Abstract
The storage life of optimum quality in postharvest hardy kiwifruit (Actinidia aruguta) is short. The effect of 0.8 μL L−1 1-Methylcyclopropene (1-MCP) on the storage quality of harvested hardy kiwifruit was investigated at temperature of 1 °C for 70 days. The results indicated that 1-MCP treatment maintained the firmness and total soluble solids content of hardy kiwifruit as well as inhibited the respiratory rate and the decrease of vitamin C and glutathione contents. The antioxidant enzymes activity of superoxide dismutase, peroxidase, catalase, and glutathione reductase were enhanced in 1-MCP treatment hardy kiwifruit, meanwhile, 1-MCP treatment induced the radical scavenging capacity (DPPH radical scavenging rate, hydroxyl radical scavenging rate, and superoxide anion scavenging capacity) in fruit during storage. These results demonstrated that hardy kiwifruit with 1-MCP treatment stimulated a series of physiological responses to delay ripening and senescence and improve storage quality. Therefore, 1-MCP treatment could be used to extend the shelf-life of commercially produced hardy kiwifruit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Actinidia arguta (Sieb. & Zucc) Planch. ex Miq., also known as hardy kiwifruit, baby kiwi, kiwiberry or mini kiwi, is a genus of berry-bearing shrubs and vines [1, 2]. Currently the hardy kiwifruit is widespread cultivated in China, US, Australia, New Zealand and most European countries [3]. Hardy kiwifruit, with smooth, edible, thin, and green skin, has a highly aromatic flavor of well balanced sour and sweet taste [4]. Hardy kiwifruit, a “healthy fruit”, because of high nutritional value, is a rich source of vitamin C, minerals, lutein, and phenolics, with health-promoting attributes including anti-inflammatory and anti-oxidative, prevention of cancer, lowering of blood pressure, thus, it has increases in commercial production year by year as a very promising fruit species [4,5,6]. However, the notable disadvantage of hardy kiwifruit is short storability as well as fast loss of quality due to rapid softening and skin wrinkling of fruit, which is detrimental to the promotion of nutritional and health value of the hardy kiwifruit [7]. Hence it is of great significance to find effective methods to prolong the storage freshness period of the hardy kiwifruit. Varieties of preservation methods, such as low temperature storage, edible coatings, controlled atmosphere, exhibited obvious extension of the postharvest life of hardy kiwifruit [8], but problems relating to high costs and food safety have emerged and some postharvest technologies have marked effects on the sensory properties of the fruit. Therefore, it is necessary to seek a technology, being simpler, cheaper, safer and more effective, to ameliorate the postharvest quality of hardy kiwifruit.
Hardy kiwifruit is typical climacteric fruit, consisting of ethylene, acting as the main regulator of ripening, which accelerates the process of fruit aging and softening [3]. The application of ethylene action inhibitors may be a good way to store this climacteric fruit. 1-Methylcyclopropene (1-MCP), a cyclic alkene able to prevent ethylene action, is useful to protect horticultural products from exogenous and self-produced ethylene, prolonging their shelf-life during storage, transportation and retail with flexibility [9]. 1-MCP has a non-toxic mode of action, negligible residue and is active at very low concentrations [10], which is registered for use on a wide range of fruits and vegetables in several countries [11]. It is reported that 1-MCP has been widely used as a postharvest treatment technology to regulate the ripening and softening of a variety of fruits, and improve postharvest quality. Application of 1-MCP has been revealed to suppress fruit softening and the process of yellowing in pear [12], inhibit the respiratory rate and the decrease of total soluble solid in peach [13], and increase levels of antioxidant enzymes activities in mango and jujube [14, 15]. However, little information is available about the effects of 1-MCP on the physiological quality and storage life of hardy kiwifruit. In this study, we evaluated the effects of 1-MCP treatments on hardy kiwifruit fruit physiological property, and the biochemical characteristics of the antioxidant defense system during storage.
Materials and methods
Plant material and treatments
‘‘Huan optimal No. 1” hardy kiwifruit was harvested from the research base in Liaoning Agricultural Technical School, Xiongyue, China, and transported to the laboratory within three hours. Hardy kiwifruit fruits were selected for use in our experiments with uniform size, and absence of visible insect and diseases as well as mechanical damage.
In a preliminary experiment, hardy kiwifruits were treated with 0.4, 0.8, 1.2 μL L−1 1-MCP during storage. Results of the preliminary experiment indicated that 0.8 μL L−1 1-MCP had the most significant effect on postharvest quality attributes. Therefore, the 0.8 μL L−1 1-MCP treatment was used as a basis for more extensive studies. In the main experiment, experimental materials were randomly divided into two groups. One group of hardy kiwifruit was sealed in plastic box, and exposed to 1-MCP with the concentration of 0.8 μL L−1 for 24 h at 1 ± 1 °C, relative humidity (RH) 80–85%. While the control group was placed in plastic tents without 1-MCP treatment under the same storage conditions. After 24 h, the samples were packed into polyethylene film bags (0.03 mm) and stored at 1 ± 1 °C (RH 85–85%) for 70 days. Firmness, respiratory rate, total soluble solids content were determined on day 0, 14, 28, 42, 56, 70, and hardy kiwifruit tissue was collected for each time, immediately frozen in liquid nitrogen, then stored at − 80 °C for the subsequent analysis. Average value is obtained from triplicate experiments.
Firmness, respiratory rate, total soluble solids content
The firmness was tested on the approximate centre of the surface of hardy kiwifruit fruit by using a TA-XT plus texture analyzer (Stable Micro Systems, UK), with a 5 mm diameter cylindrical P/5 probe, with the penetration depth 8 mm and the speed at 1.0 mm/s.
The respiration rate of hardy kiwifruit was determined by GC-2010 gas chromatograph with TCD detector according to the report of Xu et al. [16]. A 200 g of fruit was placed in a closed container for 30 min and then the CO2 concentration was measured and was expressed as mg CO2 kg−1 h−1 fresh weight.
Total soluble solids content (TSS) was measured by Abbe's refractometer (Atago, Japan) to evaluate TSS content in juice from a combined extract of 9 fruit, and result was expressed in percentage.
Vitamin C and glutathione content
Vitamin C (Vc) content was measured by the report of Wang et al. [17]. Hardy kiwifruit tissues powder (1.0 g) was homogenized in 5 mL 0.05 mol L−1 oxalic acid–0.2 mM EDTA, and centrifuged. Then, 2 mL supernatant was mixed with 3 mL oxalic acid-EDTA, 1 mL 5% H2SO4, 0.5 mL metaphosphoric acid-acetic acid and 2 mL 5 mg L−1 ammonium molybdate, then incubated at 80 °C for 10 min, and the absorbance was read at 760 nm using spectrophotometer (Shimadzu UV-1800, Japan).
Measurement of glutathione (GSH) is based on the production of a yellow color obtained when 5, 5′-dithiobis (2-nitrobenzoic acid) reacts with GSH. The content of GSH was determined using GSH kit (Comin Biotechnology Co., Ltd, Suzhou, China). A 2.0 g of samples were homogenized in 10 mL 5% (W/V) 5-Sulfosalicylic acid dihydrate extraction buffer, then centrifuged. The supernatant was used for the GSH measurement according to the manufacturer’s protocol. The absorbance was recorded at 412 nm after 2 min of the mixture reaction.
Enzymatic activities of superoxide dismutase, peroxidase, catalase and glutathione reductase
To determine the enzymatic activities of superoxide dismutase, peroxidase, catalase and glutathione reductase, a 4.0 g of powder was homogenized in 20 mL 0.1 mol L−1 PBS (pH 7.8) with 0.5% polyvinylpyrrolidone dissolved, then centrifuged. The supernatant was used for enzymes assay.
Superoxide dismutase (SOD) activity was measured as reported by Savoie et al. [18] with a modification. A 0.1 mL supernatant was mixed with 1 mL nitrotetrazolium blue chloride (NBT) (1 g L−1 in 0.01 mol L−1 PBS, pH 7) and 1 mL of riboflavin solution (1 mg riboflavin and 0.4 mL tetramethylethylenedi-amine in 100 mL of 0.01 mol L−1 PBS, pH 7), and the absorbance at 560 nm was recorded, and the SOD activity was expressed as units.
Peroxidase (POD), Catalase (CAT), and Glutathione reductase (GR) activity were assayed by the method of Shi et al. [19]. POD enzyme extract of 0.1 mL was mixed with 1 mL 0.3% (v/v) H2O2, 1 mL PBS (pH 7.8, 0.1 mol L−1), 0.9 mL 0.2% (v/v) guaiacol, then reading the absorbance at 470 nm. One POD unit was calculated as the enzyme amount generated with an increase of one absorbance unit at 470 nm within 1 min. A 0.1 mL of supernatant was mixed with 1 mL 0.3% H2O2, and 1.9 mL 0.05 mol L−1 PBS (pH 7.8) to measure the CAT activity by recording the decrease of absorbance at 240 nm due to H2O2 consumption. One CAT unit was expressed as the enzyme amount gained with decreased 0.01 absorbance unit at 240 nm within 1 min. The reaction mixture of GR activity, consisting of 0.2 mL extract, 2.7 mL of 1 mmol L−1 EDTA (in PBS, pH 7.5), 0.1 mL 5 mmol L−1 oxidized glutathione, and 40 mL 4 mmol L−1 NADPH, is used to test the activity of GR by recording the absorbance at 340 nm. One GR unit was expressed as the enzyme amount gained with reduced 0.01 absorbance at 340 nm within 1 min.
DPPH radical scavenging rate, hydroxyl radical scavenging rate and superoxide anion scavenging capacity
DPPH radical scavenging rate was determined with a modified method described by Wang et al. [20]. A 2.0 g of samples were added to 20 mL 50% ethanol, and centrifuged, then a 0.1 mL supernatant was mixed with 2.9 mL of methanol containing 120 μmol L−1 1-diphenyl-2-picrylhydra-zyl radicals. The absorbance was measured at 515 nm, DPPH dissolved in ethanol as the control. DPPH radical scavenging rate was expressed as (%) = 100 − (absorbance of sample/absorbance of control) × 100.
To analyze hydroxyl radical scavenging rate, a 2.0 g fruit tissues were homogenized with 10 mL 50 mmol L−1 PBS (pH 7.0), and centrifuged, and the supernatant was used for hydroxyl radical scavenging rate assay. Add reagents with the instructions on the kit (Comin Biotechnology Co., Ltd, Suzhou, China) and read the absorbance of hydroxyl radical scavenging rate at 536 nm after 60 min incubation at 37 °C in water bath. H2O2/Fe2+ generates hydroxyl radicals through fenton reaction that Fe2+ is oxidized in the aqueous solution of phenanthroline-Fe2+ to Fe3+, resulting in a decrease of absorbance at 536 nm and the reading was used to calculate the hydroxyl radical scavenging rate.
Superoxide anion (O2−) scavenging capacity was measured by using the manufacturer’s protocol came with the O2− scavenging capacity assay kit (Comin Biotechnology Co., Ltd, Suzhou, China). The absorbance of O2− scavenging capacity was measured at 536 nm after 60 min incubation at 37 °C and O2− scavenging capacity was calculated as (%) = 100 − (absorbance of sample/ absorbance of control) × 100.
Statistical analysis
A one-way analysis of variance was performed with LSD tests at p < 0.05 to evaluate the differences between the treatments. Presented data are mean values with standard deviation. The data were analyzed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Firmness, respiratory rate, total soluble solids content
A reduction of firmness was observed in 1-MCP-treated and untreated hardy kiwifruit, however, the firmness was significantly higher in the 1-MCP treatment hardy kiwifruit than that in the control group stored at 1 °C for 70 days. (p < 0.05; Fig. 1a). The firmness of control group was only 63.38% as much as that of the 1-MCP treatment group on day 70.
The respiration rate in both groups reached a peak value on day 42 and thereafter decreased, and the peak value of control group was 1.24 times higher, compared with peak value in 1-MCP treatment fruit (Fig. 1b). 1-MCP treatment group exhibited a significantly lower respiration rate than that in the control group, with less significance at day 28 (p < 0.05).
As shown in Fig. 1c, the TSS content generally increased in both groups until day 42, then decreased. TSS content was significantly lower in the 1-MCP-treated hardy kiwifruit than that in the control fruit on days 14 and 28, but was obviously higher (p < 0.05) in hardy kiwifruit with 1-MCP treatment after 42 days until the end of the storage.
Vitamin C and glutathione content
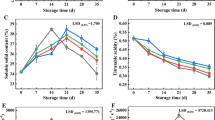
There was a clear reduction in the Vc content of hardy kiwifruit over the entire storage period, and the degree of decline in Vc content of 1-MCP treatment fruit was significantly less than that in the control fruit on days 14–56 (p < 0.05; Fig. 2a).
GSH content initially increased and then decreased, and the both groups reached the peak values on day 42 (Fig. 2b). However, GSH content was significantly higher in the 1-MCP-treated hardy kiwifruit than that in the control fruit stored at 1 °C for 70 days (p < 0.05), and the GSH content of hardy kiwifruit was 3.78 µmol g−1 in the control group and 6.27 µmol g−1 in the 1-MCP-treated group.
Enzymatic activities of superoxide dismutase, peroxidase, catalase and glutathione reductase
Peak SOD activity was observed on day 28 for the control group, while that for the 1-MCP group was observed on day 42, and the peak value of 1-MCP group increased by 78.45% compared with control fruit (Fig. 2c). POD activity tended to fluctuate, but generally increased and then declined; it was significantly higher in 1-MCP treatment group than that in the control group on days 28 and 42 (p < 0.05; Fig. 2d). CAT activity in both groups exhibited an increasing trend until day 42, then decreased (Fig. 2e). The 1-MCP treatment group maintained a higher level of CAT activity (p < 0.05), which was about 1.48 times higher than that of the control group on day 42. The change of GR activity was similar to the trend of SOD activity, and the control and 1-MCP treatment samples reached the maximum values on the 14th and 28th days, respectively (Fig. 2f). The 1-MCP group exhibited a significantly higher GR activity throughout the storage time than that in the control group (p < 0.05).
DPPH radical scavenging rate, hydroxyl radical scavenging rate and O2 − scavenging capacity
There was a general increase and then decline in DPPH radical scavenging rate, and reached the peak value on day 42. DPPH radical scavenging rate was obviously lower in control fruit compared with 1-MCP group on days 28–70 (p < 0.05; Fig. 3a). Hydroxyl radical scavenging rate had a decrease trend in the control group, while that for the 1-MCP-treated group reached a maximum on day 28 and thereafter decreased (Fig. 3b). The 1-MCP group showed significantly higher hydroxyl radical scavenging rate compared with that in the control on days 28–70 (p < 0.05). O2− scavenging capacity increased gradually until day 42, then decreased sharply in both groups, where O2− scavenging capacity was higher in 1-MCP treatment fruit than that in the control on days 42, 56, 70 (p < 0.05; Fig. 3c).
Correlation analysis
The pearson coefficient was used to evaluate the relevance of different indexes in the current study (Fig. 4). The data showed that firmness was negatively associated with TSS content greatly, and positively associated with Vc content. Furthermore, respiratory rate was positively correlated with TSS content greatly in control group (Fig. 4a), while antioxidant enzymes (SOD, CAT, POD and GR) was positively correlated with GSH content, DPPH radical scavenging rate, hydroxyl radical scavenging rate and O2− scavenging capacity in 1-MCP group (Fig. 4b).
Discussion
The major reason for the short-storage period and fast loss of storage quality is fruit softening and skin wrinkling caused by the ripening and decay of hardy kiwifruit [3]. 1-MCP has been proven to be beneficial in inhibiting ripening and senescence of a variety of fruit, as well as maintaining their postharvest quality and prolonging shelf life [21] (Xu et al. 2017). The current study revealed that the treatment of harvested hardy kiwifruit with 0.8 μL L−1 1-MCP for 24 h resulted in a higher firmness and TSS content, inhibited respiratory rate, and maintained the antioxidant substances content (Vc and GSH), antioxidant enzymes activity (SOD, CAT, POD and GR) and antioxidant capacity (DPPH radical scavenging rate, hydroxyl radical scavenging rate and O2− scavenging capacity), as well as prolonged the shelf life of hardy kiwifruit, compared with the control. Our results are similar to the report for 1-MCP treatment peach [22] and Huanghua pears [23] (Chen et al. 2015).
Hardy kiwifruit showed a typical climacteric characteristic, firmness, respiration rate and TSS content are also important indexes to evaluate the postharvest ripening and senescence of the fruit [3]. Fruit softening was prevented or delayed by 1-MCP, and the climacteric increase retarded, resulting in delay in the increase of TSS content during ripening [10, 24]. Results of the current study demonstrated that treatment of hardy kiwifruit with 0.8 μL L−1 1-MCP significantly suppressed the firmness, decreased the respiration rate, and delayed the increase of TSS content, furthermore, TSS content was negatively correlated with firmness greatly, and positively correlated with respiration rate. Therefore, the application of 1-MCP showed a beneficial effect on the inhibition of ripening and senescence in hardy kiwifruit fruit. These findings are similar to the results obtained in a research of guava [25].
Hardy kiwifruit has edible skin, being thin and hairless, which is susceptible to mechanical damage in the process of ripening and senescence, leading to the acceleration in the production of reactive oxygen species (ROS) [26]. Plant cells have an array of antioxidant systems that can eliminate ROS and prevent themselves from oxidative damage [27] (Fan et al. 2016). SOD acts as the front line of defense, which converts O2− into H2O2, then POD and CAT removed the excess H2O2 during stress [28]. As antioxidants, ascorbic acid (AsA) and GSH are crucial for the plant defense against oxidative stress because of the higher concentrations of AsA and GSH in cellular compartments, and a high reduced per oxidized ratio of AsA and GSH is maintained by GR with reducing power, which is essential for the proper scavenging of ROS in cells [28]. Results of our analysis showed that the levels of antioxidant substances content (Vc and GSH), antioxidant enzymes activity (SOD, CAT, POD and GR) were higher in the 1-MCP treated hardy kiwifruit, which may indicate that 1-MCP effectively inhibited the production of ROS by promoting antioxidant metabolism, thus suppressing the membrane oxidative stress-induced damage, and thereby delaying fruit softening and senescence (Fig. 5). These results are consistent with the report by Setha et al. that 1-MCP promoted increases in antioxidant enzymes in pineapple fruit [29]. Interestingly, antioxidant substances and antioxidant enzymes were positively correlated with respiratory rate, which may be due to a substantial increase in respiration of the climacteric fruit, thus, probably leading to an increase in oxygen free radical production with ripening, thereby inducing the antioxidant system of the fruit [14].
Free radicals are the major factors in activating biological damage such as DNA, RNA, protein, or lipid oxidation [30]. Most antioxidants that exist in plants have the ability to scavenge free radicals. The radical scavenging activities of DPPH, hydroxyl and O2− have been extensively used in measuring horticultural crops antioxidant capacity [31]. Previous investigations demonstrated that peach fruit treated with 1-MCP effectively stimulated the scavenge capacity of DPPH, O2−, and HO•, which were crucial for the regulation of defense system and the maintenance of normal intracellular homeostasis [22]. Cao et al. [32] proposed that 1-MCP treatment effectively maintained higher antioxidant activity as measured by the scavenging capacity against DPPH and O2− radicals in loquat fruit. In the current study, 1-MCP treatment maintained a higher radical scavenging rate in hardy kiwifruit fruit throughout the storage time, and radical scavenging rate was positively correlated with antioxidant enzyme activity, suggesting that the application of 1-MCP effectively enhanced radical scavenging rate, suppressed the accumulation of ROS, and maintained membrane integrity in hardy kiwifruit, as well as delayed the ripening and senescence of fruit (Fig. 5).
Conclusions
The 1-MCP treatment effectively suppressed the respiration rate of the hardy kiwifruit, delayed the decrease of firmness and TSS content, and maintained the postharvest quality when being stored at 1 °C for 70 days. Based on the data obtained in the study, the regulation pathway of 1-MCP on hardy kiwifruit has been established involving key enzymes and catabolites along the pathway (Fig. 5). The beneficial effects of 1-MCP on inhibiting the ripening along with senescence of hardy kiwifruit and reducing physiological disorders, which might be due to the increase of antioxidant enzyme activity and reduction of free radical damage.
References
P. Latocha, T. Krupa, P. Jankowski, J. Radzanowska, Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol Technol. 88, 21–33 (2014)
A.C. Lindhorst, M. Steinhaus, Aroma-active compounds in the fruit of the hardy kiwi (Actinidia arguta) cultivars Ananasnaya, Bojnice, and Dumbarton Oaks: differences to common kiwifruit (Actinidia deliciosa ‘Hayward’). Eur Food Res Technol. 242(6), 967–975 (2016)
S. Lim, S.H. Han, J. Kim, H.J. Lee, J.G. Lee, E.J. Lee, Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 190, 150–157 (2016)
A.N. Kim, H.J. Kim, J. Chun, H.J. Heo, S.G. Choi, Degradation kinetics of phenolic content and antioxidant activity of hardy kiwifruit (Actinidia arguta) puree at different storage temperatures. LWT-Food Sci Technol. 89, 535–541 (2017)
T. Krupa, P. Latocha, A. Liwińska, Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci Hortic. 130(2), 410–417 (2011)
Y.H. Liu, C.J. Liu, Current situation and potential of wild Actinidia argute resources in northeastern china. Acta Hortic. 1096, 443–449 (2015)
A. White, H.N.D. Silva, C. Requejo-Tapia, F.R. Harker, Evaluation of softening characteristics of fruit from 14 species of actinidia. Postharvest Biol Technol. 35(2), 143–151 (2005)
Y. Wang, F. Xu, X. Feng, R.L. Macarthur, Modulation of Actinidia arguta fruit ripening by three ethylene biosynthesis inhibitors. Food Chem. 173, 405–413 (2015)
J.F. Massolo, A. Concellón, A.R. Chaves, A.R. Vicente, 1-methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol Technol. 59(1), 10–15 (2011)
C.B. Watkins, The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv. 24(4), 389–409 (2006)
C.B. Watkins, B. Chris, 1-methylcyclopropene (1-MCP) based technologies for storage and shelf life extension. Acta Hortic. 687, 201–208 (2006)
J. Deell, B. Ehsani-Moghaddam, Timing of postharvest 1-methylcyclopropene treatment affects Bartlett pear quality after storage. Can J Plant Sci. 91(5), 853–858 (2011)
X.Z. Yang, W.W. Wei, P. Lv, J.H. Feng, Effectiveness of 1-methylcyclopropene treatment on peach fruit (Prunus persica L.) for extending storage life. Adv Mater Res. 1089, 159–162 (2015)
R. Singh, U.N. Dwivedi, Effect of ethrel and 1-methylcyclopropene (1-MCP) on antioxidants in mango (Mangifera indica var. Dashehari) during fruit ripening. Food Chem. 111(4), 951–956 (2008)
Z. Zhang, S. Tian, Z. Zhu, Y. Xu, G. Qin, Effects of 1-methylcyclopropene(1-MCP) on ripening and resistance of jujube (Zizyphus jujuba cv. Huping) fruit against postharvest disease. LWT-Food Sci Technol. 45(1), 13–19 (2012)
D.Y. Xu, S.T. Gu, F.H. Zhou, W.Z. Hu, K. Feng, C. Chen, A.L. Jiang, Mechanism underlying sodium isoascorbate inhibition of browning of fresh-cut mushroom (Agaricus bisporus). Postharvest Biol Technol (2020). https://doi.org/10.1016/j.postharvbio
Q. Wang, T. Ding, J.H. Zuo, L.P. Gao, L.L. Fan, Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol Technol. 112, 114–120 (2016)
J.M. Savoie, N. Minvielle, L.L. Michèle, Radical-scavenging properties of extracts from the white button mushroom Agaricus bisporus. J Sci Food Agr. 88(6), 970–975 (2010)
J. Shi, L. Gao, J. Zuo, Q. Wang, L. Fan, Exogenous sodium nitroprusside treatment of broccoli florets extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharvest Biol Technol. 116, 98–104 (2016)
L. Wang, H. Zhang, P. Jin, X. Guo, Y. Li, C. Fan, J. Wang, Y.H. Zheng, Enhancement of storage quality and antioxidant capacity of harvested sweet cherry fruit by immersion with β-aminobutyric acid. Postharvest Biol Technol. 118, 71–78 (2016)
X. Xu, H. Lei, X. Ma, T. Lai, H. Song, X. Shi, J.G. Li, Antifungal activity of 1-methylcyclopropene (1-MPC) against anthracnose (Colletotrichum gloeosporioides) in postharvest mango fruit and its possible mechanisms of action. Int J Food Microbiol. 241, 1–6 (2017)
X. Wu, X. An, M. Yu, R. Ma, Z. Yu, 1-methylcyclopropene treatment on phenolics and the antioxidant system in postharvest peach combined with the liquid chromatography/mass spectrometry technique. J Agric Food Chem. 66(25), 6364–6372 (2018)
Y.H. Chen, H.T. Lin, J. Shi, S. Zhang, Y.F. Lin, T. Lin, Effects of a feasible 1-methylcyclopropene postharvest treatment on senescence and quality maintenance of harvested Huanghua pears during storage at ambient temperature. LWT-Food Sci Technol. 64(1), 6–13 (2015)
F. Guillén, S. Castillo, P.J. Zapata, D. Martínez-Romero, M. Serrano, D. Valero, Efficacy of 1-MCP treatment in tomato fruit: 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol Technol. 43(1), 23–27 (2007)
S.P. Singh, R.K. Pal, Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharvest Biol Technol. 47(3), 307–314 (2008)
R. Sabban-Amin, O. Feygenberg, E. Belausov, E. Pesis, Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’ apples. Postharvest Biol Technol. 62(3), 295–304 (2011)
L. Fan, J. Shi, J. Zuo, L. Gao, J. Lv, Q. Wang, Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol Technol. 120, 76–83 (2016)
R. Mittler, Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9), 405–410 (2002)
S. Setha, A. Kongsuwan, V. Srilaong, Reduced internal browning in pineapple fruit by 1-MCP pretreatment and the antioxidant response. Acta Hortic 1012(1012), 573–579 (2013)
Z. Fang, J. Bouwkamp, J.C. Bouwkamp, T. Solomos, T. Solomos, Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of L. J Exp Bot. 49(320), 503–510 (1998)
L.L. Zuo, Z.Y. Wang, Z.L. Fan, S.Q. Tian, J.R. Liu, Evaluation of antioxidant and Antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro. Int J Mol Sci 13(5), 5506–5518 (2012)
S. Cao, Y. Zheng, Z. Yang, Effect of 1-MCP treatment on nutritive and functional properties of loquat fruit during cold storage. New Zeal J Crop Hort 39(1), 61–70 (2011)
Acknowledgements
This research was supported by the National Key R & D Program of China (No. 2016YFD0400903).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, D., Zhou, F., Gu, S. et al. 1-Methylcyclopropene maintains the postharvest quality of hardy kiwifruit (Actinidia aruguta). Food Measure 15, 3036–3044 (2021). https://doi.org/10.1007/s11694-021-00893-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00893-y