Abstract
Key message
Reactive oxygen species (ROS) oxidize methionine to methionine sulfoxide (MetSO) and thereby inactivate proteins. Methionine sulfoxide reductase (MSR) enzyme converts MetSO back to the reduced form and thereby detoxifies the effect of ROS. Our results show that Arabidopsis thaliana MSR enzyme coding gene MSRB8 is required for effector-triggered immunity and containment of stress-induced cell death in Arabidopsis.
Abstract
Plants activate pattern-triggered immunity (PTI), a basal defense, upon recognition of evolutionary conserved molecular patterns present in the pathogens. Pathogens release effector molecules to suppress PTI. Recognition of certain effector molecules activates a strong defense, known as effector-triggered immunity (ETI). ETI induces high-level accumulation of reactive oxygen species (ROS) and hypersensitive response (HR), a rapid programmed death of infected cells. ROS oxidize methionine to methionine sulfoxide (MetSO), rendering several proteins nonfunctional. The methionine sulfoxide reductase (MSR) enzyme converts MetSO back to the reduced form and thereby detoxifies the effect of ROS. Though a few plant MSR genes are known to provide tolerance against oxidative stress, their role in plant–pathogen interaction is not known. We report here that activation of cell death by avirulent pathogen or UV treatment induces expression of MSRB7 and MSRB8 genes. The T-DNA insertion mutant of MSRB8 exaggerates HR-associated and UV-induced cell death and accumulates a higher level of ROS than wild-type plants. The negative regulatory role of MSRB8 in HR is further supported by amiRNA and overexpression lines. Mutants and overexpression lines of MSRB8 are susceptible and resistant respectively, compared to the wild-type plants, against avirulent strains of Pseudomonas syringae pv. tomato DC3000 (Pst) carrying AvrRpt2, AvrB, or AvrPphB genes. However, the MSRB8 gene does not influence resistance against virulent Pst or P. syringae pv. maculicola (Psm) pathogens. Our results altogether suggest that MSRB8 function is required for ETI and containment of stress-induced cell death in Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved a multi-layered defense system to counter invading pathogens. Pattern-triggered immunity (PTI) is activated when plants recognize non-self-conserved molecular patterns that are present in invading microbes, through pattern recognition receptors (PRRs) (Jones and Dangl 2006; Nimchuk et al. 2003). PTI is sufficient to suppress the growth of a vast majority of microbes that interact with plants (Zipfel 2008). Successful pathogens release effector molecules to overcome PTI. To counteract these effectors, plants reciprocate by recruiting several multi-protein complexes that can recognize the presence of effectors and activate effector-triggered immunity (ETI) (Hein et al. 2009; Jones and Dangl 2006; Zhang and Zhou 2010). ETI depends on the presence of matching resistance gene (R) in the plant and avirulent gene (Avr) in the pathogen. A virulent pathogen, which is compatible with a host plant, becomes incompatible in the presence of R–Avr combinations (Jones and Dangl 2006; Nimchuk et al. 2003).
Recognition of either patterns or effectors leads to the activation of signaling cascades, which eventually results in elevated expression of defense-related compounds like phytoalexins and pathogenesis-related (PR) proteins (Spoel and Dong 2012). Plant hormones such as salicylic acid, ethylene, and jasmonic acid play crucial roles in modulating defense responses induced by both compatible and incompatible pathogens (Bari and Jones 2009). The contrasting visible feature of PTI and ETI is the progression of cell death. Incompatible interactions often induce the hypersensitive response (HR), a rapid programmed cell death at the site of infection (Nimchuk et al. 2003). In contrast, the compatible pathogens result in a slower cell death which develops into disease symptoms in infected tissues. Visible HR is preceded by the high-level accumulation of reactive oxygen species (ROS). Pathogen-induced ROS function as signaling molecules for activation of other defense responses in plants (Lamb and Dixon 1997).
ROS can significantly harm cells by damaging biomolecules. Methionine is the most vulnerable amino acid that gets oxidized into methionine sulfoxide (MetSO) by ROS (Gao et al. 1998; Vieira Dos Santos et al. 2005). All organisms including bacteria, yeast, mammals, and plants have developed repair enzymes, known as methionine sulfoxide reductase (MSR), to reduce methionine sulfoxide back into methionine (Moskovitz 2005). MetSOs that are generated upon oxidation stress are the diastereomeric mixture of methionine-R-sulfoxide (Met-R-SO) and methionine-S-sulfoxide (Met-S-SO) (Stadtman et al. 2003). Methionine sulfoxide reductase A (MSRA) and MSRB preferentially use Met-S-SO and Met-R-SO stereoisomers, respectively (Kumar et al. 2002; Neiers et al. 2007; Sharov et al. 1999). Overexpression of rice OsMSRA4.1 provides enhanced salt resistance in transgenic rice plants (Guo et al. 2009). A pepper (Capsicum annuum) MSRB gene (CaMsrB2) is an important regulator of defense against oxidative stress and pathogen attack (Oh et al. 2010). CaMsrB2-silenced pepper plants showed accelerated cell death and enhanced ROS accumulation upon pathogen inoculation (Oh et al. 2010). Expression of maize MSR coding genes (ZmMSRs) enhances upon abiotic stresses induced by polyethylene glycol (PEG) or NaCl treatment (Zhu et al. 2015). AtMSRA2 (alias PMSR2) gene function is required for oxidative stress management in short-day-grown plants (Bechtold et al. 2004). The pmsr2-1, a null mutant of AtMSRA2, exhibits increased protein oxidation, nitration, and glycation of specific amino acid residues during darkness (Bechtold et al. 2004, 2009). Overexpression of AtMSRA4 (alias PMSR4) provides resistance against oxidative stress (Romero et al. 2004), whereas its antisense lines are compromised for growth under high-light stress (Laugier et al. 2013). Arabidopsis genome contains nine genes, named MSRB1 to MSRB9, encoding proteins structurally similar to earlier reported MSRB of Drosophila, yeast, and mouse (Vieira Dos Santos et al. 2005). Physiological roles of only a few MSRB genes are known. Arabidopsis MSRB1 and MSRB2 are chloroplast localized (Laugier et al. 2010; Vieira Dos Santos et al. 2005). The msrB1 msrB2 double mutant transgenic plants are normal under stress-free conditions but growth compromised under high light or low temperature (Laugier et al. 2010). MSRB7 (At4g21830) and MSRB8 (At4g21840) genes are expressed abundantly in root and take part in ROS metabolism (Li et al. 2012). However, the roles of MSRBs in pathogen-induced ROS metabolism or disease defense are not known. We report here that MSRB8 modulates ETI-induced HR and is required for defense against avirulent pathogens.
Results
Avirulent pathogen inoculation and UV treatment induce expression of MSRB7 and MSRB8 genes
By analyzing differentially regulated genes of Arabidopsis thaliana leaves undergoing incompatible interaction with Pseudomonas syringae pv. tomato DC3000 (Pst) carrying AvrRpt2 gene (Pst-AvrRpt2) and mock treatment (unpublished data from the laboratory), we identified MSRB7 and MSRB8 genes, which showed increased expression in the inoculated leaves compared to the control leaves. To compare between virulent and avirulent pathogen-induced expression of these two genes, we analyzed Arabidopsis leaf samples after Pst and Pst-AvrRpt2 inoculation by quantitative real-time PCR (qRT-PCR). Both the pathogens were suspended in 10 mM MgCl2 at 106 CFU/ml. As a control of the experiment, we analyzed 10 mM MgCl2-treated samples. We observed rapid induction in MSRB8 and MSRB7 expression upon Pst-AvrRpt2 inoculation (Fig. 1a, b). We also observed a significant enhancement in the expression of these MSRB genes upon virulent pathogen inoculation, but the pattern of expression was different from that of avirulent pathogen inoculation. Incompatible interaction induced MSRB7 and MSRB8 expression as early as 3 h post-inoculation, which reached a high level within 6–9 h of inoculation. In contrast, a high-level expression of MSRB genes was observed only after 24 h of compatible interaction. Incompatible interaction leads to rapid HR-associated cell death, whereas compatible interaction leads to the development of disease, a process of slow cell death. We also observed rapid induction in the expression of MSRB7 and MSRB8 genes upon UV treatment (Fig. 1c, d). Both pathogen infection and UV treatment lead to ROS accumulation in plants. The results altogether suggested that activation of cell death or ROS accumulation induces expression of MSRB7 and MSRB8 genes in Arabidopsis.
Relative transcript abundance of MSRB8 and MSRB7 genes followed by pathogen and UV treatment. a MSRB8 and b MSRB7 transcript levels after infiltration with 10 mM MgCl2 (Mock) or Pst or Pst-AvrRpt2. c MSRB8 and d MSRB7 transcript levels after UV treatment. Plants were treated with UV light for 20 min and samples were collected at indicated hours after the treatment. Transcript abundance was determined by qRT-PCR. Each bar indicates mean ± standard deviation (n = 3). Experiments were repeated at least two times with similar results. *(P < 0.05) and **(P < 0.001) indicate the mean values that are significantly different from 0 h or respective mock treated sample as determined by students t test
msrB8 mutant enhances ETI-induced HR and H2O2 accumulation
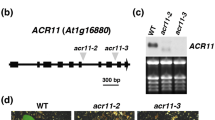
For functional analysis of MSRB genes, we searched for their mutants from the stock center. The Arabidopsis stock center had no insertion mutant having T-DNA insertion in the exonic region of the MSRB7 gene. The closest insertion to the MSRB7 coding sequences was in SALK_014786, which contained a T-DNA insertion within 180 bp upstream of the transcription start site (Supplementary Fig. S1A, S1B). However, SALK_014786 plants were not defective in transcription of MSRB7 gene (Supplementary Fig. S1C). For the MSRB8 gene, there was one line, SALK_081173 with T-DNA insertion in the 3′ untranslated region (Fig. 2a, Supplementary Fig. S1D). UV-induced expression of MSRB8 was observed by reverse-transcription PCR (RT-PCR) in WT plants but not in SALK_081173 plants (Fig. 2b). Thus, we considered the SALK_081173 line as a mutant of MSRB8 gene. The mutant plants grew like normal wild-type Col-0 plants (Fig. 2c), suggesting that MSRB8 gene was either non-essential for survival or redundant in Arabidopsis under stress-free growth conditions.
Confirmation of msrB8 mutation and HR-induced ion leakage in WT and msrB8 mutant plants. a Confirmation of T-DNA insertion in MSRB8 gene in SALK_081173 line. LP and RP are gene-specific primers and LB is a T-DNA border-specific primer. Plants from T-DNA insertion lines are numbered. b Expression of MSRB8 in SALK_081173 (msrB8) line. c Morphology of 5-week-old soil-grown wild-type plants and homozygous T-DNA insertion mutants of msrB8. d Ion leakage in WT and msrB8 after Pst-AvrRpt2 inoculation. Each bar represents the mean ± standard deviation of three biological samples each containing eight leaf discs of 8 mm diameter. e DAB staining in WT and msrB8 after mock and Pst-AvrRpt2 inoculation. f Quantitative analysis of DAB stain intensity. Each bar represents relative mean intensity of five whole leaves of the indicated genotype and the treatment. *(P < 0.05) and **(P < 0.001) indicate the mean values that are significantly different from respective WT sample as determined by students t test
Since our results linked the MSRB8 expression to cell death induction in Arabidopsis, we investigated HR in msrB8 mutant plants. When a tissue undergoing HR is floated on water, leakage of cellular contents from dead cells enhance the electrical conductivity of the water, which quantitatively reflects the extent of cell death (Torres et al. 2002). We infiltrated WT and msrB8 mutant plants with either Pst-AvrRpt2 at 107 CFU/ml suspended in 10 mM MgCl2 or only 10 mM MgCl2 as control and monitored ion leakage. As expected, WT leaves inoculated with Pst-AvrRpt2 showed enhanced ion leakage, but the control/mock-inoculated leaves did not (Fig. 2d). In comparison to WT plants, the msrB8 mutant plants showed a much higher level of ion leakage (Fig. 2d). The results suggest that the MSRB8 gene functions as a negative regulator for HR-induced cell death. To further support our observation, we measured relative H2O2 accumulation by DAB staining, after mock or Pst-AvrRpt2 inoculation in WT and msrB8 plants. In agreement with higher HR-induced cell death, we observed a significantly higher level of H2O2 accumulation in msrB8 than WT plants (Fig. 2e, f).
amiRNA and overexpression lines support negative regulatory roles of MSRB8 gene in HR- and UV-induced cell death
We wanted to investigate the redundancy in function between MSRB7 and MSRB8 genes by generating double mutants. Since we could not get a mutant of MSRB7, we took the artificial miRNA (amiRNA) approach to downregulate both the genes simultaneously (Supplementary Fig. S2). Suppression of MSRB genes was tested in the homozygous T2 progeny of transgenic amiRNA lines after inducing the expression of MSRB genes with Pst-AvrRpt2. Out of five lines tested, two lines, i.e., #1 and #5, showed high-level suppression of MSRB8 expression (Fig. 3a). However, we could not generate any amiRNA lines with near complete suppression of MSRB7 expression. Incidentally, lines #1 and #5 also showed the least level of MSRB7 mRNA accumulation compared to the other lines (Fig. 3a) and thus were taken for further studies. These two amiRNA lines also did not show UV-induced MSRB expression as shown for line #5 (Fig. 3b). We also did not observe any morphological defect in the amiRNA lines (Fig. 3c). No growth defect in the amiRNA lines may be attributed to functional redundancy or partial suppression of the MSRB7 gene. Nevertheless, we studied the effect of simultaneous downregulation of MSRB7 and MSRB8 in HR, after inoculating amiRNA lines with Pst-AvrRpt2. As shown earlier for the msrB8 mutant (Fig. 2d), both the amiRNA lines (#1 and #5) displayed more HR-induced ion leakage than WT plants (Fig. 3d, and Supplementary Fig. S3). Interestingly, the extent of ion leakage in both the amiRNA lines was similar to that of the single mutant msrB8 plants (Fig. 3d, and Supplementary Fig. S3), which suggests that either MSRB7 has no additional role in controlling cell death or the low level of expression that was observed in the amiRNA lines was sufficient for its function. Nevertheless, the results reconfirmed the importance of the MSRB8 gene in regulating HR in Arabidopsis.
Expression of MSRB genes and HR-induced ion leakage in amiRNA lines. a Expression of MSRB7 and MSRB8 in WT plants and T2 generation of transgenic plants carrying amiRNA construct. Plants were inoculated with Pst-AvrRpt2 at the dose of 5 × 106 CFU/ml and samples harvested 8 h post-inoculation. b Expression of MSRB7 and MSRB8 in line # 5 after UV treatment. WT and transgenic plants were treated with UV light for 20 min. Samples for expression analysis were collected at 6 hpt. c Photo of 5-week-old WT and amiRNA MSRB line #5, in normal growth conditions. d Ion leakage in WT, msrB8, and amiRNA MSRB line #5. Leaves were pressure infiltrated with Pst-AvrRpt2 suspension (1 × 107 CFU/ml) or 10 mM MgCl2 as mock control. Infected leaf discs were weighed before floating on distilled water. Electrical conductivity was measured at the indicated time points. Each bar represents the mean ± standard deviation of three biological samples each containing eight leaf discs of 8 mm diameter. *(P < 0.05) and **(P < 0.001) indicate the mean values that are significantly different from respective WT sample as determined by students t test
To substantiate our observations, we generated a transgenic Arabidopsis line with overexpression of MSRB8 under a constitutive cauliflower mosaic virus 35S (CaMV35S) promoter. The cDNA of MSRB8 was cloned in pCXSN-HA vector (Chen et al. 2009) and transgenic plants were generated. The transgenic plants showed high-level accumulation of MSRB8 transcript under stress-free normal growth conditions (Fig. 4a). Moreover, similar to the mutant, overexpression lines also did not show any growth defect (Fig. 4b). With the observations from the mutant and the amiRNA lines, we anticipated suppression of HR in the MSRB8 overexpression lines. As anticipated, upon Pst-AvrRpt2 inoculation, the CaMV35S:MSRB8 plants showed reduced ion leakage compared to the WT plants (Fig. 4c). To visualize the influence of MSRB8 gene on HR-induced cell death, we stained mock or Pst-AvrRpt2 inoculated leaves of WT, msrB8 mutant, and MSRB8-Oex plants with trypan blue, which stains dead cells dark blue (Nandi et al. 2003). We observed a significantly higher level of stained cells in Pst-AvrRpt2 inoculated msrB8 mutant plants than WT plants (Fig. 5a). In contrast, the MSRB8 overexpression leaves showed fewer dead cells than WT plants. The results were in accordance with ion leakage studies. To investigate whether the influence of MSRB8 in cell death was restricted to HR, we performed trypan blue staining after UV treatment. The plants were treated with UV or mock for 50 min and cell death was monitored 2 h after the treatment. Very similar to avirulent pathogen, UV treatment also resulted in more cell death in msrB8 and less cell death in MSRB8-Oex compared to WT plants (Fig. 5b). However, the mock-treated leaves of both the experiments did not show any significant cell death (Fig. 5a, b). The results altogether showed that Arabidopsis MSRB8 negatively regulates stress-induced cell death.
Overexpression of MSRB8 and its influence in HR-induced ion leakage. a Expression of MSRB8 gene by RT-PCR in WT and two transgenic lines, #1 and #9, under normal growth condition. b Morphological phenotype of 5-week-old WT and overexpression (MSRB8 Oex) line. c Ion leakage in WT and MSRB8 OeX line. Each bar represents the mean ± standard deviation of three biological samples each containing eight leaf discs of 8 mm diameter. *(P < 0.05) and **(P < 0.001) indicate the mean values that are significantly different from respective WT sample as determined by students t test
MSRB8 positively regulates resistance against avirulent pathogens
Since we observed the influence of MSRB8 on pathogen-induced cell death and ROS accumulation, we anticipated its involvement in disease defense. We monitored bacterial numbers in WT and msrB8 mutant plants after inoculating with Pst-AvrRpt2 at 5 × 105 CFU/ml. We observed 5- to 7-fold more bacterial growth in msrB8 mutants than WT plants after 3 days of inoculation (Fig. 6a). Higher bacterial load also yielded more visible disease symptoms in msrB8 plants than WT plants (Fig. 6a). The similar loss of resistance against Pst-AvrRpt2 was also observed in amiRNA lines (Fig. 6b, Supplementary Fig. S4). The observation was further validated using overexpression lines. The MSRB8 overexpression lines were significantly more resistant than the WT plants (Fig. 6c, Supplementary Fig. S4). Disease defense against the hemibiotrophic pathogen is often accompanied by the induction of PR1 gene expression, a marker gene of SA-mediated defense signaling (Giri et al. 2014; Swain et al. 2011). Compared to WT plants, we observed enhanced and reduced accumulation of PR1 transcript in MSRB8 overexpression and mutant plants respectively, after Pst-AvrRpt2 inoculation (Fig. 6d). PR1 expression pattern was in agreement with bacterial growth in these plants. The results suggested that MSRB8 positively regulates ETI against Pst-AvrRpt2. To further investigate whether the influence of MSRB8 was specific against AvrRpt2 effector, we challenged WT and msrB8 plants with Pst carrying AvrB or AvrPphB effectors. We observed a similar loss-of-resistance phenotype in msrB8 plants against both of these avirulent pathogens (Fig. 6e, f). However, in contrast to avirulent pathogens, the effect of msrB8 mutation on the growth of virulent pathogens was negligible. We did not observe any significant difference in bacterial load in the mutant or overexpression lines of MSRB8 when challenged with Pst DC3000 (Fig. 6g) or P. syringae pv. maculicola ES4326 (Fig. 6h). The results demonstrate that MSRB8 function is required for the ETI induced by avirulent pathogens, but not for general resistance induced by virulent pathogens.
Influence of MSRB8 on disease resistance. a Pst-AvrRpt2 counts and disease symptoms at 3 days post inoculation (dpi) in WT and msrB8 mutant plants. b Pst-AvrRpt2 counts and disease symptoms at 3 dpi in WT and amiRNA plants. c Pst-AvrRpt2 counts and disease symptoms at 3 dpi in WT and MSRB8 overexpression plants. d Relative transcript accumulation of PR1 gene in WT, msrB8 mutant, and MSRB8 overexpression lines. e Pst-AvrB counts and disease symptoms at 3 dpi in WT and msrB8 plants. f Pst-AvrPphB counts and disease symptoms at 3 dpi in WT and msrB8 plants. g Pst DC3000 counts at 3 dpi in WT, msrB8, and MSRB8-OE plants. h Psm ES4326 counts at 3 dpi in WT, msrB8, and MSRB8-OE plants. For all the experiments, overnight grown pathogens were infiltrated at 5 × 105 CFU/ml. Each bar of bacterial counts represents the mean ± standard deviation of four samples, in which each sample consist of five leaf disc of 5 mm diameter. Different letters above the bars indicated statistically significant difference (P < 0.05) as obtained by one-way ANOVA (Holm–Sidak method). In d relative transcript accumulation of PR1 was quantified by qRT-PCR. *(P < 0.05) and **(P < 0.001) indicate the mean values that are significantly different from respective WT sample as determined by students t test
MSRB8 function is essential under high-ROS condition
Avirulent pathogens produce a much higher level of ROS than virulent pathogens in WT Arabidopsis (Fig. 7a; Lamb and Dixon 1997; Torres et al. 2006). And compared to WT plants, msrB8 mutants accumulated a higher level of ROS after avirulent pathogen inoculation (Figs. 2e, f, 7a). However, there was no significant enhancement of ROS accumulation in msrB8 plants after virulent pathogen inoculation (Fig. 7a). These results suggest that MSRB8 function may be essential only under high-level ROS conditions. To support this assumption, we germinated WT seedlings in the presence of methyl viologen (MV) that enhances ROS and thereby induces MetSO production (Ozgur et al. 2015; Romero et al. 2004). MV application inhibited germination and seedling growth in a dose-dependent manner, both in WT and msrB8 mutant (Fig. 7b). We observed that at low concentrations of MV (up to 0.1 µM supplemented with MS medium) there was no significant difference in seedling growth between WT and msrB8 mutant. However, at higher concentrations (0.5 and 1 µM), msrB8 had more severe growth retardation than WT plants. A similar difference was observed in primary root elongation when 4-day-old seedlings were transferred to MV-containing plates and grown for another 3 days (Fig. 7c). Together these results show that under low-level ROS, MSRB8 function is redundant, but is essential under high-level ROS conditions (Fig. 7d).
Role of MSRB8 under different ROS levels. a DAB staining of WT and msrB8 leaves after Pst-AvrRpt2 (106) and Pst (5 × 105) and mock inoculation. b Effect of methyl viologen on seed germination and seedling growth. WT and msrB8 mutant plants were germinated on MS plates supplemented with different concentration of methyl viologen. Photograph was taken 14 days after germination. c Primary root length of WT and msrB8 mutant plants grown under different concentrations of methyl viologen. Seeds were germinated on MS plates and 4 days after germination were transferred onto methyl viologen-supplemented plates and grown of another 3 days. Each bar represents mean ± standard deviation of five seedlings. **(P < 0.001) indicates that the mean value of msrB8 is significantly different from the corresponding WT plants, as determined by Student’s t test. d Model depicting the role of MSRB8. Under the low-level ROS conditions, several MSRBs redundantly function to revert Met-R-SO to Met; however, under the high-ROS conditions, the MSRB8 function is essential
Discussion
Expression of MSRB7 and MSRB8 genes
HR is the most prominent visible defense response that a plant exerts against avirulent pathogens. Our results showed that HR or UV treatment induce expression of MSRB7 and MSRB8 genes (Fig. 1). Pathogenesis in plants is associated with ROS generation (Alvarez et al. 1998; Grant et al. 2000). Since MSRB functions are related to undoing the effects of ROS, it is tempting to believe that ROS trigger the expression of MSRB genes; which is also supported by methyl viologen treatment that enhances ROS accumulation (Li et al. 2012). However, our results suggest that it is not ROS per se, but the associated cellular damage that may induce expression of MSRB genes. Plants show a biphasic ROS accumulation upon avirulent pathogen inoculation (Baker and Orlandi 1995; Chandra et al. 1996; Grant and Loake 2000). Within the first hour of inoculation with either an avirulent or virulent pathogen, plants accumulate a substantial amount of ROS. When plants are challenged with a virulent pathogen, accumulated ROS gets rapidly scavenged, and within a few hours of inoculation, the ROS level returns to almost pre-inoculation levels. On the contrary, the ROS accumulation is sustained over an extended period when plants are challenged with an avirulent pathogen (Chandra et al. 1996; Grant and Loake 2000; Grant et al. 2000). Since we observed early induction of MSRB7 and MSRB8 genes upon inoculation only with the avirulent pathogen, and a delayed induction by the virulent pathogen, there is no obvious correlation between time of ROS accumulation and expression of MSRB genes. However, there is a correlation between induction of cell death and expression of these genes. Avirulent pathogen induces cell death in the form of HR rapidly within a few hours of inoculation. In contrast, virulent pathogen induces a slow cell death process taking over several days (Al-Daoude et al. 2005; Nandi et al. 2004; Singh et al. 2013a; Swain et al. 2011). Since MSRB7 and MSRB8 genes play important roles in recovering cellular processes to the normal state, it may be possible that the expression of these genes is regulated by the ROS scavenging system.
Role of MSRB8 gene in disease defense
MSRs are induced during oxidative stress to protect the functional activity of proteins by reducing methionine sulfoxide to methionine (Vieira Dos Santos et al. 2005). This enzyme-coding gene is included in the minimal gene set that is required for life, as determined by comparing several bacterial genomes (Mushegian and Koonin 1996). Since the Arabidopsis genome contains multiple copies of MSR-coding genes, knockdown lines do not show any morphological or physiological defect under normal stress-free conditions (Fig. 2; Bechtold et al. 2004; Li et al. 2012). Overexpression of MSRB7 and MSRB8 yields elevated resistance against oxidative stress (Li et al. 2012). Our observations of increased HR-induced ion leakage in the knockdown lines (Figs. 2, 3) and reduced stress-induced cell death in the overexpression plants (Figs. 4, 5) are in accordance with the proposed role of MSRB genes. Plants produce ROS at much higher levels when ETI is activated by avirulent pathogens, compared to virulent pathogen-induced PTI (Chandra et al. 1996; Lamb and Dixon 1997; Torres et al. 2002, 2006). No significant difference in virulent bacterial growth between WT and msrB8 suggests that under low-level ROS conditions, activity of other MSRB gene(s) is sufficient to detoxify the effect of ROS. However, under high-level ROS conditions, such as avirulent pathogen infection, UV, or MV treatment, MSRB8 function becomes non-redundant (Figs. 2, 3, 4, 5, 6, 7).
Proteins are the primary targets of ROS in the cell (Davies 2005). Since MSRB would reduce methionine sulfoxide to methionine, loss of MSRB function would be predicted to increase methionine sulfoxide levels in the proteins of msrB mutant plants. Protein oxidation may have a wide range of consequences, including fragmentation, dimerization, and unfolding (Davies 2005). In addition, protein oxidation leads to the formation of superoxide radical, H2O2, and other reactive species, as well as other oxidized proteins in chain reactions (Davies 2005; Neužil et al. 1993). Thus accumulation of methionine sulfoxides is likely to contribute in HR-induced membrane disintegration and ion leakage. HR is often associated with the resistance response. However, certain experimental results uncouple cell death from resistance in incompatible interactions (Al-Daoude et al. 2005; Van Poecke et al. 2007). Overexpression of RPM1 Interacting Protein 13 (RIN13) suppresses HR, but provides resistance against avirulent pathogens, carrying the AvrB or AvrRpm1 gene (Al-Daoude et al. 2005). A study with ten different accessions of Arabidopsis did not find any correlation between HR and bacterial growth, after Pst-AvrRpt2 inoculation (Van Poecke et al. 2007). We also observed a similar lack of association between cell death and resistance. Bacterial growth in MSRB8 overexpression and knockdown lines showed an inverse relationship with HR-associated ion leakage and disease defense against avirulent pathogen. The msrB8 mutant and amiRNA MSRB lines showed a higher level of HR than WT plants, but also showed susceptibility towards avirulent pathogens. On the contrary, overexpression of MSRB8 suppressed HR and also suppressed bacterial growth. Thus, our studies reiterate that HR can either be a resistance response or a means of susceptibility towards pathogens (Heath 2000; Mur et al. 2008; Stakman 1915). Despite a significant number of reports showing the positive association between HR and resistance reaction, how cell death promotes resistance remains elusive. Often, cell death is believed to generate signals for defense activation, rather than functioning directly as a defense mechanism (Heath 2000). Thus, it may be possible that MSRB8 activity delays the process of cell death, which in turn favors generation of such defense signals.
Materials and methods
Plant growth conditions and pathogen inoculation
The growth of plants, bacterial pathogens, and inoculation experiments were carried out as described previously (Singh et al. 2013b; Swain et al. 2011). In brief, plants were grown in Soilrite and vermiculite mixture (4:1) in the growth room under controlled environmental conditions at 22 °C, 70 % humidity, and 12 h alternating light (80 µE/m2/s) and dark period. All bacterial pathogen strains were grown in King’s B medium with appropriate antibiotics. Overnight grown bacterial pathogens were suspended in 10 mM MgCl2 and pressure infiltrated through the abaxial surface of leaves with a needleless syringe. Bacterial counts in inoculated leaves were determined at 3 dpi.
UV treatment, HR induction, and ion leakage
For UV treatment, 5-week-old soil-grown plants were placed below a UV bulb (TUV 30 W UV-C, Philips, the Netherlands) at 36 cm distance from the tube. Plants were exposed to the UV for 20 min and then placed back in the growth room before harvesting for expression analysis. For HR-induced ion leakage study, Pst-AvrRpt2 (1 × 107 CFU/ml suspended in 10 mM MgCl2) was pressure infiltrated through the abaxial surface of the leaves. Only 10 mM MgCl2 was infiltrated as mock treatment. Plants were covered with a plastic dome and kept under dark conditions in the growth room for 7 h, after which, inoculated leaves were punched with a 8-mm-diameter cork borer and weighed before extensive washing for 45 min in distilled water. Leaf discs were floated on distilled water in a six-well plate. Each sample containing eight leaf discs with three biological replications was taken for each set. The conductivity of water was measured to check ion leakage at different time points.
Trypan blue staining
For studying HR-induced cell death, leaves were infiltrated with the overnight grown culture at 106 CFU/ml. Plants were incubated in the dark for 10 h and then in the light for 2 h before harvesting samples. For UV treatment, whole plants were exposed to UV as mentioned above for 50 min, transferred to the growth room, and kept under light by covering with a plastic dome. Samples were harvested 2 h after completion of the UV treatment. Trypan blue staining was carried out as described earlier (Nandi et al. 2003).
RNA isolation and gene expression studies
RNA was extracted by the guanidine-phenol method and treated with DNase I (Thermo Scientific, USA) for 30 min at 37 °C, which was subsequently inactivated by adding 5 mM EDTA and heating at 65 °C. cDNA synthesis was carried out with the iScript cDNA Synthesis Kit (170–8891 BIO-RAD, USA) taking 1 µg of RNA per reaction. Fivefold-diluted cDNA was used for gene expression analysis by either semiquantitative reverse-transcription PCR (RT-PCR) or quantitative real-time PCR (qRT-PCR). qRT-PCR reactions were carried out in 96-well plates in 10 µl of reaction using SYBR green mix (Applied Biosystem, USA) in a Fast7500 Real Time PCR system from Applied Biosystems. Gene expression was normalized with the expression of ACTIN2 (At3g18780). As a usual practice, each experiment set consisted of three biological samples with two technical replicates.
Generation of amiRNA lines
Primers were designed with the help of microRNA designer software (http://wmd3.weigelworld.org). The amiRNA construct was generated using a pRS300 plasmid as the template which contains the miR319a precursor in pBSK vector (Schwab et al. 2006) and finally cloned in binary vector pBI121 between SacI and SmaI under the control of CaMV35S promoter. C58 strain of Agrobacterium was transformed with the amiRNA construct. Transgenic Arabidopsis plants were generated through the Agrobacterium-mediated floral dip method (Zhang et al. 2006). Inflorescence of Arabidopsis Col-0 ecotype was dipped in Agrobacterium suspension. Transformed T1 seeds were screened on MS media supplemented with kanamycin (50 mg/l). Presence of the transgene in antibiotic-resistant plants was confirmed later on by PCR and expression analysis.
Generation of overexpression lines
MSRB8 coding sequence of 432 bp was PCR amplified using a high fidelity DNA polymerase (Phusion, NEB) from Arabidopsis cDNA and cloned into the pCXSN-HA binary vector (Chen et al. 2009) between two XcmI sites under a constitutive CaMV35S promoter. Overexpression lines were generated as described above. Transformed T1 seeds were screened on MS media supplemented with hygromycin (25 mg/l). The presence of the transgene in antibiotic resistant plants was confirmed by PCR and expression analysis. Primers used for gene expression analysis, miRNA and overexpression vectors construction are given in Supplementary Table S1.
Detection of H2O2 by DAB staining
Leaves of 5-week-old plants were syringe infiltrated with 10 mM MgCl2 or Pst-AvrRpt2 at 106 CFU/ml or Pst at 5 × 105 CFU/ml. Inoculated plants were kept in the growth room overnight. Infiltrated leaves were cut along with petiole and floated on water. After 2 h, the water was replaced with DAB solution (1 mg/ml DAB in 10 mM Na2HPO4 and 0.05 % Tween 20), and vacuum infiltrated for 5 min at 100 mmHg pressure. The leaves were incubated in the dark for 4–5 h with gentle shaking. The DAB solution was replaced with 95 % ethanol and boiled for 5–10 min to remove chlorophyll. Stained leaves were transferred to 50 % ethanol and photographed. Quantitative analysis of DAB stain intensity was done by ImageJ software.
Methyl viologen treatment
To study the effect of methyl viologen on seedling growth, seeds were germinated on MS medium only or MS supplemented with methyl viologen. Photographs of seedlings were taken after 14 days of germination. For root length assay, seeds were germinated on MS medium for 4 days and then transferred onto methyl viologen-supplemented plates, and grown vertically for next 3 days. Primary root length was recorded for five plants of each genotype.
Abbreviations
- amiRNA:
-
Artificial microRNA
- Avr:
-
Avirulent
- CaMV35S:
-
Cauliflower mosaic virus 35S
- ETI:
-
Effector-triggered immunity
- hpi:
-
Hours post inoculation
- HR:
-
Hypersensitive response
- Met-R-SO:
-
Methionine-R-sulfoxide
- Met-S-SO:
-
Methionine-S-sulfoxide
- MSR:
-
Methionine sulfoxide reductase
- PR:
-
Pathogenesis related
- Pst:
-
Pseudomonas syringae pv. tomato
- PTI:
-
Pattern triggered immunity
- qRT-PCR:
-
Quantitative real-time PCR
- RIN13:
-
RPM1 interacting protein 13
References
Al-Daoude A, de Torres Zabala M, Ko JH, Grant M (2005) RIN13 is a positive regulator of the plant disease resistance protein RPM1. Plant Cell 17:1016–1028
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33:299–321
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Bechtold U, Murphy DJ, Mullineaux PM (2004) Arabidopsis peptide methionine sulfoxide reductase2 prevents cellular oxidative damage in long nights. Plant Cell 16:908–919
Bechtold U, Rabbani N, Mullineaux PM, Thornalley PJ (2009) Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J 59:661–671
Chandra S, Martin GB, Low PS (1996) The Pto kinase mediates a signaling pathway leading to the oxidative burst in tomato. Proc Natl Acad Sci USA 93:13393–13397
Chen S, Songkumarn P, Liu J, Wang GL (2009) A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol 150:1111–1121
Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703:93–109
Gao J, Yin D, Yao Y, Williams TD, Squier TC (1998) Progressive decline in the ability of calmodulin isolated from aged brain to activate the plasma membrane Ca-ATPase. BioChemistry 37:9536–9548
Giri MK, Swain S, Gautam JK, Singh S, Singh N, Bhattacharjee L, Nandi AK (2014) The Arabidopsis thaliana At4g13040 gene, a unique member of the AP2/EREBP family, is a positive regulator for salicylic acid accumulation and basal defense against bacterial pathogens. J Plant Physiol 171:860–867
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124:21–29
Grant JJ, Yun BW, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24:569–582
Guo X, Wu Y, Wang Y, Chen Y, Chu C (2009) OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta 230:227–238
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hein I, Gilroy EM, Armstrong MR, Birch PR (2009) The zig-zag-zig in oomycete-plant interactions. Mol Plant Pathol 10:547–562
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kumar RA, Koc A, Cerny RL, Gladyshev VN (2002) Reaction mechanism, evolutionary analysis, and role of zinc in Drosophila methionine-R-sulfoxide reductase. J Biol Chem 277:37527–37535
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Ann Rev Plant Physiol Plant Mol Biol 48:251–275
Laugier E, Tarrago L, Vieira Dos Santos C, Eymery F, Havaux M, Rey P (2010) Arabidopsis thaliana plastidial methionine sulfoxide reductases B, MSRBs, account for most leaf peptide MSR activity and are essential for growth under environmental constraints through a role in the preservation of photosystem antennae. Plant J 61:271–282
Laugier E et al (2013) Involvement of thioredoxin y2 in the preservation of leaf methionine sulfoxide reductase capacity and growth under high light. Plant Cell Environ 36:670–682
Li CW, Lee SH, Chieh PS, Lin CS, Wang YC, Chan MT (2012) Arabidopsis root-abundant cytosolic methionine sulfoxide reductase B genes MsrB7 and MsrB8 are involved in tolerance to oxidative stress. Plant Cell Physiol 53:1707–1719
Moskovitz J (2005) Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 1703:213–219
Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520
Mushegian AR, Koonin EV (1996) A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc Natl Acad Sci USA 93:10268–10273
Nandi A, Krothapalli K, Buseman CM, Li M, Welti R, Enyedi A, Shah J (2003) Arabidopsis sfd mutants affect plastidic lipid composition and suppress dwarfing, cell death, and the enhanced disease resistance phenotypes resulting from the deficiency of a fatty acid desaturase. Plant Cell 15:2383–2398
Nandi A, Welti R, Shah J (2004) The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell 16:465–477
Neiers F, Sonkaria S, Olry A, Boschi-Muller S, Branlant G (2007) Characterization of the amino acids from Neisseria meningitidis methionine sulfoxide reductase B involved in the chemical catalysis and substrate specificity of the reductase step. J Biol Chem 282:32397–32405
Neužil J, Gebicki JM, Stocker R (1993) Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J 293:601–606
Nimchuk Z, Eulgem T, Holt BF 3rd, Dangl JL (2003) Recognition and response in the plant immune system. Annu Rev Genet 37:579–609
Oh SK et al (2010) CaMsrB2, pepper methionine sulfoxide reductase B2, is a novel defense regulator against oxidative stress and pathogen attack. Plant Physiol 154:245–261
Ozgur R, Uzilday B, Sekmen AH, Turkan I (2015) The effects of induced production of reactive oxygen species in organelles on endoplasmic reticulum stress and on the unfolded protein response in Arabidopsis. Ann Bot 116:541–553
Romero HM, Berlett BS, Jensen PJ, Pell EJ, Tien M (2004) Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol 136:3784–3794
Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18:1121–1133
Sharov VS, Ferrington DA, Squier TC, Schoneich C (1999) Diastereoselective reduction of protein-bound methionine sulfoxide by methionine sulfoxide reductase. FEBS Lett 455:247–250
Singh S, Giri MK, Singh PK, Siddiqui A, Nandi AK (2013a) Down-regulation of OsSAG12-1 results in enhanced senescence and pathogen-induced cell death in transgenic rice plants. J Biosci 38:583–592
Singh V, Roy S, Giri MK, Chaturvedi R, Chowdhury Z, Shah J, Nandi AK (2013b) Arabidopsis thaliana FLOWERING LOCUS D is required for systemic acquired resistance. Mol Plant Microbe Interact 26:1079–1088
Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol 12:89–100
Stadtman ER, Moskovitz J, Levine RL (2003) Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal 5:577–582
Stakman EC (1915) Relation between Puccina graminis and plants highly resistant to its attack. J Agric Res 4:193–199
Swain S, Roy S, Shah J, Van Wees S, Pieterse CM, Nandi AK (2011) Arabidopsis thaliana cdd1 mutant uncouples the constitutive activation of salicylic acid signalling from growth defects. Mol Plant Pathol 12:855–865
Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Van Poecke RM, Sato M, Lenarz-Wyatt L, Weisberg S, Katagiri F (2007) Natural variation in RPS2-mediated resistance among Arabidopsis accessions: correlation between gene expression profiles and phenotypic responses. Plant Cell 19:4046–4060
Vieira Dos Santos C, Cuine S, Rouhier N, Rey P (2005) The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol 138:909–922
Zhang J, Zhou JM (2010) Plant immunity triggered by microbial molecular signatures. Mol Plant 3:783–793
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Zhu J, Ding P, Li Q, Gao Y, Chen F, Xia G (2015) Molecular characterization and expression profile of methionine sulfoxide reductase gene family in maize (Zea mays) under abiotic stresses. Gene 562:159–168
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Acknowledgments
We thank Zeeshan Z. Banday for comments on the manuscript. We acknowledge Arabidopsis Biological Resource Center, Ohio State University, USA for the mutant seeds. This work is supported by financial assistance from DST projects (F. No.SERB/SR/SO/PS/150/2012). SR is a recipient of a Council for Scientific and Industrial Research (CSIR) fellowship.
Author contributions
SR performed the experiments, analyzed data, and wrote the manuscript. AKN conceptualized the project, designed the experiments, and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roy, S., Nandi, A.K. Arabidopsis thaliana methionine sulfoxide reductase B8 influences stress-induced cell death and effector-triggered immunity . Plant Mol Biol 93, 109–120 (2017). https://doi.org/10.1007/s11103-016-0550-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0550-z