slowing down the decrease in total phenols and ascorbic acid content. This is consistent with the results of Liu et al. (2019) and Ma et al. (2019). It suggests that 1-MCP treatment could suppress metabolic activity and ultimately delay fruit ripening. Ozturk et al. (2021) found that a high concentration of 1-MCP (1000 nL L−1) could suppress the production of ethylene-promoting PAL to disrupt the biosynthesis of phenolic substances and reduce their content. This is similar to the results of our study. We found that the effect of 1.5 µL L−1 1-MCP treatment on the production of ethylene was weaker than that of 1.0 µL L−1 1-MCP treatment. Moreover, Habibi et al. (2020) found that the decrease in ascorbic acid content during storage may be caused by endogenous oxidation under the action of various enzymes such as APX and POD. Baswal et al. (2020) found that 1.5 µL L−1 1-MCP treatment could significantly delay the decrease in ascorbic acid content in 'Kinnow' mandarin fruit during storage. Ozturk et al. (2021) also found that 1000 nL L−1 1-MCP treatment could maintain a high ascorbic acid content in jujube. Our study results are consistent with their results.
Excessive reactive oxygen species (ROS) such as H2O2 and O2·− are produced due to environmental stress during cold storage, which causes oxidative damage to the cell membrane, loss of membrane integrity and functionality, fruit senescence, and quality loss. Moreover, excessive accumulation of ROS and MDA could lead to tissue dysfunction and metabolic disorders (Gao et al. 2016). MDA, an indicator of membrane damage, could be used to evaluate the integrity of cell membranes under oxidative stress (Cheng et al. 2020). In this study, 1-MCP treatments suppressed the accumulation of MDA (Fig. 3A) and the production of ROS (H2O2 and O2·−) (Fig. 3B and C), and MDA content was positively correlated with H2O2 and O2·− content. This indicates that 1-MCP treatments could suppress cell membrane peroxidation by suppressing the excessive production of ROS (Xu et al. 2020). In addition, respiration rate was negatively correlated with the content of H2O2 and O2·−, and the content of MDA, H2O2, and O2·− were negatively correlated with the activity of SOD, CAT, and APX. This indicates that the accumulated ROS could be scavenged by the fruit through respiration, and the activated antioxidant enzymes could also scavenge ROS and convert them into water and oxygen to suppress cell membrane peroxidation and reduce ROS-mediated oxidative damage (Sun et al. 2018). Moreover, it was found that the ascorbic acid content was negatively correlated with the content of H2O2 and O2·−. This may be due to the utilization of ascorbic acid and H2O2 by antioxidant enzymes to produce water and dehydroascorbic acid (Xu et al. 2019). Cheng et al. (2020) reported that 1-MCP treatment with chitosan application could improve antioxidant enzyme activity and reduce the accumulation of MDA in Chinese jujube. Feng et al. (2018) found that 1-MCP treatment could reduce the accumulation of MDA in “Yali” pears.
The synergistic action of antioxidant enzymes such as SOD, CAT, APX, and POD is an important mechanism for scavenging ROS and protecting cell membranes. It has been found that high SOD activity could inhibit the accumulation of free radicals in the process of H2O2 formation, leading to a reduced O2·− production rate and thus protecting cells from oxidative stress. To alleviate oxidative stress, excessive H2O2 must be converted into non-toxic molecules by enzymes such as CAT, APX, and POD (Cheng et al. 2020). Chen et al. (2015) found that antioxidant enzyme activity in “Huanghua” pears was increased by 1-MCP treatment, the accumulation of ROS was suppressed, and the aging process was delayed. Cheng et al. (2020) also found that 1.0 µL L−1 1-MCP treatment could increase the activities of APX, SOD, and POD in Chinese jujube. In this study, the 1-MCP treatments, especially the 1.0 µL L−1 1-MCP treatment, maintained higher CAT and APX activities during storage and reduced the accumulation of H2O2 compared with the control group. Moreover, correlation analysis showed that SOD activity was positively correlated with CAT and APX activities. The high SOD activity in P. domestica fruit induced by 1-MCP treatments could convert O2·− to H2O2, CAT and APX could decompose H2O2 into water and oxygen, and POD could oxidize toxic substances such as phenols into non-toxic substances, thus scavenging ROS and detoxifying the body.
5 Conclusion
1-MCP paper treatment could reduce the loss of bioactive substances in P. domestica fruit and effectively prolong shelf life. A 1.0 µL L−1 1-MCP paper treatment especially delays water loss, fruit color change, and anthocyanin degradation, maintains firmness and a high content of SSC, TA, ascorbic acid, and total phenol, reduces fruit respiration rate and ethylene production, and enhances the activities of antioxidant enzymes (SOD, CAT, APX, and POD). Moreover, it could also reduce the accumulation of ROS (H2O2 and O2·−) and the degree of lipid peroxidation in the cell membrane. The role of 1-MCP in antagonizing senescence and protecting physiological quality could be attributed to the increase in antioxidant enzyme activity, reduction of ROS, and alleviation of lipid peroxidation of the cell membrane. Therefore, 1.0 µL L−1 1-MCP treatment could be used to prolong the storage life of P. domestica fruit at 4 °C, and is an effective measure to improve commercial quality.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Agehara S, Crosby K, Holcroft D, Leskovar DI (2018) Optimizing 1-methylcyclopropene concentration and immersion time to extend shelf life of muskmelon (Cucumis melo L. var. reticulatus) fruit. Sci Hortic 230:117–125. https://doi.org/10.1016/j.scienta.2017.09.021
Baswal AK, Dhaliwal HS, Singh Z, Mahajan BVC, Gill KS (2020) Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’ mandarin (Citrus nobilis L. X C. deliciosa L) fruit. Postharvest Biol. Technol 161:111064. https://doi.org/10.1016/j.postharvbio.2019.111064
Cai H, Han S, Jiang L, Yu M, Ma R, Yu Z (2019) 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res Int 122:573–584. https://doi.org/10.1016/j.foodres.2019.01.026
Cheemaa MUA, Rees D, Colgan R, Taylorb M, Westby A (2013) The effects of ethylene, 1-MCP and AVG on sprouting in sweetpotato roots. Postharvest Biol Technol 85:89–93. https://doi.org/10.1016/j.postharvbio.2013.05.001
Chen Y, Lin H, Shi J, Zhang S, Lin Y, Lin T (2015) Effects of a feasible 1-methylcyclopropene postharvest treatment on senescence and quality maintenance of harvested Huanghua pears during storage at ambient temperature. LWT Food Sci Technol 64:6–13. https://doi.org/10.1016/j.lwt.2015.05.021
Chen Y, Sun J, Lin H, Hung Y-C, Zhang S, Lin Y, Lin T (2016) Paper-based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. J Sci Food Agr 97:2547–2552. https://doi.org/10.1002/jsfa.8072
Cheng Y, Liu L, Feng Y, Dong Y, Guan J (2019) Effects of 1-MCP on fruit quality and core browning in ‘Yali’ pear during cold storage. Sci Hortic 243:350–356. https://doi.org/10.1016/j.scienta.2018.08.041
Cheng S, Yu Y, Guo J, Chen G, Guo M (2020) Effect of 1-methylcyclopropene and chitosan treatment on the storage quality of jujube fruit and its related enzyme activities. Sci Hortic 265:109281. https://doi.org/10.1016/j.scienta.2020.109281
Chu W, Gao H, Chen H, Fang X, Zheng Y (2018) Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem 239:68–74. https://doi.org/10.1016/j.foodchem.2017.06.024
Escribano S, Sugimoto N, Macnish AJ, Biasi WV, Mitcham EJ (2017) Efficacy of liquid 1-methylcyclopropene to delay ripening of ‘Bartlett’ pears. Postharvest Biol Technol 126:57–66. https://doi.org/10.1016/j.postharvbio.2016.11.007
Fan X, Shu C, Zhao K, Wang X, Cao J, Jiang W (2018) Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci Hortic 232:63–70. https://doi.org/10.1016/j.scienta.2017.12.061
Feng Y-X, Cheng Y-D, He J-G, Li L-M, Guan J-F (2018) Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage. J Integr Agric 17:1667–1675. https://doi.org/10.1016/S2095-3119(18)61940-9
Gao H, Zhang ZK, Chai HK, Cheng N, Yang Y, Wang DN, Yang T, Cao W (2016) Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol Technol 118:103–110. https://doi.org/10.1016/j.postharvbio.2016.03.006
Giménez MJ, Serrano M, Valverde JM, Martínez-Romero D, Castillo S, Valero D, Guillén F (2017) Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J Sci Food Agric 97:1220–1228. https://doi.org/10.1002/jsfa.7853
Habibi F, Ramezanian A (2017) Vacuum infiltration of putrescine enhances bioactive compounds and maintains quality of blood orange during cold storage. Food Chem 227:1–8. https://doi.org/10.1016/j.foodchem.2017.01.057
Habibi F, Ramezanian A, Guillén F, Serrano M, Valero D (2020) Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem 306:125634. https://doi.org/10.1016/j.foodchem.2019.125634
Hanxu P, Wang R, Li L, Wang J, Cao J, Jiang W (2016) Manipulation of ripening progress of different plum cultivars during shelf life by post-storage treatments with ethylene and 1-methylcyclopropene. Sci Hortic 198:176–182. https://doi.org/10.1016/j.scienta.2015.11.007
Khaleghnezhad V, Yousefi AR, Tavakoli A, Farajmand B (2019) Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Sci Hortic 250:302–309. https://doi.org/10.1016/j.scienta.2019.02.057
Ku V, Wills R, Ben-Yehoshua S (1999) 1-Methylcyclopropene can differentially affect the postharvest life of strawberries exposed to ethylene. HortScience 34(1):119–120. https://doi.org/10.21273/HORTSCI.34.1.119
Lien N, Hitka G, Zsom T, Kókai Z (2016) Application of 1-MCP on apricots at different temperatures and days after harvest. Acta Aliment 45:542–550. https://doi.org/10.1556/066.2016.45.4.11
Lin Y, Lin Y, Lin H, Lin M, Li H, Yuan F, Chen Y, Xiao J (2018) Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem 264:1–8. https://doi.org/10.1016/j.foodchem.2018.05.031
Liu R, Gao H, Chen H, Fang X, Wu W (2019) Synergistic effect of 1-methylcyclopropene and carvacrol on preservation of red pitaya (Hylocereus polyrhizus). Food Chem 283:588–595. https://doi.org/10.1016/j.foodchem.2019.01.066
Ma Y, Ban Q, Shi J, Dong T, Jiang C-Z, Wang Q (2019) 1-Methylcyclopropene (1-MCP), storage time, and shelf life and temperature affect phenolic compounds and antioxidant activity of ‘Jonagold’ apple. Postharvest Biol Technol 150:71–79. https://doi.org/10.1016/j.postharvbio.2018.12.015
Ozturk B, Yildiz M, Yildiz K, Gun S (2021) Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv. ‘Li’) fruit by applying 1-methylcyclopropene. Sci Hortic 275:109671. https://doi.org/10.1016/j.scienta.2020.109671
Pan H, Wang R, Li L, Wang J, Cao J, Jiang W (2016) Manipulation of ripening progress of different plum cultivars during shelf life by post-storage treatments with ethylene and 1-methylcyclopropene. Sci Hortic 198:176–182. https://doi.org/10.1016/j.scienta.2015.11.007
Petriccione M, Sanctis F, Pasquariello MS, Mastrobuoni F, Rega P, Scortichini M, Mencarelli F (2015) The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol 8:394–408. https://doi.org/10.1007/s11947-014-1411-x
Rupavatharam S, East AR, Heyes JA (2015) Re-evaluation of harvest timing in ‘Unique’ feijoa using 1-MCP and exogenous ethylene treatments. Postharvest Biol Technol 99:152–159. https://doi.org/10.1016/j.postharvbio.2014.08.011
Sabir F, Agar I (2011) Influence of different concentrations of 1-methylcyclopropene on quality of tomato harvested at different maturity stages. J Sci Food Agric 91:2835–2843. https://doi.org/10.1002/jsfa.4529
Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, Clarke SL, Cullen DM, Lucas EA (2014) A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone 58:151–159. https://doi.org/10.1016/j.bone.2013.10.005
Sun J, Lin H, Zhang S, Lin Y, Wang H, Lin M, Hung Y-C, Chen Y (2018) The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem 247:16–22. https://doi.org/10.1016/j.foodchem.2017.12.017
Thongkum M, Imsabai W, Burns P, McAtee PA, Schaffer RJ, Allan AC, Ketsa S (2018) The effect of 1-methylcyclopropene (1-MCP) on expression of ethylene receptor genes in durian pulp during ripening. Plant Physiol Biochem 125:232–238. https://doi.org/10.1016/j.plaphy.2018.02.004
Tiryaki D, Aydın İ, Atici O (2019) Psychrotolerant bacteria isolated from the leaf apoplast of cold-adapted wild plants improve the cold resistance of bean (Phaseolus vulgaris L.) under low temperature. Cryobiology 86:111–119. https://doi.org/10.1016/j.cryobiol.2018.11.001
Xu F, Liu S, Liu Y, Xu J, Liu T, Dong S (2019) Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit. Food Chem 288:201–207. https://doi.org/10.1016/j.foodchem.2019.03.024
Xu Y, Li SE, Huan C, Jiang T, Zheng X, Brecht J (2020) Effects of 1methylcyclopropene treatment on quality and anthocyanin biosynthesis in plum (Prunus salicina cv. Taoxingli) fruit during storage at a non-chilling temperature. Postharvest Biol Technol 169:111291. https://doi.org/10.1016/j.postharvbio.2020.111291
Xu F, Zhang K, Liu S (2020) Evaluation of 1-methylcyclopropene (1-MCP) and low temperature conditioning (LTC) to control brown of Huangguan pears. Sci Hortic 259:108738. https://doi.org/10.1016/j.scienta.2019.108738
Yang W, Liu Y, Sang Y, Ma Y, Guo M, Bai G, Cheng S, Chen G (2021) Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol Technol 180:111614. https://doi.org/10.1016/j.postharvbio.2021.111614
Zhang JQ, Shen M, Zhu CC, Yu FX, Liu HL (2014) 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS ONE 9:e86589. https://doi.org/10.1371/journal.pone.0086589
Zhang Q, Liu Y, He C, Zhu S (2015) Postharvest exogenous application of ABA reduces internal browning in pineapple. J Agric Food Chem 63:5313–5320. https://doi.org/10.1021/jf506279x
Zhu C, Hu W, Wu H, Hu X (2014) No evident dose-response relationship between cellular ROS level and its cytotoxicity–A paradoxical issue in ROS-based cancer therapy. Sci Rep 4:5029. https://doi.org/10.1038/srep05029
Acknowledgements
This work was supported by High level talents research start project of Shihezi University (RCZK2021B37); the scientific and technological research plan in key areas of the Corps, research on the key technology of cold chain logistics and deep processing and creation of new products (2019AB024); the Science and technology research plan for key fields of XPCC (2020AB009); and the Innovation project for the south-forward development for Xinjiang production and construction corps (2018DB002).
Author information
Authors and Affiliations
Contributions
YM: Investigation, Writing—original draft; WZ and SC: Methodology, Writing—review & editing; WY and YL: Supervision, Software; SY and XZ: Investigation, Conceptualization; MG: Methodology, Writing—review & editing, Validation; GC: Data curation Formal analysis, Funding acquisition. All authors contributed jointly to all aspects of the work reported in the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
All authors declare that there are no conflict of interest.
Additional information
Communicated by Jinwook Lee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, Y., Zhang, W., Cheng, S. et al. 1-methylcyclopropene treatment improves postharvest quality and antioxidant activity of Prunus domestica L. cv. Ximei fruit. Hortic. Environ. Biotechnol. 63, 857–867 (2022). https://doi.org/10.1007/s13580-022-00442-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-022-00442-6