Abstract
The effects of γ-aminobutyric acid (GABA) on promotion of chilling tolerance and the methionine sulfoxide reductase (MSR)-thioredoxin reductase (TrxR) system in peach (Prunus persica) fruit were investigated. The peaches were immersed in distilled water (as a control) and GABA solution followed by chilling treatment for 35 days. GABA treatment suppressed the augmentation of chilling injury (CI) index and weight loss rate, and the decline of firmness and total soluble solids content in peaches caused by chilling. The superoxide anion (O2·−) production rate, hydrogen peroxide (H2O2) content and reactive oxygen species accumulation were also retarded following GABA application. GABA treatment elevated the activity and gene expression of methionine sulfoxide reductase A (MSRA) and MSRB. Moreover, the activity and gene expression of TrxR were enhanced by GABA application. Additionally, GABA treatment increased the reduced nicotinamide adenine dinucleotide phosphate (NADPH) content and decreased the oxidized nicotinamide adenine dinucleotide phosphate (NADP) content, consequently boosting the NADPH/NADP ratio. Also, the activity and gene expression of glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6GPDH) were promoted following GABA application. Thus, GABA treatment induced the MSR-TrxR system, consequently reducing CI in peaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cold storage is commonly used to retard fruit decay and prolong shelf life (Jannatizadeh et al. 2019). However, peaches (Prunus persica) are prone to chilling injury (CI) when exposed to cold stress (Zhu et al. 2019). CI symptoms in peaches mainly include internal browning (Huang et al. 2020), which leads to economic losses. Thus, approaches to ameliorate CI in peaches are necessary.
Use of γ-aminobutyric acid (GABA) in food products is permitted in Japan, the United States and China. GABA has been used in food products in Japan since 2001, has been listed as a recognized safe substance in the United States since 2008, and as a new resource in China since 2009. GABA, a crucial plant effector molecule, has been shown to accelerate a series of physiological reactions (Kinnersley and Turano 2000), especially reduction of CI (Shang et al. 2011; Yang et al. 2011; Wang et al. 2014; Habibi et al. 2020). Accordingly, GABA treatment significantly promoted endogenous GABA and proline production (Shang et al. 2011), and boosted the antioxidant capacity and energy status (Yang et al. 2011), thus retarding CI in peaches. Additionally, GABA treatment alleviated CI by ameliorating membrane damage and elevating the antioxidant capacity in banana peel (Wang et al. 2014). Also, GABA application modified the abundance of aroma volatile compounds, thus ameliorating CI in blood oranges (Habibi et al. 2020). However, the biochemical and molecular mechanisms involved in the GABA treatment-mediated reduction of CI in postharvest fruit remain to be revealed.
Oxidative damage caused by reactive oxygen species (ROS) overproduction is one of the early reactions in fruit susceptible to CI (Jiao et al. 2019; Yao et al. 2019). In order to maintain ROS homeostasis, it is necessary to employ measures to accelerate the ROS scavenging capacity in cold sensitive fruit. GABA treatment promoted the levels of antioxidant enzymes in peaches (Yang et al. 2011) and banana peels (Wang et al. 2014), indicating that GABA application is an effective technology to elevate the ROS scavenging capacity in fruit vulnerable to CI. Apart from antioxidant enzymes, the methionine sulfoxide reductase (MSR) family is one of the main plant ROS scavenging systems (Sadanandom et al. 2019). Methionine (Met) in proteins is prone to oxidation by ROS into methionine-S-sulfoxide and methionine-R-sulfoxide (Met-S-SO and Met-R-SO) (Fig. 1a), thus changing the configuration and activity of corresponding proteins, which can be reverted by the MSR family (Ding et al. 2019). There are two main types of MSRs in organisms, MSRA and MSRB, which specifically reduce Met-S-SO and Met-R-SO, respectively. MSRs in plants could be triggered upon exogenous applications of certain substances. Accordingly, methyl viologen, ozone, and high light activated MSR, and thereby retarded oxidative damage in Arabidopsis thaliana (Romero 2004). In addition, nitric oxide (NO) (Jiao et al. 2019) and gibberellic acid (GA3) (Jiao and Duan 2019) treatments boosted MSR gene expression, thus ameliorating CI in peaches. However, the induction of MSR family enzymes following GABA treatment in peaches remains to be elucidated.
Thioredoxin (Trx) is a reducing agent for MSR family enzymes (Kim and Kim 2008). When serving as a reducing agent for MSRA or MSRB, Trx is oxidized (Fig. 1b). Subsequently, the oxidized Trx is reduced by thioredoxin reductase (TrxR) (Fig. 1c). TrxR, a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent dimer selenium enzyme, is a member of the pyridine nucleotide-disulfide oxidoreductase family. It can reduce the oxidized Trx by catalyzing the transfer of electrons from NADPH to oxidized Trx (Fig. 1c). This is a crucial detoxification reaction to protect organisms from damage by excessive ROS. MSR family proteins, TrxR and NADPH form the MSR-TrxR system, which plays an important role in maintaining a stable redox state in organisms. TrxR in plants could be promoted following certain exogenous treatments. Accordingly, hydrogen peroxide (H2O2) treatment boosted the TrxR activity, NADPH content and activity of glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) (NADPH-regenerating enzymes in the pentose phosphate pathway) in guava fruit (Chumyama et al. 2019). In addition, fluctuating light modulated TrxR activity, thus affecting photosynthesis in Arabidopsis (Diaz et al. 2020). However, the modulation of the MSR-TrxR system by GABA treatment in peaches remains to be elucidated.
The aims of this research were to explore the CI severity and induction of the MSR-TrxR system following GABA treatment in peaches.
2 Materials and methods
2.1 Plant material and postharvest applications
Peaches (Prunus persica Batsch cv. ‘Yuhua No. 3’), with characteristics of early maturity, medium juice, hard solute, and semi-freestone fruit, were handpicked at commercial maturity from an orchard in Nanjing, China. The selected uniform peach samples free from mechanical injury were divided into two groups, each with three biological replicates, at random. For each biological replicate, three technical replicates were carried out. For each technical replicate of each biological replicate of each treatment, 400 fruit were used.
-
(1)
Control (CK): The peaches were immersed in distilled water.
-
(2)
GABA: The peaches were immersed in 5 mM GABA solution.
The GABA concentration was determined according to my preliminary experiments (Figure S1 of the Supporting Information). The fruit were treated for 10 min and subsequently air dried for 40 min. The samples were then treated at 4 ± 1 °C and 80–90% relative humidity for 35 d. For each technical replicate of each biological replicate of each treatment, 30 peaches were used to calculate the CI index, weight loss rate, firmness and total soluble solids content every 7 d. For each technical replicate of each biological replicate of each treatment, 20 peaches were sampled to analyze physiological indicators every 7 d, which were kept at − 80 °C.
2.2 CI index, weight loss rate, total soluble solids content and firmness calculation
Peaches were removed to 20 °C for 3 d after each cold storage period. The CI index was calculated in accordance with Jiao et al. (2019). The CI index was assessed by recording the degree of internal browning: 0 = none, 1 = < 5%, 2 = 6%-25%, 3 = 26%-50%, 4 = > 50%. CI index = Σ (CI degree × number of peaches at the CI degree)/(5 × total number of peaches in the replicate).
For weight loss rate calculation, the peaches were weighed before and after each storage period.
Firmness was assayed by a firmness analyzer (FT327, Effegi, Alfonsine, Italy) using a probe with a 7.5 mm penetration depth.
For total soluble solids content, 5.0 g peach samples were ground, followed by centrifugation at 9000g for 15 min. Then, the collected supernatant was detected using a WYT-4 hand-held refractometer (Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China).
2.3 O2 ·− production rate, H2O2 content and ROS accumulation determination
For the O2·− production rate assay, 5.0 g peach samples were ground using 50 mM phosphate buffer (pH 7.8), and centrifuged at 11,000g for 20 min at 4 °C. The O2·− production rate was detected in accordance with Wang et al. (2020). The results were expressed as mM kg−1 FW.
For the H2O2 content assay, 5.0 g peach samples were extracted in cold acetone, and centrifuged at 11,000g for 20 min at 4 °C. The H2O2 content was detected in accordance with Yang et al. (2016). The results were expressed as μM kg−1 FW.
The ROS production was measured using a fluorescence spectrophotometer (Cary Eclipse, VARIAN, America) in accordance with Jing et al. (2016). The maximum excitation/emission wavelength was 485/530 nm, respectively, and the slit width was 5 nm. The results were expressed as a.u. mg−1 FW, where a.u. represents the relative fluorescence intensity.
2.4 MSRA and MSRB activity assay
5.0 g peach samples were extracted in 15 mM HEPES (pH 8.0) containing 30 mM KCl, 10 mM MgCl2 and 1 mM PMSF, and centrifuged at 28,000g for 35 min at 4 °C. Then, the collected supernatant was added to 15 mM HEPES (pH 8.0) containing 30 mM KCl, 10 mM MgCl2, 20 mM DTT and 0.5 mM dabsyl-Met-S, R-SO at 37 °C for 1 h. Then, to stop the reaction, ethanol:acetate buffer was added. The reaction mixture was centrifuged at 28,000g for 35 min at 4 °C. The activity of MSRA and MSRB was detected in accordance with Ding et al. (2019). The results were expressed as mM min−1 kg−1 FW.
2.5 TrxR activity determination
5.0 g peach samples were extracted in 25 mM potassium phosphate buffer (pH 7.0) containing 2.5 mM EDTA at 4 °C, and centrifuged at 11,000g for 15 min at 4 °C. TrxR activity was detected in accordance with Chumyama et al. (2019). The results were expressed as mM min−1 kg−1 FW.
2.6 NADPH and NADP contents determination
For NADP extraction, 5.0 g peach samples were extracted in 10% methanol containing 0.5 M perchloric acid. For NADPH extraction, 5.0 g peach samples were extracted in 10% methanol containing 0.5 M NaOH. The homogenate was centrifuged at 12,000g for 20 min at 4 °C. For the NADP extraction, the collected supernatant was adjusted to pH 5.0 with 6 M KOH. For the NADPH extraction, the collected supernatant was adjusted to pH 8.0 with 1 M HCl. NADPH and NADP contents were detected in accordance with Chumyama et al. (2019). The results were expressed as mg kg−1 FW.
2.7 G6PDH and 6PGDH activity assay
5.0 g peach samples were extracted in extraction buffer containing 50 mM Tris–HCl (pH 7.8), 0.1 mM EDTA, 0.2% TritonX-100 and 10% glycerol at 4 °C, and centrifuged at 18,000g for 15 min at 4 °C. The activity of G6PDH and 6GPDH was assayed in accordance with Chumyama et al. (2019). The results were expressed as mM min−1 kg−1 FW.
2.8 Gene expression assay
Total RNA in peaches was acquired using an E.Z.N.A. Plant RNA Kit (Omega, Norcross, GA, USA; R6827-01) following the manufacturer’s instructions. First-strand cDNA was obtained in accordance with Jiao et al. (2019). Primers for quantitative real-time polymerase chain reaction tests for MSRA, MSRB, TrxR, G6PDH, 6GPDH and β-actin were designed via Primer Premier 5.0 (Table 1). The test process was set per Jiao et al. (2019). Each assay was performed with three replicates.
2.9 Statistical analysis
Statistical analysis was carried out using t-test performed in SPSS 22.0 (SPSS Inc., Chicago, IL, USA) program at a significance level of p < 0.05 and p < 0.01.
3 Results
3.1 Effects of GABA on the CI degree, weight loss rate, firmness and total soluble solids content in peaches
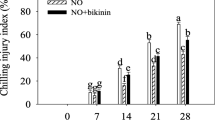
The CI disorder in peaches was initially observed at 14 d. The CI index and weight loss rate continuously increased following control and GABA treatments throughout storage. GABA treatment markedly delayed the increase in the CI index and weight loss rate. Following 35 d of storage, GABA treatment decreased the CI index and weight loss rate by 31% and 17%, respectively (Fig. 2a and b).
The firmness continuously decreased in peaches following control and GABA treatments throughout storage. GABA treatment markedly delayed the decrease in the firmness. Following 35 d of storage, GABA treatment boosted the firmness by 56% compared to the control (Fig. 2c).
The total soluble solids content first increased and then decreased in peaches following control and GABA treatments throughout storage. GABA treatment markedly retained the total soluble solids content. Following 35 d of storage, GABA treatment elevated the total soluble solids content by 38% compared to the control (Fig. 2d).
3.2 Effects of GABA on the O2 ·− production rate, H2O2 production and ROS accumulation in peaches
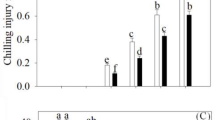
The O2·− production rate, H2O2 production and ROS accumulation continuously increased following control and GABA applications throughout storage in peaches. GABA suppressed the increase in the O2·− production rate, H2O2 production and ROS accumulation by 27%, 21% and 13%, respectively, after 35 d of storage (Fig. 3).
3.3 Effects of GABA on the activity and gene expression of MSRA and MSRB in peaches
The activity of MSRA and MSRB first increased and then decreased following control and GABA applications throughout storage in peaches. GABA elevated the activity of MSRA and MSRB. The activity of MSRA and MSRB after GABA treatment peaked at 28 d and 21 d, which was 1.4 and 1.5 times that of the control, respectively. Following 35 d of storage, the activity of MSRA and MSRB in GABA-immersed peaches was 1.2 and 1.6 times that of the control, respectively (Fig. 4a and b).
MSRA gene expression first increased and then decreased following control and GABA treatments throughout storage. MSRB gene expression continuously increased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage. GABA elevated the expression of MSRA and MSRB. The expression of MSRA and MSRB following GABA treatment peaked at 21 d and 21 d, which was 1.8 and 2.0 times that of the control, respectively. Following 35 d of storage, the expression of MSRA and MSRB in GABA-immersed peaches was 1.8 and 1.3 times that of the control, respectively (Fig. 4c and d).
3.4 Effects of GABA on activity and gene expression of TrxR in peaches
The activity and gene expression of TrxR continuously decreased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage in peaches. GABA upregulated the activity and gene expression of TrxR. The activity and gene expression of TrxR following GABA treatment peaked at 21 d and 14 d, which were 2.9 and 2.6 times that of the control, respectively. Following 35 d of storage, the activity and gene expression of TrxR in GABA-immersed peaches were 2.2 and 2.3 times that of the control, respectively (Fig. 5).
3.5 Effects of GABA on NADPH and NADP contents in peaches
The NADPH content continuously decreased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage. GABA boosted the NADPH content. The highest NADPH content following GABA treatment was acquired at 14 d, which was 1.5 times that of the control. Following 35 d of storage, the NADPH content in GABA-immersed peaches was 1.6 times that of the control (Fig. 6a).
The NADP content continuously increased following the control treatment, while it first decreased and then increased following GABA treatment throughout storage. GABA downregulated the NADPH content. The lowest NADP content following GABA treatment was acquired at 14 d, which decreased by 50% compared with the control. Following 35 d of storage, the NADP content in GABA-immersed peaches decreased by 19% compared with the control (Fig. 6b).
The NADPH/NAPD ratio continuously decreased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage. GABA promoted the NADPH/NAPD ratio. The highest NADPH/NAPD ratio following GABA treatment was acquired at 14 d, which was 2.9 times that of the control. Following 35 d of storage, the NADPH/NAPD ratio in GABA-immersed peaches was 2.4 times that of the control (Fig. 6c).
3.6 Effects of GABA on the activity and gene expression of G6PDH and 6PGDH in peaches
The activity of G6PDH and 6PGDH continuously decreased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage. GABA upregulated the activity of G6PDH and 6PGDH. The activity of G6PDH and 6PGDH following GABA treatment peaked at 21 d and 28 d, which was 2.4 and 2.2 times that of the control, respectively. Following 35 d of storage, the activity of G6PDH and 6PGDH in GABA-immersed peaches was 2.5 and 2.1 times that of the control, respectively (Fig. 7a and b).
The gene expression of G6PDH and 6PGDH continuously decreased following the control treatment, while it first increased and then decreased following GABA treatment throughout storage. GABA upregulated the expression of G6PDH and 6PGDH. The expression of G6PDH and 6PGDH following GABA treatment peaked at 14 d and 21 d, which was 2.2 and 2.5 times that of the control, respectively. Following 35 d of storage, the expression of G6PDH and 6PGDH in GABA-immersed peaches was 1.7 and 1.9 times that of the control, respectively (Fig. 7c and d).
4 Discussion
This work suggested that GABA treatment markedly retarded the elevation of the CI index and weight loss rate, and maintained firmness and total soluble solids content in peaches following 35 d of cold storage (Fig. 2), verifying that application of GABA could be considered to boost chilling tolerance in peaches.
Cold stress destroys the redox state in plants, thus giving rise to oxidative damage, which is an early reaction in fruit vulnerable to CI (Wang et al. 2014). Thus, to enhance chilling tolerance, it is crucial to alleviate oxidative damage in fruit prone to CI. GABA application suppressed the increase in the O2·− production rate, H2O2 production and ROS accumulation in peaches (Fig. 3), suggesting that GABA application would be an effective way to ameliorate oxidative damage.
The data demonstrated that GABA application modulated the activity and gene expression of MSRA and MSRB in peaches (Fig. 4). Accordingly, MSR was involved in the amelioration of oxidative damage in Arabidopsis (Li et al. 2012). Hence, GABA application-activated MSRA and MSRB may play a role in maintaining a stable redox state in peaches. ROS overproduction upon chilling stress oxidizes Met into Met-S-SO or Met-R-SO (Fig. 1a), causing the inactivation of relevant proteins, which can be reverted by MSRA or MSRB (Roy and Nandi 2017). Therefore, MSR family enzymes in cold-sensitive fruit may not only scavenge excessive ROS, but also detoxify the effects of ROS. Additionally, GABA application triggered the calcium pathway in tobacco pollen tubes (Yu et al. 2014). GABA treatment induced an increase in calcium and calmodulin content in germinated hulless barley (Ma et al. 2019). These results indicated that GABA might be a potential regulator of Ca2+ levels in plants. Accordingly, the interaction between MSR and Ca2+/CAM-binding kinase has been shown to reduce the ROS level upon carbonate alkaline stress in Glycine soja (Sun et al. 2016). Thus, it could be inferred from these reports that Ca2+ might mediate the GABA application-boosted MSR family in peaches.
Trx is a reducing agent for MSRA and MSRB (Kim and Kim 2008). When acting as a reducing agent for MSRA or MSRB to reduce Met-S-SO or Met-R-SO into Met, Trx is oxidized (Fig. 1b). Then, the oxidized Trx is reduced by TrxR, which is dependent on NADPH (Fig. 1c). Thus, the effects of GABA treatment on TrxR levels and NADPH redox equilibrium were further investigated. Data in this work suggested that the activity and gene expression of TrxR were enhanced by GABA application in peaches (Fig. 5). GABA application also upregulated the NADPH content, and downregulated the NADP content, thus boosting the NADPH/NADP ratio (Fig. 6). TrxR reduces the oxidized Trx by transferring electrons from NADPH to the oxidized Trx (Fig. 1c). The reduced Trx may be involved in certain reactions that maintain redox equilibrium (Sang et al. 2016). NADPH redox equilibrium is vital for the reduction reaction by TrxR. Thus, the regulation of NADPH-regenerating enzymes upon GABA application was revealed. The results showed that the activity and gene expression of G6PDH and 6PGDH in peaches were promoted by GABA treatment (Fig. 7). The pentose phosphate pathway could produce NADPH in eukaryotes (Tian et al. 2021). G6PDH converts glucose 6-phosphate into gluconolactone 6-phosphate. Then, spontaneous transformation or 6-phosphoglucolactase converts gluconolactone 6-phosphate into 6-phosphogluconic acid. This process produces a molecule of NADPH. 6PGDH converts 6-phosphogluconic acid into ribulose 5-phosphate. This process also produces a molecule of NADPH. Hence, GABA treatment promoted NADPH redox equilibrium via the induction of NADPH-regenerating enzymes (G6PDH and 6PGDH), thus facilitating the reduction reaction by TrxR in peaches. Moreover, TrxR reduced ROS levels in Arabidopsis (Lepistö et al. 2013). Additionally, TrxR has been verified to act as an electron donor for 2-Cys peroxiredoxin, thus modifying redox equilibrium in Anabaena cells (Mihara et al. 2016). Therefore, TrxR may not only reduce the oxidized Trx, but also directly maintain ROS equilibrium. In summary, this work demonstrated that GABA application decelerated the CI by boosting the MSR-TrxR system in peaches, which is consistent with the previous reports suggesting that GABA treatment could elevate the levels of antioxidant enzymes in peaches (Yang et al. 2011) and banana peel (Wang et al. 2014). Therefore, GABA application can ameliorate oxidative damage in fruit susceptible to CI and expand our knowledge on the effects of GABA on cold resistance.
5 Conclusions
GABA application retarded the increase in the CI index and weight loss rate, and the decrease in firmness and total soluble solids content in peaches. O2·− and H2O2 production was also delayed after GABA application. GABA application boosted the activity and gene expression of MSRA, MSRB, TrxR, G6PDH and 6GPDH. Also, GABA application elevated the NADPH content and reduced the NADP content, ultimately upregulating the NADPH/NADP ratio. Thus, GABA application facilitated chilling tolerance by promoting the MSR-TrxR system in peaches. This study would lay a theoretical foundation for the construction of a cold resistant technology system for fruits and vegetables.
Data availability
Data in this paper are available from the corresponding author by request.
References
Chumyama A, Faiyueb B, Saengnil K (2019) Reduction of enzymatic browning of fresh-cut guava fruit by exogenous hydrogen peroxide-activated peroxiredoxin/thioredoxin system. Sci Hortic 255:260–268. https://doi.org/10.1016/j.scienta.2019.05.042
Diaz M, Nikkanen L, Himanen K, Toivola J, Rintamki E (2020) Two chloroplast thioredoxin systems differentially modulate photosynthesis in Arabidopsis depending on light intensity and leaf age. Plant J 104:718–734. https://doi.org/10.1111/tpj.14959
Ding P, Fang L, Wang G, Li X, Huang S, Gao Y, Zhu J, Xiao L, Tong J, Chen F, Xia G (2019) Wheat methionine sulfoxide reductase A4.1 interacts with heme oxygenase 1 to enhance seedling tolerance to salinity or drought stress. Plant Mol Biol 101:200–220. https://doi.org/10.1007/s11103-019-00901-2
Habibi F, Ramezanian A, Guillén F, Serrano M, Valero D (2020) Effect of various postharvest treatment on aroma volatile compounds of blood orange fruit exposed to chilling temperature after long-term storage. Food Bioprocess Technol 13:2054–2064. https://doi.org/10.1007/s11947-020-02547-1
Huang D, Tian W, Feng J, Zhu H (2020) Interaction between nitric oxide and storage temperature on sphingolipid metabolism of postharvest peach fruit. Plant Physiol Biochem 151:60–68. https://doi.org/10.1016/j.plaphy.2020.03.012
Jannatizadeh A, Aghdam M, Luo Z, Razavi F (2019) Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioprocess Technol 12:741–750. https://doi.org/10.1007/s11947-019-2247-1
Jiao C, Duan Y (2019) The role of glycogen synthase kinase-3 in gibberellic acid-induced chilling tolerance and defense response in postharvest fruit. Food Bioprocess Technol 12:1733–1740. https://doi.org/10.1007/s11947-019-02338-3
Jiao C, Chai Y, Duan Y (2019) Inositol 1,4,5-trisphosphate mediates nitric-oxide-induced chilling tolerance and defense response in postharvest peach fruit. J Agric Food Chem 67:4764–4773. https://doi.org/10.1021/acs.jafc.9b00153
Jing G, Zhou J, Zhu S (2016) Effects of nitric oxide on mitochondrial oxidative defence in postharvest peach fruits. J Sci Food Agric 96:1997–2003. https://doi.org/10.1002/jsfa.7310
Kim H, Kim J (2008) Thioredoxin as a reducing agent for mammalian methionine sulfoxide reductases b lacking resolving cysteine. Biochem Biophys Res Commun 371:490–494. https://doi.org/10.1016/j.bbrc.2008.04.101
Kinnersley A, Turano F (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509. https://doi.org/10.1080/07352680091139277
Lepistö A, Pakula E, Toivola J, Krieger-Liszkay A, Vignols F, Rintamäki E (2013) Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot 64:3843–3854. https://doi.org/10.1093/jxb/ert216
Li W, Lee S, Chieh P, Lin C, Wang Y, Chan M (2012) Arabidopsis root-abundant cytosolic methionine sulfoxide reductase B genes MsrB7 and MsrB8 are involved in tolerance to oxidative stress. Plant Cell Physiol 53:1707–1719. https://doi.org/10.1093/pcp/pcs114
Ma Y, Wang P, Gu Z, Tao Y, Shen C, Zhou Y, Han Y, Yang R (2019) Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem X 2:10023. https://doi.org/10.1016/j.fochx.2019.100023
Mihara S, Yoshida K, Higo A, Hisabori T (2016) Functional significance of NADPH-thioredoxin reductase C in the antioxidant defense system of Cyanobacterium anabaena sp. PCC 7120. Plant Cell Physiol 58:86–94. https://doi.org/10.1093/pcp/pcw182
Romero M (2004) Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol 136:3784–3794. https://doi.org/10.1104/pp.104.046656
Roy S, Nandi A (2017) Arabidopsis thaliana methionine sulfoxide reductase B8 influences stress-induced cell death and effector-triggered immunity. Plant Mol Biol 93:109–120. https://doi.org/10.1007/s11103-016-0550-z
Sadanandom A, Poghosyan Z, Fairbairn D, Murphy D (2019) Differential regulation of plastidial and cytosolic isoforms of peptide methionine sulfoxide reductase in Arabidopsis. Plant Physiol 123:255–264. https://doi.org/10.1104/pp.123.1.255
Sang T, Shan X, Li B, Shu S, Sun J, Guo S (2016) Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep 35:1769–1782. https://doi.org/10.1007/s00299-016-1995-x
Shang H, Cao S, Yang Z, Cai Y, Zheng Y (2011) Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem 59:1264–1268. https://doi.org/10.1021/jf104424z
Sun X, Sun M, Jia B, Qin Z, Zhu Y (2016) A Glycine soja methionine sulfoxide reductase B5a interacts with the Ca2+/CAM-binding kinase GsCBRLK and activates ROS signaling under carbonate alkaline stress. Plant J 86:514–529. https://doi.org/10.1111/tpj.13187
Tian Y, Peng K, Bao Y, Zhang D, Jing C (2021) Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis. Plant Physiol Biochem 161:86–97. https://doi.org/10.1016/j.plaphy.2021.02.005
Wang Y, Luo Z, Huang X, Yang K, Du R (2014) Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic 168:132–137. https://doi.org/10.1016/j.scienta.2014.01.022
Wang Y, Yang Q, Jiang H, Wang B, Bi Y, Li Y, Prusky D (2020) Reactive oxygen species-mediated the accumulation of suberin polyphenolics and lignin at wound sites on muskmelons elicited by benzo (1, 2, 3)-thiadiazole-7-carbothioic acid S-methyl ester. Postharvest Biol Technol 170:111325. https://doi.org/10.1016/j.postharvbio.2020.111325
Yang A, Cao S, Yang Z, Cai Y, Zheng Y (2011) γ-aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem 129:1619–1622. https://doi.org/10.1016/j.foodchem.2011.06.018
Yang Q, Wang F, Rao J (2016) Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in kiwifruit. PLoS ONE 11:e0162159. https://doi.org/10.1371/journal.pone.0162159
Yao W, Xu T, Farooq S, Jin P, Zheng Y (2019) Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol Technol 144:20–28. https://doi.org/10.1016/j.postharvbio.2018.05.007
Yu G, Zou J, Feng J, Peng X, Wu J, Wu Y, Palanivelu R, Sun M (2014) Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J Exp Bot 65:3235–3248. https://doi.org/10.1093/jxb/eru171
Zhu Y, Wang K, Wu C, Zhao Y, Yin X, Zhang B, Grierson D, Cheng K, Xu C (2019) Effect of ethylene on cell wall and lipid metabolism during alleviation of postharvest chilling injury in peach. Cells 8:1612. https://doi.org/10.3390/cells8121612
Acknowledgements
This project was supported by research start-up funds for high-level talents of Anhui Agricultural University.
Author information
Authors and Affiliations
Contributions
CJ: Conceptualization, Formal analysis, Investigation, Methodology, Data curation, Writing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript.
Additional information
Communicated by Heakeun Yun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiao, C. γ-Aminobutyric acid boosts chilling tolerance by promoting the methionine sulfoxide reductase-thioredoxin reductase system in peach fruit. Hortic. Environ. Biotechnol. 63, 353–361 (2022). https://doi.org/10.1007/s13580-021-00408-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00408-0