Abstract
Osteoporosis is a clinical disease characterized by decreased bone density due to a disrupted balance between bone formation and resorption, which increases fracture risk and negatively affects the quality of life of a patient. LncRNAs are RNA molecules over 200 nucleotides in length with non-coding potential. Many studies have demonstrated that numerous biological processes involved in bone metabolism are affected. However, the complex mechanisms of action of lncRNAs and their clinical applications in osteoporosis have not yet been fully elucidated. LncRNAs, as epigenetic regulators, are widely involved in the regulation of gene expression during osteogenic and osteoclast differentiation. LncRNAs affect bone homeostasis and osteoporosis development through different signaling pathways and regulatory networks. Additionally, researchers have found that lncRNAs have great potential for clinical application in the treatment of osteoporosis. In this review, we summarize the research results on lncRNAs for clinical prevention, rehabilitation treatment, drug development, and targeted therapy for osteoporosis. Moreover, we summarize the regulatory modes of various signaling pathways through which lncRNAs affect the development of osteoporosis. Overall, these studies suggest that lncRNAs can be used as novel targeted molecular drugs for the clinical treatment of osteoporosis to improve symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Osteoporosis is a systemic skeletal disease characterized by decreased bone density and degeneration of bone tissue microarchitecture, leading to increased bone fragility and fracture risk [1]. Osteoporosis is caused by several factors, including genetic and environmental factors. In most cases, these factors cause osteoporosis by interfering with the differentiation and function of osteoblasts and osteoclasts [2]. Globally, around 200 million people, mostly elderly women, have osteoporosis [3]. Moreover, patients with osteoporosis experience devastating hip and vertebral fractures [4]. With the world's population aging, osteoporosis is becoming more prevalent [5] and this is a major public health issue. To control the occurrence and development of osteoporosis, currently, pharmaceutical intervention is mainly used to reduce bone resorption or increase bone formation. Commonly used pharmaceuticals include calcium tablets and vitamin D, estrogen replacements, calcitonin, bisphosphonates, alendronate (ALN), and risedronate (RIS) [6, 7]. Vitamin D and estrogen replacements can promote calcium absorption, accelerate calcium salt deposition, and improve bone mineralization [8, 9]. In addition, estrogen affects osteogenic differentiation and osteoclast absorption. It is often used for the treatment and prevention of postmenopausal osteoporosis [9, 10]. The combination of calcitonin and calcitonin receptors in osteoclasts leads to a reduction in the combination of osteoclasts and mineralized bone as well as in osteoclast activity [11]. Bisphosphonates prevent the formation of osteoclasts and reduce their function by affecting the recruitment, differentiation, and resorption activity of osteoclasts and cell apoptosis, thus interfering with the process of bone resorption [12]. However, the efficacy of pharmaceutical treatments is limited, many adverse reactions occur, and there is currently no effective treatment for osteoporosis. Therefore, in-depth exploration of the molecular mechanisms underlying osteoporosis development is important.
In the past few years, scientists have focused on a group of regulatory RNAs that do not encode proteins, known as the non-coding RNAs (ncRNAs), including intronic RNAs, microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and extracellular RNA [13]. NcRNAs have been shown to be associated with the development of various diseases, such as cancer, nervous system, cardiovascular system, and skeletal system diseases [2, 14, 15]. They regulate gene expression via epigenetic modifications. For example, lncRNAs can act as chromatin, transcriptional, and post-transcriptional regulators involved in multiple biological processes including cell development, differentiation, proliferation, metabolism, and cell cycle regulation [16]. In one such study, 743 lncRNAs (461 upregulated lncRNAs and 282 downregulated lncRNAs) were screened for significantly different expression in a postmenopausal osteoporosis mouse model compared with healthy controls [17], suggesting that lncRNAs play a pivotal role in the development and occurrence of osteoporosis. Recently, many studies have been conducted on the mechanisms by which lncRNAs affect osteoporosis development. Researchers are actively moving the research content closer to clinical applications, making the idea of using lncRNAs for the clinical treatment of osteoporosis more realistic. In this review, we summarize these new research results to provide a theoretical basis and direction guide for the search for new clinical treatment options.
Classification, characteristics, and mechanism of action of lncRNAs
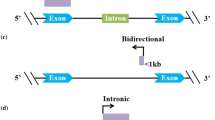
LncRNAs are transcripts over 200 nucleotides in length with no or little potential to encode proteins. Approximately 30,000 lncRNA transcripts have been identified in the human genome [18]. LncRNAs can be generated by various means, such as chromosomal recombination, non-coding genes, and disruption of the translation reading frame of protein-coding genes through reverse transcription. Based on their distance from protein-coding sequences and their relative positions, lncRNAs can be categorized into five types: synonymous, antisense, bidirectional, intronic, and intergenic lncRNAs [19]. In contrast to mRNAs, lncRNAs undergo much more alternative splicing, which increases the range of possible isoforms [20], which also illustrates the possibility of rich and diverse functions of lncRNAs. LncRNAs are typically expressed at lower levels than mRNAs [21]; however, they show stronger tissue-specific expression patterns, thereby playing an integral role in cell specificity [22, 23]. LncRNAs frequently lack the high sequence exhibited across species conservation [24], and although lncRNAs have the same sequence in human and mouse embryonic stem cells, they are localized in different subcellular regions and ultimately function differently in mouse and human cells [25]. LncRNAs play biological roles mainly through the following mechanisms. (1) They participate in epigenetic regulation mechanisms and regulate gene transcription by recruiting chromatin remodeling complexes to regulate histone and DNA modification or by interacting with histone-modifying enzymes [20]. (2) They participate in transcription regulation. They directly bind to the promoter region or interact with RNA-binding proteins and target the region to interfere with gene expression [26]. Moreover, lncRNAs can directly interfere with the transcription of adjacent genes or affect the activity of transcription factors to regulate gene expression [27]. (3) They participate in post-transcriptional splicing, modification, and translation. They interact with shear factors to regulate alternative splicing of mRNA [28]. Furthermore, they can interact with RNA methyltransferases or demethylases to regulate mRNA methylation or downstream gene expression by competitively binding to mRNA [29]. lncRNAs can also interact with mRNA and siRNAs to form complementary double strands to silence gene expression [30]. (4) At post-translational level, lncRNAs interact with proteins to regulate their localization, phosphorylation, acetylation, and ubiquitination [31]. (5) LncRNAs can perform biological functions as miRNAs or siRNA precursors [29]. The number of lncRNAs is enormous, and the regulatory mechanisms involved in the development of various diseases are complex and diverse, which are not yet fully understood.
LncRNAs in bone development and homeostasis
During bone development, the deposition and resorption of the bone matrix and minerals are in a dynamic balance to maintain the stability of bone mass in adults. This highly coordinated process is precisely regulated by osteoblasts, osteoclasts, and osteocytes throughout their life cycle [32]. Osteoclasts are differentiated from hematopoietic stem cells, while osteoblasts are differentiated from bone marrow mesenchymal stromal cells (BMSCs). BMSCs gradually differentiate into osteoprogenitors, pre-osteoblasts, and osteoblasts after being stimulated by cytokines, which produce a matrix, repair the tissue microenvironment, and enhance bone regeneration [33]. LncRNAs can be widely involved in the differentiation of BMSCs to osteoblasts through Wnt/β-catenin, mitogen-activated protein kinase (MAPK), Notch and TGF-β/BMP signaling pathway, and then affect osteoporosis development [34]. For example, the Wnt/β-catenin and TGF-β /BMP pathways can regulate Runt-related transcription factor 2 (RUNX2) and transcription factor osterix (OSX) to induce an osteogenic phenotype [35].
Additionally, lncRNAs regulate osteoclast differentiation. Recent studies suggest that osteoclast differentiation is mainly regulated by the RANKL/RANK/OPG signaling axis [36]. Osteprotegerin (OPG), nuclear factor κB (NF-κB /RANK), and RANK ligand (RANKL) play a prominent role in determining bone quality and strength. Polypeptide-related factor CSF-1 (colony stimulating factor 1) and RANKL produced by osteoblasts induce gene expression of specific osteoclast lineages, recruit multinucleated polykaryons to adhere to the bone surface, and promote osteoclast maturation. The initiation of this series of processes depends on the binding of RANKL to the RANK receptor on the surface of osteoclasts, thereby activating the signaling cascade in osteoclasts. OPG is produced by osteoblasts and inhibits osteoclast formation. Bone resorption can be prevented, and RANKL/RANK interaction can be blocked by OPG binding to RANKL [37]. The lncRNA Bmncr was downregulated in the bone marrow and spleen of osteoporotic mice and was gradually decreased during RANKL-induced osteoclast differentiation. Overexpression of Bmncr reduces the number of osteoclasts and inhibits bone resorption, demonstrating that Bmncr can alleviate osteoporosis progression by inhibiting RANKL-induced osteoclast differentiation [38].
In many studies, lncRNAs have been linked with osteoporosis through complex regulatory networks [34, 39, 40]. With the development of various experimental technologies such as high-throughput sequencing and deepening of research, an increasing number of lncRNA molecules and their detailed mechanisms have become available, and downstream targets have been identified. Moreover, promoting the osteogenic differentiation of BMSCs or inhibiting the activity of osteoclasts could be effective methods for treating bone degenerative diseases (Fig. 1).
Mechanisms of lncRNAs in bone development and homeostasis. LncRNAs regulate osteogenic differentiation through Wnt/β-catenin, MAPK, Notch and TGF-β/BMP signaling pathway. Runx2 induces osteogenic phenotypes. LncRNAs regulate osteoclast differentiation through RANKL/RANK/OPG signaling axis. CSF-1 and RANKL together promote osteoclast generation, activation, and maturation. OPG competitively binds to RANKL, blocks the interaction between RANKL and RANK, and prevents excessive bone resorption
LncRNAs in osteogenic differentiation
LncRNAs involved in osteogenic differentiation through lncRNA–miRNA network
Long non-coding RNAs (lncRNAs) regulate gene expression at the post-transcriptional level by acting as competing endogenous RNAs (ceRNAs). The ceRNA hypothesis states that RNA transcripts with the same miRNA response element (MRE) can compete for miRNA binding, acting as RNA sponges that prevent miRNAs from binding to their target sites [41]. MiR-138 acts as a negative factor during osteogenic differentiation and can inversely regulate the FAK-ERK1/2-RUNX2 signaling pathway, whereas lncRNA H19 releases its inhibitory effect on focal adhesion kinase (FAK) by sponging miR-138. In conclusion, H19 can upregulate FAK engagement and promote tension-induced osteogenesis in hBMSCs by competitively binding to miR-138 [42]. miR-320a, by interacting directly with catenin beta 1 (CTNNB1) and inhibiting Wnt/β-catenin signaling, negatively regulates osteogenic differentiation of BMSCs, while lncRNA DANCR can abolish the inhibitory effect of miR-320a [43]. Similarly, DANCR has been shown to inhibit the osteogenic differentiation of hBMSCs via the miR-1301-3p/PROX1 axis [44].
MALAT1 can increase the expression of OSX in hBMSCs by competitively binding to miR-143, miR-96, and miR-124-3p, thereby promoting their osteogenic differentiation [45,46,47]. BMSC-driven exosomal MALAT1 may act as miR-34c sponge to upregulate the expression of SATB2, which is a specific immunohistochemical biomarker of osteoblast differentiation [48], thereby enhancing osteogenic activity and relieving osteoporosis symptoms in mouse models [49]. Moreover, MiR-140-5p can also bind to SATB2, and H19 can act as a ceRNA of miR-140-5p, leading to increased SATB2 levels in BMSCs, thereby promoting osteogenic differentiation of BMSCs [50].
SNHG5 affects the expression of RUNX3 through competitive binding with miR-582-5p. RUNX3 activates SNHG5 transcription, and the positive feedback loop of SNHG5/miR-582-5p/RUNX3 promotes osteoporosis [51]. The transcription factors belonging to the RUNX family are considered crucial for osteogenic differentiation, as they stimulate the increased expression of osteogenic markers, such as osteocalcin (OCN), osteopontin (OPN), and OSX. H19 upregulates SDF-1 by binding to miR-149 and enhances the expression of RUNX2, thereby promoting osteogenic differentiation of BMSCs [52]. Furthermore, lncRNA CCAT1 can competitively bind to miR-34a-5p to inhibit the proliferation and differentiation of osteoblasts in osteoporotic rats [53]. The lncRNAs LOC100126784 and POM121L9P are associated with increased osteogenic differentiation of BMSCs via the miR-503-5p/SORBS1 pathway [54]. HCG18 inhibits the osteoporosis-induced osteogenic differentiation of BMSCs through the miR-30a-5p/NOTCH1 axis [55]. These studies indicate that the lncRNA–miRNA sponge mechanism plays an important role in the regulation of osteoporosis development.
Moreover, lncRNAs and miRNAs can function without endogenous competitive mechanisms. For example, H19 is a precursor of miR-675 and is involved in osteogenesis. H19/miR-675 promotes osteogenic differentiation by negatively regulating TGF-β1, while H19/miR-675 downregulates Smad3 phosphorylation and HDAC4/5 expression. TGF-β phosphorylates Smad3, which subsequently recruits HDAC4/5 to RUNX2 and forms a stable complex on the RUNX2-binding DNA sequence, thereby downregulating RUNX2 and OCN gene expression to form H19/miR-675/TGF-β1/Smad3/HDAC Signaling pathways regulate the osteogenic differentiation of human mesenchymal stromal cells (hMSCs) [56]. Interestingly, contradictory results were obtained in another study. By collecting samples from patients with postmenopausal osteoporosis and healthy individuals, researchers found that the H19/miR-29a-3p axis promotes osteoporosis by regulating the expression of pro-inflammatory factors, cell proliferation, and apoptosis [57]. The above research shows that H19 can promote the development and delay the progression of osteoporosis. The detailed mechanisms and reasons for this remain to be elucidated. Wang et al. found that the expression of MEG3 and miR-133a-3p increased and is positively correlated with BMSCs derived from PMOP. Finally, MEG3 promotes the development of PMOP by targeting miR-133a-3p to inhibit the osteogenic differentiation of BMSCs [58]. Furthermore, miR-214 can be regulated by the lncRNAs H19, MALAT1, and MEG3 to affect the osteogenic differentiation of BMSCs and the development of osteoporosis [59,60,61] (Table 1).
LncRNAs involved in osteogenic differentiation through Wnt/β-catenin signaling pathway
The Wnt family comprises many secreted glycoproteins. The Wnt signaling pathway is involved in many important biological processes, such as development, cell proliferation, metabolism, and cell differentiation [62]. The Wnt/β-catenin signaling pathway critically controls bone mass by promoting bone formation. During BMSC differentiation, β-catenin has been shown to be a key trigger for osteoblast differentiation and osteogenesis [63]. Numerous studies have shown that Wnt signaling is the apical pathway of many target genes of lncRNA H19 during bone formation. Zhou et al. found that the expression of Wnt promoter can be cooperatively regulated by H19/Foxc2, which promotes the osteogenic differentiation of BMSCs through the Wnt-β-catenin pathway [64]. Additionally, the H19/Dkk4/Wnt signaling cascade has been demonstrated to play a critical role in the development of disuse osteoporosis (DOP). DKK4 is an extracellular inhibitor of Wnt signaling. Mechanical unloading leads to decreased expression of H19, promoting DKK4 expression and subsequent inhibition of Wnt signaling, resulting in decreased osteogenesis and the development of DOP [65]. Similarly, in in vitro studies, researchers have demonstrated that LncRNA SNHG1 regulates the Wnt/β-catenin signaling pathway through the miR-101/DKK1 axis to inhibit osteogenic differentiation [66]; however, its mechanism in vivo still needs to be elucidated. SP1-induced lncRNA SNHG1 can also regulate the Wnt signaling pathway mediated by SFRP1 by sponging miR-181c-5p, inhibiting osteogenic differentiation and promoting osteoclast differentiation, thus playing a role in the development of osteoporosis, in which SFRP1 is an antagonist of the Wnt signaling pathway [67]. A key component of the Wnt/β-catenin signaling pathway, WNT2B, controls the expression of RUNX2, as well as promotes the expression of osterix at gene and protein levels, thereby regulating the osteogenesis process [68]. One study showed that WNT2B is regulated by the LINC00707/miR-370-3p/WNT2B axis during osteogenesis of hBMSCs [69]. Another study demonstrated that LINC00707 could also target miR-145-mediated low-density lipoprotein receptor-related protein 5(LRP5) by activating the Wnt/β-catenin pathway, thereby promoting the osteogenic differentiation of hBMSCs [70], which is consistent with the previously mentioned results [69]. Recently, a novel lncRNA molecule, LINC01119, was identified as a negative regulator of osteogenesis in MSCs, and is thought to regulate osteogenesis via the Wnt pathway by targeting FZD4, a receptor in the Wnt signaling pathway [71]. In addition, lncRNA HOTTIP can activate the Wnt/β-catenin signaling pathway by interacting with the transcription factor WDR5 and upregulating β-catenin gene expression, thereby enhancing osteogenic differentiation [72] (Fig. 2).
LncRNA-mediated mechanisms in osteogenic differentiation. LncRNA H19, SNHG1, LINC00707, LINC01119, and HOTTIP regulate osteogenic differentiation through the Wnt/β-catenin pathway. LncRNA UCA1 and MEG3 regulate osteogenic differentiation through the TGFβ/BMPs signaling pathway. LncRNA DANCR, SNHG1, and MALAT1 regulate osteogenic differentiation through the MAPK pathway. LncRNA NKILA and MIR22HG regulate osteogenic differentiation via PI3K-AKT pathway. LncRNA H19, Rmst, and lnc-Evf2 regulate osteogenic differentiation via Notch pathway. LncRNA NKILA and HOXA-AS2 regulate osteogenic differentiation via NF-κB pathway
LncRNAs involved in osteogenic differentiation through TGFβ/BMPs family
Bone morphogenetic proteins (BMPs), which are members of the transforming growth factor (TGF) family, have been shown to play important roles in many cellular regulatory processes, including bone and cartilage [73]. TGFβ/BMP ligands activate signaling cascades by binding to transmembrane serine-threonine kinase receptors (termed as types I and II) to form complexes and work through two pathways: the classical Smad-dependent pathway (TGFβ/BMP ligands, receptors, Smads) and non-canonical Smad-independent pathways, such as the p38 MAPK pathway [74]. In the classical pathway, the activated TGFβ/BMP-Smad signaling pathway can affect the osteogenic differentiation process by regulating the downstream target Runx2 [75].
In 2015, Zhuang et al. found that overexpression of the lncRNA MEG3 can promote the osteogenic differentiation of BMSCs by targeting BMP4 [76]. BMP4 has been identified as a regulator of cartilage and bone formation [77]. MEG3, which is located near BMP4, dissociates the transcriptional repressor SOX2 from the BMP4 promoter and promotes gene expression of BMP4 [76]. Moreover, DEPTOR, an endogenous inhibitor of rapamycin (mTOR), inhibits the MEG3-mediated activation of this process via BMP4 signaling [78]. Furthermore, DNA cytosine-5-methyltransferase 1 (DNMT1) can interact with the MEG3 promoter and inhibit the expression of MEG3. The results of the study by Li et al. demonstrated that osteogenic differentiation of MSCs was inhibited through the DNMT1/MEG3/BMP4 pathway [79]. During the analysis of plasma samples from patients with osteoporosis, Zhang et al. found that the expression level of lncRNA UCA1 in the plasma of patients with OP was higher than that in normal patients. A previous study showed that the low-expression lncRNA UCA1 can activate Smad1/5/8 by promoting BMP-2 expression in osteoblasts, thereby promoting the proliferation and differentiation of osteoblasts [80]. In addition, lncRNA RAD51-AS1 was found to interact with the RNA-binding protein YBX1, then form mRNA-protein complexes with SMAD7 and SMURF2 and inhibit their translation, and finally activate the TGF-β signaling pathway and promote the proliferation and osteogenic differentiation of hBMSCs [81] (Fig. 2).
LncRNAs involved in osteogenic differentiation through MAPK signaling pathway
MAPK signaling pathways, including c-Jun N-terminal kinase (JNK), ERK 1/2 and p38, play important roles in osteogenic differentiation. Normally, activation of p38, JNK, and ERK 1/2 triggers osteogenic differentiation [82]. During osteogenic differentiation of hBMSCs, the expression of lncRNA DANCR significantly decreases. Upregulation of DANCR abnormally reduces the number of S-phase cells, alkaline phosphatase (ALP) activity, expression of osteogenic marker genes, and deposition of mineralized matrix in hBMSCs. Furthermore, the inhibitory effect of DANCR overexpression was more pronounced when it was introduced in combination with a specific inhibitor to induce p38 inactivation. Thus, DANCR regulates osteogenic differentiation independent of ERK1/2 and JNK but depends on p38 [83].
As a member of the MAPK signaling pathway, p38 influences several biological processes, including inflammation, differentiation, growth, and cell death. [84]. In a study on postmenopausal osteoporosis, researchers found that the expression level of lncRNA SNHG1 in ovariectomized (OVX) mice was much higher than that in the sham-operated group, and later studies found that overexpression of SNHG1 could enhance the interaction between p38 and the ubiquitinase Nedd4, while reducing the stability of p38 in BMSCs, thereby accelerating the degradation of p38. Therefore, lncRNA SNHG1 can inhibit p38 activation through Nedd4-mediated ubiquitination, negatively regulating the osteogenic differentiation of BMSCs [85].
In another study, the expression of MALAT1 in osteoporotic rats was found to be significantly lower than that in normal rats; western blotting results showed that the expression levels of ERK1/2 and P38 in the MALAT1 siRNA group were significantly higher than those in the negative control (NC) siRNA group, and inhibiting the expression of lncRNA MALAT1 reduced the ALP activity of BMSCs. This suggests that MALAT1 inhibits the osteogenic differentiation of BMSCs by enhancing the activation of the MAPK signaling pathway, thereby promoting osteoporosis progression [86] (Fig. 2).
LncRNAs involved in osteogenic differentiation through NF-κB, PI3K/AKT, and Notch signaling pathway
Previous studies have shown that the activation of the AKT signaling pathway can maintain the osteogenic differentiation of human dental follicle cells and is widely regarded as an osteogenic activator [87]. In contrast, NF-κB acts as a negative regulator of osteogenic differentiation and its activation can inhibit osteogenesis [88]. In a study on the regulation of osteogenic differentiation by lncRNA NKILA, it was found that during the osteogenesis of MSCs, NKILA could negatively regulate the function of NF-κB and positively regulate the activity of AKT, thereby promoting the osteogenesis of MSCs [89]. Zhu et al. identified the underlying mechanism by which lncRNA HOXA-AS2 regulates the osteogenic differentiation of MSCs. They found that NF-κB can recruit HDAC2 to the promoter of the osteogenic master transcription factor SP7 and lead to the transcriptional repression of SP7, while HOXA-AS2 positively regulates osteogenic differentiation by inactivating NF-κB activity [90]. However, another lncRNA molecule, MIR22HG, has been shown to be downregulated in osteoporosis models, and MIR22HG relieved the negative regulation of PI3K/AKT signaling from phosphatase and tensin homolog (PTEN) by downregulating PTEN, further promoting osteogenic differentiation of hBMSCs both in vitro and in vivo [91].
The Notch pathway includes Notch receptors (Notch 1–4), Delta-like (DLL) and Jagged (JAG) ligands, negative and positive regulators, and transcription factors. Notch signaling plays an important role in regulating the cell cycle, stem cell renewal and proliferation [92]. Liao et al. demonstrated that silencing H19 significantly impaired BMP9-induced osteogenic differentiation, and this process was effectively rescued by activating Notch signaling, suggesting that BMP9-induced mesenchymal differentiation can be promoted by H19 through Notch signaling [93]. The results of the in vitro and in vivo experiments were consistent. Similarly, the lncRNA Rmst was shown to be involved in the key process of BMP9-induced osteogenic differentiation of MSCs through the RMST-miRNA-Notch regulatory axis [94]. Additionally, osteogenic induction after silencing the expression of lnc-Evf2 significantly reduced the protein levels of Notch2, Notch3, and HES1 but did not change their mRNA levels, indicating that Lnc-Evf2 promotes osteogenic differentiation at the post-transcriptional level through Notch signaling [40] (Fig. 2).
The regulatory role of lncRNAs in osteoclasts
Osteoclasts are tissue-specific macrophage polykaryotes that arise from the differentiation of monocyte/macrophage precursor cells on or near the bone surface. They develop and adhere to the bone matrix and secrete acids and lyases to degrade and resorb the bone [36]. Many studies have shown that lncRNAs can regulate the process of osteoclast differentiation, disrupt the homeostasis of bone mass balance, and lead to osteoporosis (Table 2).
Liu et al. demonstrated that Lnc-AK077216 could inhibit the negative regulation of the transcription factor NFATc1 by NFAT-interacting protein (NIP45), thereby promoting RANKL-induced osteoclastogenesis and bone resorption [95]. Similarly, a recent study showed that the knockdown of lncRNA XIST inhibited the pro-osteoclastic differentiation effect of RANKL. Furthermore, knockdown of lncRNA XIST reduced the expression of sphingosine kinase 1 (SPHK1), and RIP analysis demonstrated that lncRNA XIST and SPHK1 mRNA interact with fusion in sarcoma (FUS). Therefore, lncRNA XIST interacts with FUS and promotes the SPHK1/S1P/ERK signaling pathway to promote osteoclastic differentiation [96]. Furthermore, lncRNA MALAT1 demonstrated that MALAT1 upregulated the expression of IGF2BP1 by competitively binding to miR-124-3p as a ceRNA, promoted the osteogenic differentiation of BMSCs, and inhibited the osteoclast differentiation of macrophages in osteoporosis through the Wnt/β-catenin pathway [97]. Zhang et al. demonstrated, for the first time, that the lncRNA Neat1 competes with microRNA7 (miR-7) for binding and blocks its ability to regulate the function of protein tyrosine kinase 2 (PTK2), ultimately promoting the formation of osteoclasts [98]. In a clinical study, researchers found that patients with postmenopausal osteoporosis (PMOP) had higher lncRNA cancer susceptibility 11 (CASC11) and TNF-α in plasma than healthy controls. The upregulated expression of TNF-α induces osteoclast activation and increases bone resorption. Therefore, CASC11 may promote bone resorption by upregulating TNF-α, leading to the occurrence of PMOP [99]. Similarly, another clinical study demonstrated that the overexpression of lncRNA growth arrest-specific transcript 5 (GAS5) rescued the inhibitory effect of miR-21 on apoptosis. Therefore, GAS5 may downregulate miR-21 and increase the apoptotic rate of osteoclasts, thereby alleviating OP development [100]. Many lncRNAs have been proven to be involved in the development of osteoporosis by regulating osteoclast differentiation [101,102,103]. However, most current research focuses on the role of lncRNAs in osteoporosis from the perspective of osteogenic differentiation. The discovery of additional lncRNAs and their detailed mechanisms of action in osteoclast differentiation is an important direction for future research.
LncRNAs in the treatment of osteoporosis
In recent years, research into the relationship between osteoporosis and lncRNAs has been actively moving towards clinical applications. The research directions include the following aspects (Table 3):
LncRNAs can be used as special serum markers to provide guiding information for the clinical diagnosis, prevention, and rehabilitation of osteoporosis. For example, the lncRNA CASC11 can be used as a biomarker reflecting the treatment process of PMOP, and high levels of CASC11 in plasma on the day of hospital discharge are significantly associated with a high recurrence rate. Therefore, detecting plasma levels of CASC11 may provide guidance for the prevention of PMOP recurrence [99]. Moreover, lncRNA–NEF is expressed at low levels in the plasma of patients with PMOP. In the treatment, it was found that patients with low levels of lncRNA–NEF in the plasma had a longer treatment course and a higher recurrence rate [104]. Similar to the lncRNA SNHG1, Huang et al. found that the plasma expression level of SNHG1 in postmenopausal women with osteoporosis is significantly lower than that in healthy postmenopausal women, and this difference was observed a year before osteoporosis was diagnosed. Therefore, plasma SNHG1 levels could be used as a predictor of PMOP to identify high-risk groups for early prevention and treatment [105].
LncRNAs play an essential role in the treatment of osteoporosis, which helps us better understand the pharmacological mechanisms of some drugs. Yang et al. found that the lncRNA DANCR is involved in the mechanism by which sesamin regulates osteogenesis and osteoclastogenesis. Therefore, sesamin can be developed as a new drug for the treatment of postmenopausal osteoporosis, especially in postmenopausal women with high serum DANCR levels [106]. Melatonin (MT) and estrogen have also been shown to promote the osteogenic differentiation process of BMSCs and alleviate osteoporosis through lncRNA H19 [39, 107].
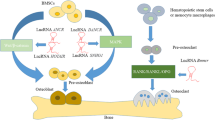
An increasing number of biomaterials have been shown to influence bone metabolism and development through epigenetic mechanisms. A recent study using bioactive glass nanoparticles (BGN) showed that Nron mediates the reversal of bone loss in postmenopausal osteoporotic mice. Researchers have found that BGN with active ions induces BMSCs to secrete heterogeneous extracellular vesicles (EVs) rich in the lncRNA Nron, which inhibits nuclear translocation of NFATc1 in osteoclasts, thereby inhibiting osteoclast differentiation and alleviating osteoporosis development [108]. Another bone implant material, TiO2 nanotubes (TNTs), promotes the osteogenic differentiation of MSCs. Many studies have indicated that this process is mediated by epigenetic mechanisms, such as the lncRNAs CCL3-AS, LINC00941, LINC01279, and ZFAS1, which are closely related to this process [111, 112]. Bone implant materials have very high requirements for biocompatibility and mechanical properties; otherwise, they can easily lead to bone implant failure. The research team of Shuai et al. found that introducing silver (Ag) into different biopolymers improved the cytocompatibility and mechanical properties of bone implant materials. For example, Ag nanoparticles, as the conductive phase of a polyvinylidene fluoride (PVDF)/barium titanate (BaTiO3) composite, enhance the piezoelectric properties and antibacterial activity of the composite and effectively promote cell proliferation and differentiation [113]. Additionally, poly-L-lactic acid-polyglycolic acid (PLLA-PGA) scaffolds were introduced with co-dispersed graphene oxide (GO)-Ag nanosystems, and polymer scaffolds based on mesoporous bioactive glass (MBG) loaded with in situ-grown Ag improved their mechanical properties and endowed them with long-term antibacterial activity through the continuous release of Ag+. Meanwhile, they also show good biocompatibility in promoting the adhesion and proliferation of osteoblasts [114, 115]. These studies provide a solution to the problem of bacterial resistance caused by the heavy use of antibiotics after bone implantation. The epigenetic regulation of biomaterials can explain the influence of new materials on cells and can be used as a potential tool for safety evaluation of biomaterials [112]. Based on this conclusion, more suitable bone implant materials can be designed in future studies based on the epigenetic mechanisms of the disease.
LncRNAs can be directly used as gene-targeted drugs in the treatment of osteoporosis. In gene-targeted therapy, exogenous nucleic acid fragments are targeted to specific sites to modulate gene expression and combat disease progression. However, the specific delivery of drugs to target tissue cells and avoidance of their degradation are a challenge in the implementation of gene-targeted therapy. The research team of Cai et al. discovered a novel targeted delivery system: Asp 8-PU. Asp 8 is a guide that targets the bone resorption area and polyurethane (PU) is a carrier for drug delivery. Asp 8-PU can specifically deliver gene drugs to the bone resorption surface and inhibit the bone resorption process, thereby alleviating osteoporosis progression [116]. Based on the above research, Jin et al. identified lncRNA Nron as a negative regulator of bone resorption and injected Nron intravenously into OVX mice via a bone-resorption surface-targeting nucleic acid delivery system (Asp 8-PU); Nron treatment significantly increased cortical bone thickness and bone strength in OVX mice. Moreover, using Nron functional motifs instead of full-length transcripts significantly reduced side effects, such as splenomegaly, while maintaining the same therapeutic effects as full-length Nron. Mechanistically, Nron interacts with the E3 ligase CUL4B through a functional motif to regulate the stability of ERα in osteoclasts [109]. Similarly, in another study, researchers identified a new lncRNA–lnc-DIF to inhibit the osteogenesis process and delivered si-lnc-DIF to the surface of bone formation through an osteogenesis-targeted delivery system, ultimately improving osteoporotic symptoms in OVX mice [110]. These studies provide a new perspective for the development of novel pharmaceuticals for the treatment of osteoporosis and for understanding their pharmacological effects.
Conclusion and future prospectives
In this review, we summarize the various functions and mechanisms of lncRNAs in the development of osteoporosis and analyze the effects and roles of different signaling pathways in osteogenic and osteoclast differentiation processes. We found that lncRNAs can not only act as competitive endogenous RNAs for many microRNAs to regulate and affect the expression of different targeted genes, but also play a crucial role in regulating the process of osteogenic differentiation through Wnt/β-catenin, TGF-β/BMP, MAPK, and Notch pathways. Moreover, lncRNAs can regulate the activity of osteoclasts through the OPG/RANK/RANKL regulatory network to affect bone resorption, resulting in the disruption of bone homeostasis and osteoporosis. We also summarized the clinical application of lncRNAs in osteoporosis, including clinical diagnosis, prevention and rehabilitation, and drug development and targeted therapy. However, the studies reviewed herein reveal that they were characterized by certain limitations. First, most studies on the mechanisms of lncRNAs in osteogenic differentiation are indirect mechanisms; the downstream targets of lncRNAs and detailed molecular mechanisms involved need to be further elucidated. Second, most current experimental models for osteoporosis mechanism research involve vertebrates, such as rats. Owing to the low conservation characteristics of lncRNAs, it is extremely difficult to replicate the in vitro research results in clinical treatments. Therefore, more research and/or new experimental methods need to be developed to address such problems. Finally, the implementation of precise treatment in patients with osteoporosis through lncRNAs, while reducing side effects, must be the focus of future research. Therefore, we hope that further research will be conducted on targeted therapies for osteoporosis using lncRNAs in the future.
Data availability
Not applicable.
Abbreviations
- lncRNAs:
-
Long non-coding RNAs
- miRNAs:
-
MicroRNAs
- circRNAs:
-
Circular RNAs
- BMSCs:
-
Bone marrow mesenchymal stromal cells
- RUNX2:
-
Runt-related transcription factor 2
- OPG:
-
Osteprotegerin
- NF-κB:
-
Nuclear factor κB
- RANK:
-
Receptor activator of NF-kappaB
- RANKL:
-
Receptor activator of NF-kappaB ligand
- OSX:
-
Osterix
- TNF:
-
Transforming growth factor
- ceRNAs:
-
Competing endogenous RNAs
- MRE:
-
MiRNA response element
- FAK:
-
Focal adhesion kinase
- ERK:
-
Extracellular signal-regulated kinase
- hBMSCs:
-
Human bone marrow mesenchymal stromal cells
- CTNNB1:
-
Catenin beta 1
- PROX1:
-
Prospero homeobox 1
- SORBS1:
-
SH3 domain containing 1
- HDAC:
-
Histone deacetylase
- hMSCs:
-
Human mesenchymal stromal cells
- PMOP:
-
Postmenopausal osteoporotic
- DOP:
-
Disuse osteoporosis
- DKK:
-
Dickkopf
- Foxc2:
-
Forkhead box C2
- SFRP1:
-
Secreted frizzled-related protein 1
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- FZD4:
-
Frizzled class receptor 4
- WDR5:
-
WD repeat-containing protein 5
- BMP:
-
Bone morphogenetic protein
- TGF:
-
Transforming growth factor
- DNMT1:
-
DNA cytosine-5-methyltransferase
- ALP:
-
Alkaline phosphatase
- OVX:
-
Ovariectomy
- PI3K:
-
Phosphoinositide 3-kinase
- PTEN:
-
Phosphatase and tensin homolog
- NIP45:
-
NFAT-interacting protein
- FUS:
-
Fused in sarcoma
- PTK2:
-
Protein tyrosine kinase 2
- APN:
-
Adiponectin
- SIRT1:
-
Sirtuin 1
References
Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Yang Y, Yujiao W, Fang W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53(1):40.
Sözen T, Özışık L, Başaran N. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56.
Black DM, Rosen CJ. Clinical practice. Postmenopausal Osteoporos N Engl J Med. 2016;374(3):254–62.
Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International osteoporosis foundation (IOF) and the European federation of pharmaceutical industry associations (EFPIA). Arch Osteoporos. 2013;8(1):136.
Lewiecki EM, Binkley N, Bilezikian JP. Treated osteoporosis is still osteoporosis. J Bone Miner Res. 2019;34(4):605–6.
Eiken P, Vestergaard P. Treatment of osteoporosis after alendronate or risedronate. Osteoporos Int. 2016;27(1):1–12.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408.
Balasch J. Sex steroids and bone: current perspectives. Hum Reprod Update. 2003;9(3):207–22.
Trouvin AP, Goëb V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–54.
Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FA, Martin TJ. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest. 1986;78(2):355–60.
Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19.
Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–79.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18.
Silva AM, Moura SR, Teixeira JH, Barbosa MA, Santos SG, Almeida MI. Long noncoding RNAs: a missing link in osteoporosis. Bone Res. 2019;7:10.
Gu H, Huang Z, Zhou K, et al. Expression profile analysis of long non-coding RNA in OVX models-derived BMSCs for postmenopausal osteoporosis by RNA sequencing and bioinformatics. Front Cell Dev Biol. 2021;9: 719851.
Frankish A, Diekhans M, Ferreira AM, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47(D1):D766–73.
Peng S, Cao L, He S, et al. An overview of long noncoding RNAs involved in bone regeneration from mesenchymal stem cells. Stem Cells Int. 2018;2018:8273648.
Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2): e202009045.
Mukherjee N, Calviello L, Hirsekorn A, de Pretis S, Pelizzola M, Ohler U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat Struct Mol Biol. 2017;24(1):86–96.
Cabili MN, Dunagin MC, McClanahan PD, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16(1):20.
Zuckerman B, Ulitsky I. Predictive models of subcellular localization of long RNAs. RNA. 2019;25(5):557–72.
Hezroni H, Koppstein D, Schwartz MG, Avrutin A, Bartel DP, Ulitsky I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep. 2015;11(7):1110–22.
Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–72.
Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21(5):542–51.
Nojima T, Proudfoot NJ. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev Mol Cell Biol. 2022;23(6):389–406.
Ben Amor B, Wirth S, Merchan F, et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009;19(1):57–69.
Zhang X, Wang W, Zhu W, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20(22):5573.
Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310.
Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond). 2021;41(2):109–20.
Suzuki A, Minamide M, Iwaya C, Ogata K, Iwata J. Role of metabolism in bone development and homeostasis. Int J Mol Sci. 2020;21(23):8992.
Zhou Z, Hossain MS, Liu D. Involvement of the long noncoding RNA H19 in osteogenic differentiation and bone regeneration. Stem Cell Res Ther. 2021;12(1):74.
Chen S, Liu D, Zhou Z, Qin S. Role of long non-coding RNA H19 in the development of osteoporosis. Mol Med. 2021;27(1):122.
Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112(12):3491–501.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59(2):99–107.
Chen RS, Zhang XB, Zhu XT, Wang CS. LncRNA Bmncr alleviates the progression of osteoporosis by inhibiting RANML-induced osteoclast differentiation. Eur Rev Med Pharmacol Sci. 2019;23(21):9199–206.
Han H, Tian T, Huang G, Li D, Yang S. The lncRNA H19/miR-541-3p/Wnt/β-catenin axis plays a vital role in melatonin-mediated osteogenic differentiation of bone marrow mesenchymal stem cells. Aging (Albany NY). 2021;13(14):18257–73.
Zhang Z, Qi H, Xia H, et al. Preosteoblast-enriched lnc-Evf2 facilitates osteogenic differentiation by targeting Notch. Acta Biochim Biophys Sin (Shanghai). 2021;53(2):179–88.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–8.
Wu J, Zhao J, Sun L, Pan Y, Wang H, Zhang WB. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62–70.
Wang CG, Hu YH, Su SL, Zhong D. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/β-catenin signaling pathway. Exp Mol Med. 2020;52(8):1310–25.
Weng W, Di S, Xing S, et al. Long non-coding RNA DANCR modulates osteogenic differentiation by regulating the miR-1301-3p/PROX1 axis. Mol Cell Biochem. 2021;476(6):2503–12.
Zang LY, Yang XL, Li WJ, Liu GL. Long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1 promotes the osteoblast differentiation of human bone marrow-derived mesenchymal stem cells by targeting the microRNA-96/osterix axis. J Craniofac Surg. 2021;33(3):956–61.
Gao Y, Xiao F, Wang C, et al. Long noncoding RNA MALAT1 promotes osterix expression to regulate osteogenic differentiation by targeting miRNA-143 in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2018;119(8):6986–96.
Li X. LncRNA metastasis-associated lung adenocarcinoma transcript-1 promotes osteogenic differentiation of bone marrow stem cells and inhibits osteoclastic differentiation of Mø in osteoporosis via the miR-124-3p/IGF2BP1/Wnt/β-catenin axis. J Tissue Eng Regen Med. 2022;16(3):311–29.
Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63(1):36–49.
Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019;11(20):8777–91.
Bi HU, Wang D, Liu X, Wang G, Wu X. Long non-coding RNA H19 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by regulating microRNA-140-5p/SATB2 axis. J Biosci. 2020;45:56.
Zheng J, Guo H, Qin Y, et al. SNHG5/miR-582–5p/RUNX3 feedback loop regulates osteogenic differentiation and apoptosis of bone marrow mesenchymal stem cells. J Cell Physiol. 2020. https://doi.org/10.1002/jcp.29527.
Li G, Yun X, Ye K, et al. Long non-coding RNA-H19 stimulates osteogenic differentiation of bone marrow mesenchymal stem cells via the microRNA-149/SDF-1 axis. J Cell Mol Med. 2020;24(9):4944–55.
Hu F, Jiang C, Bu G, Fu Y, Yu Y. Silencing long noncoding RNA colon cancer-associated transcript-1 upregulates microRNA-34a-5p to promote proliferation and differentiation of osteoblasts in osteoporosis. Cancer Gene Ther. 2021;28(10–11):1150–61.
Xu Y, Xin R, Sun H, et al. Long non-coding RNAs LOC100126784 and POM121L9P derived from bone marrow mesenchymal stem cells enhance osteogenic differentiation via the miR-503-5p/SORBS1 axis. Front Cell Dev Biol. 2021;9: 723759.
Che M, Gong W, Zhao Y, Liu M. Long noncoding RNA HCG18 inhibits the differentiation of human bone marrow-derived mesenchymal stem cells in osteoporosis by targeting miR-30a-5p/NOTCH1 axis. Mol Med. 2020;26(1):106.
Huang Y, Zheng Y, Jia L, Li W. Long noncoding RNA H19 promotes osteoblast differentiation via TGF-β1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cells. 2015;33(12):3481–92.
Li Z, Hong Z, Zheng Y, et al. An emerging potential therapeutic target for osteoporosis: LncRNA H19/miR-29a-3p axis. Eur J Histochem. 2020;64(4):3155.
Wang Q, Li Y, Zhang Y, et al. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother. 2017;89:1178–86.
Huang XZ, Huang J, Li WZ, Wang JJ, Song DY, Ni JD. LncRNA-MALAT1 promotes osteogenic differentiation through regulating ATF4 by sponging miR-214: Implication of steroid-induced avascular necrosis of the femoral head. Steroids. 2020;154: 108533.
Yang C, Gu Z, Ding R, et al. Long non-coding RNA MEG3 silencing and microRNA-214 restoration elevate osteoprotegerin expression to ameliorate osteoporosis by limiting TXNIP. J Cell Mol Med. 2021;25(4):2025–39.
He Q, Li R, Hu B, et al. Stromal cell-derived factor-1 promotes osteoblastic differentiation of human bone marrow mesenchymal stem cells via the lncRNA-H19/miR-214-5p/BMP2 axis. J Gene Med. 2021;23(9): e3366.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50.
Kim HY, Yoon JY, Yun JH, et al. CXXC5 is a negative-feedback regulator of the Wnt/β-catenin pathway involved in osteoblast differentiation. Cell Death Differ. 2015;22(6):912–20.
Zhou P, Li Y, Di R, et al. H19 and Foxc2 synergistically promotes osteogenic differentiation of BMSCs via Wnt-β-catenin pathway. J Cell Physiol. 2019;234(8):13799–806.
Li B, Liu J, Zhao J, et al. LncRNA-H19 modulates Wnt/β-catenin signaling by targeting Dkk4 in hindlimb unloaded rat. Orthop Surg. 2017;9(3):319–27.
Xiang J, Fu HQ, Xu Z, Fan WJ, Liu F, Chen B. lncRNA SNHG1 attenuates osteogenic differentiation via the miR-101/DKK1 axis in bone marrow mesenchymal stem cells. Mol Med Rep. 2020;22(5):3715–22.
Yu X, Rong PZ, Song MS, et al. lncRNA SNHG1 induced by SP1 regulates bone remodeling and angiogenesis via sponging miR-181c-5p and modulating SFRP1/Wnt signaling pathway. Mol Med. 2021;27(1):141.
Jiang Z, Jiang C, Fang J. Up-regulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;495(1):238–45.
Jia B, Wang Z, Sun X, Chen J, Zhao J, Qiu X. Long noncoding RNA LINC00707 sponges miR-370-3p to promote osteogenesis of human bone marrow-derived mesenchymal stem cells through upregulating WNT2B. Stem Cell Res Ther. 2019;10(1):67.
Cai WL, Zeng W, Liu HH, Zhu BY, Liu JL, Liu Y. LncRNA LINC00707 promotes osteogenic differentiation of hBMSCs through the Wnt/β-catenin pathway activated by LINC00707/miR-145/LRP5 axis. Eur Rev Med Pharmacol Sci. 2020;24(1):18–28.
Gao H, Dong H, Zheng J, et al. LINC01119 negatively regulates osteogenic differentiation of mesenchymal stem cells via the Wnt pathway by targeting FZD4. Stem Cell Res Ther. 2022;13(1):43.
Liu R, Li Z, Song E, et al. LncRNA HOTTIP enhances human osteogenic BMSCs differentiation via interaction with WDR5 and activation of Wnt/β-catenin signalling pathway. Biochem Biophys Res Commun. 2020;524(4):1037–43.
Calvier L, Chouvarine P, Legchenko E, et al. PPARγ Links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017;25(5):1118-34.e7.
Deng ZL, Sharff KA, Tang N, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–21.
Kang Q, Song WX, Luo Q, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–59.
Zhuang W, Ge X, Yang S, et al. Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells. 2015;33(6):1985–97.
Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90(Suppl 1):14–8.
Chen S, Jia L, Zhang S, Zheng Y, Zhou Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res Ther. 2018;9(1):185.
Li H, Xu X, Wang D, et al. Hypermethylation-mediated downregulation of long non-coding RNA MEG3 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells and promotes pediatric aplastic anemia. Int Immunopharmacol. 2021;93: 107292.
Zhang RF, Liu JW, Yu SP, et al. LncRNA UCA1 affects osteoblast proliferation and differentiation by regulating BMP-2 expression. Eur Rev Med Pharmacol Sci. 2019;23(16):6774–82.
Li B, Wang J, Xu F, et al. LncRNA RAD51-AS1 regulates human bone marrow mesenchymal stem cells via interaction with YBX1 to ameliorate osteoporosis. Stem Cell Rev Rep. 2022;19(1):170–87.
Robaszkiewicz A, Valkó Z, Kovács K, et al. The role of p38 signaling and poly(ADP-ribosyl)ation-induced metabolic collapse in the osteogenic differentiation-coupled cell death pathway. Free Radic Biol Med. 2014;76:69–79.
Zhang J, Tao Z, Wang Y. Long non-coding RNA DANCR regulates the proliferation and osteogenic differentiation of human bone-derived marrow mesenchymal stem cells via the p38 MAPK pathway. Int J Mol Med. 2018;41(1):213–9.
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13.
Jiang Y, Wu W, Jiao G, Chen Y, Liu H. LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019;228:208–14.
Zheng S, Wang YB, Yang YL, et al. LncRNA MALAT1 inhibits osteogenic differentiation of mesenchymal stem cells in osteoporosis rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):4609–17.
Viale-Bouroncle S, Klingelhöffer C, Ettl T, Morsczeck C. The AKT signaling pathway sustains the osteogenic differentiation in human dental follicle cells. Mol Cell Biochem. 2015;406(1–2):199–204.
Lin TH, Gibon E, Loi F, et al. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-κB activity. J Orthop Res. 2017;35(2):281–8.
Zhang Y, Cao X, Li P, et al. LncRNA NKILA integrates RXFP1/AKT and NF-κB signalling to regulate osteogenesis of mesenchymal stem cells. J Cell Mol Med. 2020;24(1):521–9.
Zhu X, Yu J, Du J, Zhong G, Qiao L, Lin J. LncRNA HOXA-AS2 positively regulates osteogenesis of mesenchymal stem cells through inactivating NF-κB signalling. J Cell Mol Med. 2019;23(2):1325–32.
Jin C, Jia L, Tang Z, Zheng Y. Long non-coding RNA MIR22HG promotes osteogenic differentiation of bone marrow mesenchymal stem cells via PTEN/ AKT pathway. Cell Death Dis. 2020;11(7):601.
Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13(9):654–66.
Liao J, Yu X, Hu X, et al. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017;8(32):53581–601.
Zhang Z, Liu J, Zeng Z, et al. lncRNA Rmst acts as an important mediator of BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) by antagonizing Notch-targeting microRNAs. Aging (Albany NY). 2019;11(24):12476–96.
Liu C, Cao Z, Bai Y, et al. LncRNA AK077216 promotes RANKL-induced osteoclastogenesis and bone resorption via NFATc1 by inhibition of NIP45. J Cell Physiol. 2019;234(2):1606–17.
Zhang DW, Wang HG, Zhang KB, Guo YQ, Yang LJ, Lv H. LncRNA XIST facilitates S1P-mediated osteoclast differentiation via interacting with FUS. J Bone Miner Metab. 2022;40(2):240–50.
Li X. LncRNA metastasis-associated lung adenocarcinoma transcript-1 promotes osteogenic differentiation of bone marrow stem cells and inhibits osteoclastic differentiation of Mø in osteoporosis via the miR-124-3p/IGF2BP1/Wnt/β-catenin axis. J Tissue Eng Regen Med. 2021;16(3):311–29.
Zhang Y, Chen XF, Li J, He F, Li X, Guo Y. lncRNA Neat1 stimulates osteoclastogenesis via sponging miR-7. J Bone Miner Res. 2020;35(9):1772–81.
Yu H, Zhou W, Yan W, Xu Z, Xie Y, Zhang P. LncRNA CASC11 is upregulated in postmenopausal osteoporosis and is correlated with TNF-α. Clin Interv Aging. 2019;14:1663–9.
Cong C, Tian J, Gao T, et al. lncRNA GAS5 is upregulated in osteoporosis and downregulates miR-21 to promote apoptosis of osteoclasts. Clin Interv Aging. 2020;15:1163–9.
Lee CP, Huang YN, Nithiyanantham S, Huang CM, Ko YC. LncRNA-Jak3:Jak3 coexpressed pattern regulates monosodium urate crystal-induced osteoclast differentiation through Nfatc1/Ctsk expression. Environ Toxicol. 2019;34(2):179–87.
Ling L, Hu HL, Liu KY, Ram YI, Gao JL, Cao YM. Long noncoding RNA MIRG induces osteoclastogenesis and bone resorption in osteoporosis through negative regulation of miR-1897. Eur Rev Med Pharmacol Sci. 2019;23(23):10195–203.
Yu Y, Yao P, Wang Z, Xie W. Down-regulation of FTX promotes the differentiation of osteoclasts in osteoporosis through the Notch1 signaling pathway by targeting miR-137. BMC Musculoskelet Disord. 2020;21(1):456.
Ma X, Guo Z, Gao W, et al. LncRNA-NEF is downregulated in postmenopausal osteoporosis and is related to course of treatment and recurrence. J Int Med Res. 2019;47(7):3299–306.
Huang S, Zhu X, Xiao D, et al. LncRNA SNHG1 was down-regulated after menopause and participates in postmenopausal osteoporosis. Biosci Rep. 2019;39(11):BSR20190445.
Yang Z, Feng L, Wang H, et al. DANCR mediates the rescuing effects of sesamin on postmenopausal osteoporosis treatment via orchestrating osteogenesis and osteoclastogenesis. Nutrients. 2021;13(12):4455.
Li T, Jiang H, Li Y, Zhao X, Ding H. Estrogen promotes lncRNA H19 expression to regulate osteogenic differentiation of BMSCs and reduce osteoporosis via miR-532-3p/SIRT1 axis. Mol Cell Endocrinol. 2021;527: 111171.
Yang Z, Liu X, Zhao F, et al. Bioactive glass nanoparticles inhibit osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression in the extracellular vesicles derived from bone marrow mesenchymal stem cells. Biomaterials. 2022;283: 121438.
Jin F, Li J, Zhang YB, et al. A functional motif of long noncoding RNA Nron against osteoporosis. Nat Commun. 2021;12(1):3319.
Yin C, Tian Y, Li D, et al. Long noncoding RNA Lnc-DIF inhibits bone formation by sequestering miR-489–3p. iScience. 2022;25(3):103949.
Jin Z, Yan X, Shen K, et al. TiO(2) nanotubes promote osteogenic differentiation of mesenchymal stem cells via regulation of lncRNA CCL3-AS. Coll Surf B Biointerfaces. 2019;181:416–25.
Lv L, Liu Y, Zhang P, et al. The epigenetic mechanisms of nanotopography-guided osteogenic differentiation of mesenchymal stem cells via high-throughput transcriptome sequencing. Int J Nanomed. 2018;13:5605–23.
Shuai C, Liu G, Yang Y, et al. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy. 2020;74: 104825.
Shuai C, Xu Y, Feng P, Wang G, Xiong S, Peng S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem Eng J. 2019;374:304–15.
Shuai C, Guo W, Wu P, et al. A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem Eng J. 2018;347:322–33.
Cai M, Yang L, Zhang S, Liu J, Sun Y, Wang X. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int J Nanomed. 2017;12:7469–82.
Funding
This study was funded by the National Natural Science Foundation of China [Grant No. 81972156], 345 Talent Project, and Outstanding Scientific Fund of Shengjing Hospital.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. JH wrote the first draft of the manuscript. DL, JZ, SQ, SC, and ZZ drafted the manuscript. All authors commented on the previous versions of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, J., Liu, D., Zhao, J. et al. Long non-coding RNAs in osteoporosis: from mechanisms of action to therapeutic potential. Human Cell 36, 950–962 (2023). https://doi.org/10.1007/s13577-023-00888-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00888-5