Abstract
The induction of chilling tolerance and defense response by glycogen synthase kinase-3 (GSK-3) under gibberellic acid (GA3) treatment in peach fruit was explored at 4 °C up to 28 days. The fruits were treated with exogenous GA3 and bikinin (GSK-3 inhibitor). Results showed that exogenous GA3 alleviated chilling injury, and upregulated endogenous GA3 content in peach fruit. Furthermore, GSK-3 expression was activated by exogenous GA3 treatment. GA3 also upregulated gene expression of superoxide dismutase, peroxidase, catalase, glutathione reductase, glutathione S-transferase, and ascorbate peroxidase. Additionally, GA3 enhanced gene and protein expression of small ubiquitin-like modifier and gene expression of methionine sulfoxide reductase, and weakened gene expression of lipoxygenase and phospholipase D. These above impacts stimulated by exogenous GA3 were blocked by the addition of bikinin. Overall, GSK-3 was involved in stimulation of chilling tolerance and defense response under GA3 treatment in postharvest peach fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Refrigeration is a commonly used storage technology to extend the storage life and to inhibit decay of fruits. Peach fruit is recommended to be stored at − 1 to 0 °C (depending on the variety). Nevertheless, it has been widely reported that the temperature range of 2–7 °C induce chilling injury in peach fruit (Crisosto and Mitchell 2002; Lurie and Crisosto 2005). The most common visual symptoms of CI of harvested peach fruit are internal browning and flesh mealiness, thereby shortening postharvest life and reducing consumer acceptance (Lurie and Crisosto 2005). These chilling disorders resulted in the reduction of consumer acceptance and increase in postharvest losses of the fruit. Therefore, potential approaches to alleviate chilling injury are in need for its storage and transportation.
The phytohormones regulate many physiological processes of plant growth and development (Ding et al. 2013). Accordingly, gibberellic acid (GA3) treatment effectively enhanced chilling tolerance in tomato (Zhu et al. 2016) and peach (Dagar et al. 2012; Gang et al. 2015; Pegoraro et al. 2015; Weksler et al. 2012) fruit. However, activation of endogenous signal compounds has been shown to be one of the mechanisms involved in chilling tolerance in postharvest fruit (Ruan et al. 2015). It is necessary to explore the downstream messengers involved in GA3-alleviated CI in postharvest fruit. It has reported that glycogen synthase kinase-3 (GSK-3), a critical signal compound, mediated isoflavone biosynthesis in soybean sprouts in response to UV-B exposure (Jiao et al. 2017). Nevertheless, little is available in the existing reports concerning the mediation of GA3-alleviated CI by GSK-3 in peach fruit.
Oxidative damage has been considered as an early response in sensitive plant tissues to CI (Ding et al. 2016). Plants elevate levels of both antioxidant enzymes and non-enzymatic antioxidants to detoxification of free radical (Santa-Cruz et al. 2014). It has been reported that GSK-3 regulated NF-E2-related factor (Nrf2) antioxidant response in JFH1 HCV-infected Huh7.5.1 hepatocytes (Jiang et al. 2015). This suggested that GSK-3 may be involved in antioxidant system in animals. Nevertheless, the role of GSK-3 in antioxidant system in peach fruit under GA3 treatment is not available in the literature. In addition, accordingly, small ubiquitin-like protein modifiers (SUMO) proteases regulated ROS production in Arabidopsis (Conti et al. 2007). A conserved functional SUMO interacting motif (SIM) in the gibberellin receptor has been identified in cereal crops (Nelis et al. 2015). Little attention is received about the mediation of the induction of SUMO by GSK-3 under GA3 treatment in peach fruit.
Furthermore, the ROS generation during plant growth could result in lipid peroxidation, which may ultimately affect cell membrane permeability. Lipoxygenase (LOX) and phospholipase D (PLD) are shown to be the vital enzymes leading to membrane lipid degradation in plants (Yao et al. 2018). Apart from lipid peroxidation, excess ROS generation also resulted in the conversion of methionine (Met) residue to Met sulfoxide (MetO). The methionine sulfoxide reductase (MSR) could revert S- and R-diastereoisomers of MetO into Met (the reduced form), thereby protecting proteins against oxidative stress-caused damage. The MSR could be induced constantly by abscisic acid in tomato (Dai and Wang 2010). Nevertheless, little is available in regard to the effects of GSK-3 on LOX, PLD and MSR under GA3 treatment in peach fruit.
Based on the above reports, little is available concerning the mediation by endogenous signal transduction of GA3-alleviated CI in peach fruit. Therefore, the aims of the research were to investigate the regulation of the CI index and gene expression for enzymes participated in antioxidant system, SUMO, LOX, PLD, and MSR by GSK-3 under GA3 treatment, and to explore the signal transduction mechanism in postharvest peach fruit.
Materials and Methods
Fruit Materials and Postharvest Treatments
Peach fruits (P. persica Batsch cv. Jinqiuhongmi) were harvested at commercial maturity from a local orchard in Beijing, China. The peaches were chosen for uniformity without any damage and randomly divided into three groups:
-
(1)
Control group (CK): The fruit were immersed in sterile deionized water.
-
(2)
GA3: The fruit were immersed in 0.1 mM GA3.
-
(3)
GA3+ bikinin (GSK-3 inhibitor): The fruit were immersed in 0.1 mM GA3 plus 10 μM bikinin.
The concentration of GA3 and bikinin was acquired according to our preliminary experiments (Supplemental Fig. 1). The fruit of each group were immersed for 10 min, and were dried in air for 40 min at room temperature afterwards. Subsequently, all three groups were stored at 4 °C and 80% relative humidity for 28 days. During storage, twenty fruit from each treatment were taken at 7 day intervals for determinations. Each assay was conducted three times. The fruit material were kept at − 80 °C for further analysis.
CI Index
During cold storage, CI index was determined after 0, 7, 14, 21, and 28 d, respectively. CI intensity was scored by the extent of internal browning symptom: 0 = no damage, 1 = superficial damage (damage < 5%), 2 = moderate damage (damage 6%–25%), 3 = severe damage (damage 26% to − 50%), 4 = very severe damage (damage > 50%). The CI index was calculated according to the following formula: CI index = Σ [(CI scale) × number of fruits at that CI)] / (5 × total number of fruit in each sample) × 100%.
Determination of GA3 Content
A mass of 2.0 g fresh sample was extracted by 10 mL of 0.1% acetic acid in acetonitrile, which was shaken for 1 min. Subsequently, 4 g of anhydrous magnesium sulfate was added, followed by being shaken for 1 min, and was then centrifuged at 8000 rpm for 5 min. The GA3 content in postharvest peach fruit was analyzed using high-performance liquid chromatography (HPLC) described by the method of Zhu et al. (2016). A Waters XBridge™ C18 reversed-phase column (2.1 × 150 mm, with a 5.0-μm particle size) was used, and the column temperature was maintained at 30 °C. The injection volume was 5 μL. The mobile phases consisted of 0.5 % (v/v) acetic acid (A) and methanol (B). The column was developed with stepwise linear gradient elution according to the following schedule: 0–2 min, 20% B; 3 min, 90% B; and 6.5–8 min, 20% B at a flow rate of 5 μL s−1. The results were expressed as nanograms of GA3 per gram of FW.
Gene Expression
Total RNA from 3 batches of peach fruit was obtained by an E.Z.N.A.™ Plant RNA Kit (Omega, Norcross, GA, USA; R6827-01) as described by instructions. The first-strand cDNA was obtained according to Zhu et al. (2016). cDNA sequences of the enzymes were obtained from NCBI database, and the primers designed and employed in our research are exhibited in Table 1. Quantitative real-time PCR (qRT-PCR) assay was conducted using the SYBR®Premix Ex Taq™ (TAKARA: RR420A) in the ABI 7500 sequence detection system as described by instructions (Applied Biosystems, Foster City, CA, USA). The PCR conditions were 1 cycle of 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, and then 63 °C for 25 s. Each assay was conducted with 3 biological replicates.
Western Blot
The peach samples at the end of storage (28 d) were ground with RIPA lysis buffer containing cocktail (a protease inhibitor). The mixture was then centrifuged to collect the tissue lysates. Western blot assay was conducted according to Jiao et al. (2017).
Statistical Analyses
All the data were shown as the mean ± standard deviation (SD). The assay of significant difference was conducted using the SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) via the standard ANOVA and Duncan test.
Results
Effects of Exogenous GA3 on CI in Peach Fruit at 4 °C up to 28 Days
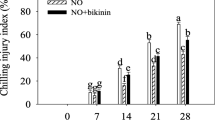
CI symptoms in peach fruit appeared after 7 days, which elevated during the whole cold storage at 4 °C (Fig. 1). The CI index was effectively reduced by exogenous GA3. At the end of cold storage, compared with the control, the CI index in peach fruit under GA3 treatment decreased by 26%. However, the CI index significantly increased by bikinin (GSK-3 inhibitor) treatment under GA3 treatment. At the end of storage, compared with GA3-treated peach fruit, the CI index in bikinin-treated peach fruit increased by 15%.
Effects of Exogenous GA3 on Endogenous GA3 Content in Peach Fruit at 4 °C up to 28 Days
During the whole cold storage at 4 °C, endogenous GA3 content gradually decreased and increased in control and exogenous GA3-treated peach fruit, respectively (Fig. 2). Compared with the control, exogenous GA3 upregulated endogenous GA3 content. At the end of cold storage, the endogenous GA3 content in peach fruit under GA3 treatment was 1.7 times higher than that of the control.
Effects of Exogenous GA3 on Gene Expression of GSK-3 at 4 °C up to 28 Days
In general, during cold storage at 4 °C, the gene expression of GSK-3 was enhanced at the early stages, and was weakened afterwards (Fig. 3). During 14 d of storage, compared with the control, exogenous GA3 upregulated GSK-3 expression. The highest GSK-3 expression in GA3-treated peach fruit was obtained on day 7, which was 2 times higher than that of the control. At the end of storage, there was no statistical difference in the GSK-3 expression between control and GA3 treatments. However, during the whole cold storage, except on day 21, bikinin inhibited the GSK-3 expression under GA3 treatment. At the end of storage, compared with GA3-treated peach fruit, the GSK-3 expression in bikinin-treated peach fruit decreased by 37%.
Effects of Exogenous GA3 on Gene Expression of SOD, POD, CAT, APX, GR, and GST at 4 °C up to 28 Days
During the whole cold storage at 4 °C, the gene expression of SOD, POD, CAT, APX, GR, and GST enzymes showed the similar trends as the GSK-3 expression (Fig. 4). Compared with the control, exogenous GA3 upregulated gene expression of SOD, POD, CAT, APX, GR, and GST. The highest gene expression of SOD, POD, and GR under GA3 treatment was obtained on day 14, which was 2.6, 1.4, and 2.1 times higher than that of the control, respectively. The highest gene expression of CAT, APX, and GST under GA3 treatment was obtained on day 21, which was 1.4, 1.6, and 5.2 times higher than that of the control, respectively. At the end of storage, the gene expression of SOD, POD, CAT, APX, GR, and GST in GA3-treated peach fruit was 1.9, 3.1, 4.0, 1.6, 1.3, and 1.2 times higher than that of the control, respectively. However, during the whole storage, the gene expression of SOD, POD, CAT, APX, GR, and GST under GA3 treatment was weakened by bikinin. At the end of storage, compared with GA3-treated peach fruit, the gene expression of POD, CAT, GR, and GST in bikinin-treated peach fruit decreased by 30%, 81%, 68%, 23%, 68% and 27%, respectively, and there were no statistical differences in the gene expression of SOD and APX between GA3 and GA3 plus bikinin treatments.
Effects of bikinin on gene expression of superoxide dismutase (A), peroxidase (B), catalase (C), ascorbate peroxidase (D), glutathione S-transferase (E) and glutathione reductase (F) under GA3 treatment in peach fruit at 4 °C up to 28 days. Values are the mean ± SD (standard deviation). Values not sharing the same letter are significantly different at p < 0.05
Effects of Exogenous GA3 on Gene and Protein Expression of SUMO at 4 °C up to 28 Days
During the whole storage at 4 °C, the gene expression of SUMO in GA3-treated peach fruit showed the similar trends as the GSK-3 expression, while except on day 21, the gene expression of SUMO in control continuously increased (Fig. 5). Compared with the control, exogenous GA3 enhanced the gene expression of SUMO. The highest gene expression of SUMO in GA3-treated peach fruit was obtained on day 14, which was 3.1 times higher than that of the control. At the end of cold storage, the gene expression of SUMO in peach fruit under GA3 treatment was 1.1 times higher than that of the control. However, during the whole storage, bikinin weakened the SUMO expression under GA3 treatment. At the end of storage, compared with GA3-treated peach fruit, the SUMO expression in bikinin-treated peach fruit decreased by 25% (Fig. 5A).
Effects of bikinin on gene (A) and protein (B and C) expression of small ubiquitin-like modifier under GA3 treatment in peach fruit at 4 °C up to 28 days. Panels show representative bands (B). Histograms represent relative protein levels of small ubiquitin-like modifier (C) normalized to the corresponding rubisco. Values are the mean ± SD (standard deviation). Values not sharing the same letter are significantly different at p < 0.05
Also, the protein expression of SUMO was upregulated by exogenous GA3. At the end of storage, the protein expression of SUMO in GA3-treated peach fruit was 1.7 times of the control. However, the protein expression of SUMO under GA3 treatment was weakened by bikinin. At the end of storage, compared with GA3-treated peach fruit, the protein expression of SUMO decreased by 35% in bikinin-treated peach fruit (Fig. 5B and C).
Effects of Exogenous GA3 on Gene Expression of LOX and PLD at 4 °C up to 28 Days
During the whole cold storage at 4 °C, the gene expression of LOX and PLD exhibited the similar trends as the GSK-3 expression (Fig. 6). Compared with the control, exogenous GA3 weakened the gene expression of LOX and PLD. The highest gene expression of LOX and PLD in GA3-treated peach fruit was obtained on days 21 and 14, which decreased by 20% and 75%, respectively compared with the control. At the end of cold storage, the gene expression of LOX and PLD in peach fruit under GA3 treatment decreased by 35% and 57%, respectively compared with the control (Fig. 6A and B). However, during the whole storage, bikinin blocked the GA3 treatment-inhibited gene expression of LOX and PLD. At the end of storage, compared with GA3-treated peach fruit, the gene expression of LOX and PLD in bikinin-treated peach fruit increased by 36% and 75%, respectively (Fig. 6A and B).
Effects of Exogenous GA3 on Gene Expression of MSR at 4 °C up to 28 Days
During the whole storage at 4 °C, the gene expression of MSR both in control and GA3-treated peach fruit continuously increased (Fig. 6C). Compared with the control, exogenous GA3 enhanced the gene expression of MSR. At the end of cold storage, the gene expression of MSR in peach fruit under GA3 treatment was 1.4 times higher than that of the control. However, during the whole storage, bikinin weakened the MSR expression under GA3 treatment. At the end of storage, compared with GA3-treated peach fruit, the MSR expression in bikinin-treated peach fruit decreased by 27% (Fig. 6C).
Discussion
Our results demonstrated that during storage, GA3 treatment significantly reduced CI (Fig. 1), and upregulated endogenous GA3 content (Fig. 2) in peach fruit. It suggested that GA3 treatment could be used as a possible method that alleviated CI in peach fruit.
The ROS production in plants can interfere cellular redox equilibrium, hence leading to oxidative damage (Shi et al. 2007). Redox homeostasis is important for alleviating the damage caused by oxidative stress in plants (Chaves and Oliveira 2004). To counterbalance ROS generation, the ROS scavenging systems were activated in plants. It turned out that during cold storage, GA3 treatment up regulated the gene expression of SOD in peach fruit (Fig. 4A). SOD has been identified as the first line of defense against ROS, which generates H2O2 and oxygen by scavenging superoxide radicals (Apel and Hirt 2004). In addition, the gene expression of POD and CAT also increased by GA3 treatment (Fig. 4B and C). The joint effects of SOD, POD and CAT are necessary for alleviating oxidative stress-caused damage as a result of their complementary roles in cell metabolism (Benavides et al. 2005). To regulate the levels of ROS, an ascorbate-glutathione pathway also exists in plants. GA3 treatment also activated APX expression (Fig. 4D). APX is the primary enzyme that participates in ROS detoxification in chloroplasts and the cytosol of plant cells. Further, the GR expression was stimulated by GA3 (Fig. 4E). GR, as an enzyme in the glutathione pathway, could reduce oxidized glutathione (GSSG) to reduced glutathione (GSH). GA3 also induced the GST expression (Fig. 4F), another enzyme in the glutathione cycle and metabolism. Additionally, the increase in gene expression of SOD, POD, CAT, APX, GR, and GST triggered by GA3 was weakened by bikinin (GSK-3 inhibitor) in different degrees (Fig. 4). These data demonstrated that GSK-3 was involved in GA3 treatment-induced antioxidant system enhancement in peach fruit. GA3 treatment, an external signal, could not directly induce defense responses in plants, instead of triggering its downstream effectors like GSK-3 (Fig. 3). Then GSK-3 transduced the GA3 signal to defense reactions (Eilert 1987) like antioxidant system enhancement through a modulation of transcripts for enzymes involved in antioxidant system (Fig. 4). Ca2+ possibly mediated the antioxidant system enhancement by GSK-3. Accordingly, AtCBL1 and AtCP1, as two kinds of the Ca2+ sensor family called AtCBLs, could be activated upon the overexpression of AtGSK1 in transgenic Arabidopsis. AtCBL1 was considered to be a Ca2+ sensor involved in stress signal transmission (Kudla et al. 1999), and AtCP1 was identified as a small Ca2+-binding protein with EF hands (Jang et al. 1998). Both of the proteins interact with CBL-interacting protein kinases (CIPKs) (Shi et al. 1999). Hence, GSK-3 acts as an effective mediator of Ca2+ mobilization in plant. Ca2+ could elevate total antioxidant capacity and DPPH* scavenging capacity (Yang et al. 2016). Thus, mediation of antioxidant system enhancement by GSK-3 might be via a Ca2+-mediated signal pathway in peach fruit under GA3 treatment. In addition, the gene and protein expression of SUMO also increased by GA3 treatment (Fig. 5). Moreover, it turned out that GA3-regulated expression of SUMO was mediated by GSK-3 (Fig. 5). SUMO, a covalent protein modification, could be attached to key protein targets to regulate their activity, thus playing an important role in cell signal transduction (Conti et al. 2007). Accordingly, SUMO proteases regulated ROS production in Arabidopsis (Conti et al. 2007). SUMO conjugation caused inhibition of NADPH oxidase, thereby decreasing in intracellular ROS production in HEK293 and HeLa cells (Kim et al. 2011). In addition, SUMO modification facilitated human SOD1 stability and aggregation (Fei et al. 2006). Hence, SUMO may be involved in GSK-3-induced antioxidant system enhancement under GA3 treatment.
ROS generated in plants may lead to lipid peroxidation, which damages membrane integrity. LOX and PLD have been considered to be critical enzymes participated in membrane lipid degradation in plants. LOX degrades polyunsaturated fatty acids, destroying the bilayer of phospholipids. PLD leads to the peroxidation of membrane phospholipids. GA3 treatment could weaken the gene expression of LOX and PLD (Fig. 6A and B), elevating unsaturated fatty acids level and maintaining membrane integrity and consequently alleviating CI in peach fruit. Our results further showed that GA3-regulated gene expression of LOX and PLD was mediated by GSK-3 (Fig. 6A and B). Ca2+ possibly modulates LOX (Shi et al. 2002) and PLD association with membranes (Zheng et al. 2000) in plants. Hence, Ca2+ may mediate GSK-3-modulated LOX and PLD. Apart from membrane lipid degradation and peroxidation, ROS production also resulted in the oxidation of Met residue to MetO, damaging the structure and function of some proteins. GA3 treatment induced the MSR expression in peach fruit (Fig. 6C). MSR could catalyze the reduction of MetO to Met, consequently protecting proteins against oxidative stress-caused damage. Moreover, our results showed that GA3-regulated gene expression of MSR was mediated by GSK-3 (Fig. 6C). Excess ROS could result in transient oxidation of CaM, downregulating energy metabolism and the further ROS generation through respiratory control mechanisms, which allows for rapid restoration of protein function through repair of oxidized Met by MSR (Bigelow and Squier 2005). Therefore, oxidation of CaM may be a vital regulator for GSK-3-activated MSR under GA3 treatment.
Conclusions
Exogenous GA3 treatment protected peach fruit against CI, and elevated endogenous GA3 content. The GSK-3 expression was enhanced by GA3 treatment. GA3 also stimulated SOD, POD, CAT, GR, GST, and APX expression. Additionally, the elevation of gene and protein expression of SUMO and gene expression of MSR and the inhibition of gene expression of LOX and PLD were in response to GA3 treatment. However, bikinin, a GSK-3 inhibitor, weakened the above GA3-triggered impacts. Overall, GSK-3 was involved in stimulation of chilling tolerance and defense response under GA3 treatment in postharvest peach fruit. However, further work is still required to reveal the complex molecular networks regulated by GSK-3 under GA3 treatment in response to chilling stress.
References
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55(1), 728–749.
Benavides, M. P., Gallego, S. M., & Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17(1), 21–34.
Bigelow, D. J., & Squier, T. C. (2005). Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochimica et Biophysica Acta-Biomembranes, 1703(2), 121–134.
Chaves, M. M., & Oliveira, M. M. (2004). Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany, 55(407), 2365–2384.
Conti, L., Donnel, E. O., Price, J., Love, A., Dominy, P., & Sadanandom, A. (2007). SUMO proteases regulate ROS production in Arabidopsis. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology, 146(4), S260–S260.
Crisosto, C. H., & Mitchell, F. G. (2002). Postharvest handling systems: stone fruits. In A. A. Kader (Ed.), Postharvest technology of horticultural crops. Davis: University of California.
Dagar, A., Weksler, A., Friedman, H., & Lurie, S. (2012). Gibberellic acid (GA3) application at the end of pit ripening: effect on ripening and storage of two harvests of ‘September snow’ peach. Scientia Horticulturae, 140, 125–130.
Dai, C., & Wang, M. H. (2010). Expression pattern of a peptide methionine sulfoxide reductase gene from tomato (Solanum lycopersicum) in response to abiotic and oxidative stresses. Journal of Korean Society for Applied Biological Chemistry, 53(2), 127–132.
Ding, Y., Wei, W., Wu, W., Davis, R. E., Jiang, Y., Lee, I. M., Hammond, R. W., Shen, L., Sheng, J. P., & Zhao, Y. (2013). Role of gibberellic acid in tomato defence against potato purple top phytoplasma infection. Annals of Applied Biology, 162(2), 191–199.
Ding, Y., Zhu, Z., Zhao, J., Nie, Y., Zhang, Y., Sheng, J., Meng, D., Mao, H., & Tang, X. (2016). Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food and Bioprocess Technology, 9(8), 1327–1334.
Eilert, U. (1987). Elicitation: methodology and aspects of application. Cell culture and somatic cell genetics of plants, Academic Press, San Diego, Calif., 4, 153–196.
Fei, E., Jia, N., Yan, M., Ying, Z., Sun, Q., Wang, H., Zhang, T., Ma, X., Ding, H., Yao, X., Shi, Y., & Wang, G. (2006). SUMO-1 modification increases human SOD1 stability and aggregation. Biochemical and Biophysical Research Communications, 347(2), 406–412.
Gang, C., Li, J., Chen, Y., Wang, Y., Li, H., Pan, B., & Odeh, I. (2015). Synergistic effect of chemical treatments on storage quality and chilling injury of honey peaches. Journal of Food Processing and Preservation, 39(6), 1108–1117.
Jang, H. J., Pih, K. T., Kang, S. G., Lim, J. H., Jin, J. B., Hai, L. P., et al. (1998). Molecular cloning of a novel Ca2+-binding protein that is induced by NaCl stress. Plant Molecular Biology, 37(5), 839–847.
Jiang, Y., Bao, H., Ge, Y., Tang, W., Cheng, D., Luo, K., Gong, G., & Gong, R. (2015). Therapeutic targeting of GSK3β enhances the Nrf2 antioxidantresponse and confers hepatic cytoprotection in hepatitis C. Gut, 64(1), 168–179.
Jiao, C., Zhu, L., & Gu, Z. (2017). GSK-3 mediates NO-cGMP-induced isoflavone production in soybean sprouts. Food Research International, 101, 203–208.
Kim, H. J., Yun, J., Lee, J., Hong, H., Jeong, J., Kim, E., Bae, Y. S., & Lee, K. J. (2011). SUMO1 attenuates stress-induced ROS generation by inhibiting NADPH oxidase 2. Biochemical and Biophysical Research Communications, 410(3), 555–562.
Kudla, J., Xu, Q., Harter, K., Gruissem, W., & Luan, S. (1999). Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4718–4723.
Lurie, S., & Crisosto, C. H. (2005). Chilling injury in peach and nectarine. Postharvest Biology and Technology, 37(3), 195–208.
Nelis, S., Conti, L., Zhang, C., & Sadanandom, A. (2015). A functional small ubiquitin-like modifier (SUMO) interacting motif (SIM) in the gibberellin hormone receptor GID1 is conserved in cereal crops and disrupting this motif does not abolish hormone dependency of the DELLA-GID1 interaction. Plant Signaling & Behavior, 10(2), e987528.
Pegoraro, C., Tadiello, A., Girardi, C. L., Chaves, F. C., Quecini, V., Costa de Oliveira, A., Trainotti, L., & Rombaldi, C. V. (2015). Transcriptional regulatory networks controlling woolliness in peach in response to preharvest gibberellin application and cold storage. BMC Plant Biology, 15(1), 279.
Ruan, J., Li, M., Jin, H., Sun, L., Zhu, Y., Xu, M., & Dong, J. (2015). UV-B irradiation alleviates the deterioration of cold-stored mangoes by enhancing endogenous nitric oxide levels. Food Chemistry, 169, 417–423.
Santa-Cruz, D. M., Pacienza, N. A., Zilli, C. G., Tomaro, M. L., Balestrasse, K. B., & Yannarelli, G. G. (2014). Nitric oxide induces specific isoforms of antioxidant enzymes in soybean leaves subjected to enhanced ultraviolet-B radiation. Journal of Photochemistry and Photobiology B: Biology, 141, 202–209.
Shi, J., Kim, K. N., Ritz, O., Albrecht, V., Gupta, R., Harter, K., Luan, S., & Kudla, J. (1999). Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. The Plant Cell, 11(12), 2393–2405.
Shi, P., Zeng, F., Song, W., Zhang, M., & Deng, R. (2002). Effects of calcium and lanthanum on ABA biosynthesis in cucumber leaves. Russian Journal of Plant Physiology, 49(5), 696–699.
Shi, Q., Fei, D., Wang, X., & Min, W. (2007). Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiology and Biochemistry, 45(45), 542–550.
Weksler, A., Dagar, A., Friedman, H., & Lurie, S. (2012). The effect of gibberellin on firmness and storage potential of peaches and nectarines. Acta Horticulturae, 962, 591–595.
Yang, R., Hui, Q., Gu, Z., Zhou, Y., Guo, L., Shen, C., & Zhang, W. (2016). Effects of CaCl2 on the metabolism of glucosinolates and the formation of isothiocyanates as well as the antioxidant capacity of broccoli sprouts. Journal of Functional Foods, 24, 156–163.
Yao, W., Xu, T., Farooq, S. U., Jin, P., & Zheng, Y. (2018). Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biology and Technology, 144, 20–28.
Zheng, L., Krishnamoorthi, R., Zolkiewski, M., & Wang, X. (2000). Distinct Ca2+ binding properties of novel C2 domains of plant phospholipase dalpha and beta. Journal of Biological Chemistry, 275(26), 19700–19706.
Zhu, Z., Ding, Y., Zhao, J., Nie, Y., Zhang, Y., Sheng, J., et al. (2016). Effects of postharvest gibberellic acid treatment on chilling tolerance in cold-stored tomato (Solanum lycopersicum L.) Fruit. Food and Bioprocess Technology, 9(7), 1–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• GA3 alleviated CI in peach fruit.
• GA3 induced GSK-3 pathway.
• GSK-3 was involved in GA3-enhanced antioxidant system.
• GSK-3 was involved in GA3-enhanced SUMO and MSR expression.
• GSK-3 was involved in GA3-weakened LOX and PLD expression.
Electronic Supplementary Material
ESM 1
(DOC 43 kb)
Rights and permissions
About this article
Cite this article
Jiao, C., Duan, Y. The Role of Glycogen Synthase Kinase-3 in Gibberellic Acid-Induced Chilling Tolerance and Defense Response in Postharvest Peach Fruit. Food Bioprocess Technol 12, 1733–1740 (2019). https://doi.org/10.1007/s11947-019-02338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02338-3