Abstract
Objective
Insecticides play an essential role in preventing crop losses. However, there is growing concern over adverse effects associated with their widespread and long-term use. Cypermethrin (CYP) and lambda-cyhalothrin (LCT) are type II pyrethroid insecticides that inhibit voltage-sensitive sodium channel function. Emamectin benzoate (EMB) is an avermectin class insecticide that stimulates γ-aminobutyric acid (GABA) receptor and glutamate-gated chloride channel.
Methods
The L-929 cells were exposed to CYP, LCT, and EMB for 48 h to measure the cytotoxic effect by MTT assay. Then, Annexin V/PI was used to evaluate apoptosis. Finally, Comet assay was used to determine the genotoxicity and DNA damage.
Results
We present the investigation results of potential cytotoxic and genotoxic effects of LCT, CYP, and EMB insecticides on normal murine fibroblast cells (L-929). Our results indicated that cell viability decreased with increasing concentrations of insecticides during 48 h. Moreover, the apoptosis induction by EMB was more potent than LCT and CYP. The comet assay findings indicated that CYP causes more DNA damage than the other two insecticides. As a result, CYP may be considered a genotoxic insecticide.
Conclusion
Our results may be included in future research to provide a more thorough assessment of the toxicity of these insecticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different insecticides are widely used to control insect vectors and pests [1]. However, their widespread and unrestricted usage contaminates the environment and results in the introduction of these chemicals into the food chain through water and soil [2]. Recent years have seen a rise in exposure to these compounds, and adverse health impacts in non-target species, including humans and animals, have become evident. Several studies showed that long-term exposure to insecticides affects health, by causing oxidative stress, genetic modifications, epigenetic changes, DNA damage, chromosomal aberration, mitochondrial dysfunction, endocrine disruption, and even cancers[3, 4]. Iran consumes approximately 50% of all pesticides [2] .According to Food and Agriculture Organization (FAO) data, Iran ranks 53rd globally and 14th in Asia regarding pesticide usage per hectare[5]. Pyrethroid insecticides are used in various pest control activities, including garden and household insecticides, as broad-spectrum compounds which represent more than 30% of globally used insecticides in the market [6]. Pyrethroid insecticides are considered safe, less harmful, and relatively non-toxic to the environment and mammalian health compared to other insecticides [7]. Despite its low toxicity to mammals, pyrethroid retention in mammalian tissues through inhalation, dermal absorption, and food/water consumption might be harmful [7, 8]. Various epidemiological studies have reported the association between prolonged high-dose exposure to pyrethroids and possible adverse health effects in recent years [9]. The mechanism of pyrethroids' action in insects and mammals is similar, which involves disrupting the normal function of voltage-sensitive sodium channels [10]. Cypermethrin (CYP) and lambda-cyhalothrin (LCT) are both type II pyrethroid insecticides [10, 11]. World Health Organization (WHO) classified these insecticides as moderately hazardous (Class II) [5, 12]. Besides, Occupational Safety and Health Administration (OSHA) and the American Conference of Governmental Industrial Hygienists (ACGIH) have not established any allowable exposure limits for CYP [5]. CYP has been shown to be neurotoxic to insects and mammals. These effects are achieved by increasing sodium permeability and hyper-exciting neurons, followed by a prolonged sodium channel opening in the neuronal membrane [13]. Reports indicated that this synthetic pyrethroid accumulates in the fatty tissue, skin, liver, kidneys, adrenal glands, ovaries and causes damage to vital organs [7]. LCT is a mixture of cyhalothrin isomers that can provide an effective immediate lethal impact against a wide variety of arthropods [3, 14]. The mechanisms of action of this synthetic pyrethroid are related to its accumulation in the biological membranes which lead to oxidative damage, as suggested by previous reports. Although LCT usually manifests a weak toxic effect, several studies showed links between adverse health effects and exposure to LCT. Therefore, the widespread application of this insecticide evokes various influences in different in vitro and in vivo models [3, 4, 14, 15]. Emamectin benzoate (EMB) is a novel macrocyclic lactone insecticide that belongs to the avermectin family, widely used against various pests [16, 17]. United States Environmental Protection Agency (U.S EPA) described EMB as a highly toxic compound for mammals [18, 19]. The WHO also classified EMB in class II as moderately hazardous [12]. According to the current analysis in rats and mice, EMB is considered non-carcinogenic and non-mutagenic, but it has strong potential to induce DNA damage and apoptosis [18, 19]. This insecticide acts as a neurotoxin via the persistent stimulation of the γ-aminobutyric acid (GABA) receptor and glutamate-gated chloride channels of the nerve cells [16]. Humans have a finite number of GABA-reactive neurons. As a result, EMB was meant to be safe [19]. However, due to EMB's lipophilicity, it may easily cross the cell membrane, causing toxicity to humans and animals [19]. Numerous investigations have shown that EMB is toxic to mammals [16, 19, 20]. Determination of the cytotoxicity and genotoxicity of insecticides in cell models may aid in the understanding of the mechanism of action of insecticides. Therefore, this study investigated the cytotoxic and genotoxic effects of LCT, CYP, and EMB insecticides on murine fibroblast cells (L-929).
Results
Cytotoxicity of insecticides on L-929 cells
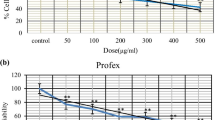
The cell viability after exposure to the different concentrations of mentioned insecticides (0, 1.95, 3.90, 7.80, 15.62, 31.25, 62.5, 125, 250, 500,1000 μg/ml) is as shown in Fig. 1. These data demonstrated a dose-dependent effect of these insecticides on the cells. IC50 values calculated for LCT, CYP and EMB were 4.46 ± 0.37, 7.48 ± 0.21, 53.41 ± 7.51 (µg/ml), respectively.
Evaluation of apoptosis induction in L-929 cells
The data showed the incubation of cells with an IC50 dose of EMB resulted in the induction of early stages of apoptosis (35.4% vs. 2.58% for the control group, p < 0.01) (Fig. 2). In comparison, the percentage of late apoptotic (6.83%) and necrotic cells (6.6%) was not statistically significant compared to the control group (p > 0.05). The incubation of cells with LCT and CYP was not statistically significant compared to the control group (p > 0.05). The results showed that EMB had induced apoptosis more than the other two insecticides.
Evaluation of the apoptosis in L-929 cells treated with an IC50 dose of CYP, LCT, and EMB after 48 h. A Flow cytometric dot plots. B The percentage of apoptotic cells of CYP, LCT, and EMB; Each value indicates the mean ± standard deviation (SD). **p < 0.001, ****p < 0.0001. *CYP: Cypermethrin, LCT:lambda-cyhalothrin, EMB: Emamectin benzoate
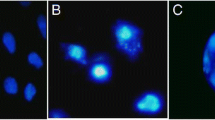
Evaluation of genotoxicity effect of insecticides on L-929 cells
The parameters of the comets in the control and exposed groups are listed in Table 1. The findings demonstrated that there was a statistically significant difference in tail length in the CYP and EMB groups compared to the control group. However, increasing the tail length of the LCT group was not significant. In CYP and EMB groups, head DNA decreased, and tail DNA increased compared to the control group, indicating the migration of DNA from the nucleus to the outside (Fig. 3). These changes were not significant in the LCT group. In the Tail moment parameter results, changes in the CYP group were significant compared to the control group, while other insecticides did not show a significant difference.
Discussion
There are a few research reports on the cytotoxicity and genotoxicity of insecticides using the normal murine fibroblast cell line. Therefore, this study aimed to evaluate the potential cytotoxic and genotoxic activity of three common insecticides, including LCT, CYP, and EMB, using MTT assay and comet assay on L-929 cell line. The present study indicated that cell growth rate decreased with increasing concentration of LCT, CYP, and EMB insecticides, which clearly showed the cytotoxic effect in a dose-dependent manner. LCT and CYP were the most cytotoxic and inhibited cell proliferation at low doses. However, EMB exhibited the least cytotoxicity. This result runs in good agreement with previous studies; LCT [21,22,23,24], CYP [1, 25, 26] and EMB [27]. On the contrary, a study showed that CYP had low cytotoxicity effects on human neuroblastoma cell line SH-SY5Y. The authors stated that differences in CYP cytotoxicity effects might vary in terms of using the pyrethroids with different degrees of purity [28]. Studies indicated that the insecticide exposure in different cell lines does not show the same pattern of toxic reactions, and insecticides inhibit cell growth using various toxic mechanisms [28].
Our results showed that in the IC50 dose, EMB showed a significant rate of apoptosis induction and caused an increase in early apoptotic cells. However, LCT and CYP also had the same apoptosis induction rate but were not statistically significant. EMB inhibits cell proliferation and promotes apoptosis, as shown in this research. Increased apoptotic rates in cortical neurons were seen in a research with increasing CYP concentrations [13]. Studies have described that EMB induces apoptosis in a time- and dose-dependent manner [18, 19]. Besides, numerous studies indicated that EMB has potent cytotoxic and apoptotic effects on Tn5B1-4 [16], QSG7701(human hepatic cancer cell line) [19], 16HBE (human bronchial epidermal cells) [20], K562, and Molt-4 (human acute lymphoblastic leukemia) [29] cells.
In the present study, the tail length results showed that CYP and EMB could induce early DNA damage in L-929 cells and CYP had a high genotoxic effect on the cells in IC50 dose. Studies suggested that the genotoxicity mechanism of CYP is related to its chemical structure. Previous studies reported CYP-induced DNA damage in non-target organisms [30,31,32,33]. Evidence showed the potential genotoxic effects of CYP via inducing DNA damage which can be regarded as a trigger of apoptosis [31]; tail formation following the nuclear fragmentation indicates apoptosis [31]. EMB induces DNA damage in a dose-dependent manner, according to a research evaluating its genotoxic impact [19]. Exposure to EMB was shown to have genotoxic potential, causing DNA damage in insect Sf-9 cells [18], human lung cells [20], and HeLa cells [18], according to reports. The other finding in our study was that contrary to the high cytotoxic effect of LCT, this insecticide had a weak genotoxic effect on cells at IC50 dose. Although, other studies showed the genotoxic effect of LCT on Sf-9 cells [11], human lymphocytes [23], mosquito erythrocytes [34], macrophages [24], and bone marrow.
Materials and methods
Chemicals and reagents
All insecticides, including LCT (50 gr/L), a capsule suspension formulation, EMB 5%, a water-soluble granule formulation, and CYP 10%, were purchased from Dogal Kimyevi Maddeler ve Zirai, Turkey. RPMI 1640 medium (RPMI) and fetal bovine serum (FBS) were purchased from Biowest (Paris, France). Low melt agarose and Annexin V-FITC were obtained from Thermo Fisher Scientific (USA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) was purchased from Alfa Aesar (Massachusetts, USA). Agarose, dimethyl sulfoxide (DMSO), ethidium bromide, and propidium iodide (PI) were obtained from Sigma-Aldrich (MO, USA).
Cell culture
L-929 cell line (a normal murine fibroblast) was purchased from Iranian Biological Resources Center and maintained in RPMI medium with 10% heat-inactivated FBS and 1% penicillin–streptomycin solution (Biosera, country) and incubated 37 °C (Memmert, Germany) in a humidified incubator with a 5% CO2 level.
MTT assay
L-929 cells were seeded 6 × 103 cells/well in 96-well culture plates. After overnight incubation, the cells were exposed to various insecticide concentrations. After 48 h of the insecticide exposure, the supernatants were removed, and 20 μL MTT (5 mg/ml in Phosphate Buffered Saline, PBS) was added to each well. The cells were then incubated at 37 °C for 3 h to create the formazan crystals. After dissolving the formazan crystals in DMSO, the absorbance was measured at 570 nm (wavelength) and 690 nm (reference) using an ELISA Plate Reader (Anthos, UK)[35].
Comet assay
The alkaline comet assay was used to evaluate the genotoxicity and DNA damage. L-929 cells (3 × 105 cells/well) were seeded in 6-well plates. After 24 h, the cells were treated with IC50 of insecticides for 48 h. Then, the cells were collected, resuspended in 75 µL PBS (2 × 103 cells/ mL), and mixed with 1% low melting agarose. Subsequently, the cells were layered onto frosted glass slides. Slides were preserved in refrigerators at 4 °C for 10 min. Then, the slides were immersed in pre-chilled lysis solution (2.5 M NaCl, 10 mM Tris–HCl, 10 mM Na2 EDTA, 1% Triton X-100, pH 7.5) for 60 min at 4 °C. The slides were soaked in denaturing solution (1 mM Na2 EDTA, 300 mM NaOH, pH 13) for 30 min at 4 °C then electrophoresis (0.66 V/cm) was carried out for 20 min, and slides were stained with ethidium bromide. The images were taken by fluorescence microscopy (Olympus)[35].
Apoptosis assay
Annexin V/PI is extensively used to evaluate apoptosis due to the high affinity to phosphatidylserine, which is transferred from the internal to the external plasma membrane during the apoptosis process. In brief, L-929 cells were seeded on 6-well plates. After 24 h, cells were treated with IC50 concentration of insecticides for 48 h. Then the cells were trypsinized and washed twice with cold PBS. Then, 91 μl of binding buffer (1X), 5 μl of Annexin V-FITC, and 4 µl PI solution (50 μg/ml) were added to the obtained cell suspension and incubated for 15 min at room temperature in the dark. Before flow cytometry, the labeled cells were diluted with 400 µl binding buffer and examined by FACS Calibur flow cytometer[35].
Statistical analysis
The results were determined as mean ± standard deviation (SD) for the parameters. The statistical analyses were performed using one-way variance (ANOVA) analysis in GraphPad Prism software (Version 5.0) to evaluate the group differences. Differences with p < 0.05 were considered to be statistically significant. The statistical analyses involving multiple comparisons among the means are conducted using Tukey–Kramer test.
Conclusions
Using insecticides on agricultural fields and in the home should be applied with caution and be limited. In general, our findings showed cytotoxicity, genotoxicity, and apoptotic effects LCT (4.46 ± 0.37 µg/ml), CYP (7.48 ± 0.21 µg/ml), and EMB (53.41 ± 7.51 µg/ml), on the cell model. These results should be considered in the safety assessments to evaluate the appropriate guide to use them. More studies are needed to understand insecticide's mechanism of action.
References
AlKahtane AA et al (2018) Cytotoxicity and genotoxicity of cypermethrin in hepatocarcinoma cells: a dose- and time-dependent study. Dose-Response 16:1559325818760880
Deihimfard R et al (2014) Evaluating risk from insecticide use at the field and regional scales in Iran. Crop Prot 65:29–36
Hsu S-S, Jan C-R, Liang W-Z (2018) The investigation of the pyrethroid insecticide lambda-cyhalothrin (LCT)-affected Ca2+ homeostasis and -activated Ca2+-associated mitochondrial apoptotic pathway in normal human astrocytes: The evaluation of protective effects of BAPTA-AM (a selective Ca2+ chelator). Neurotoxicology 69:97–107
Yahia D, Ali MF (2018) Assessment of neurohepatic DNA damage in male Sprague-Dawley rats exposed to organophosphates and pyrethroid insecticides. Environ Sci Pollut Res 25:15616–15629
Taheri E, Yousefinejad S, Dehghani F, & Jafari S (2021) Inhalation health risk assessment of occupational exposure to cypermethrin in farmers. International Journal of Environmental Analytical Chemistry: 1–11.
Bao W, Liu B, Simonsen DW, Lehmler H-J (2020) Association between exposure to pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general US Adult population. JAMA Intern Med 180:367–374
Chrustek A et al (2018) Current research on the safety of pyrethroids used as insecticides. Medicina 54:61
Kaneko H (2011) Pyrethroids: mammalian metabolism and toxicity. J Agric Food Chem 59:2786–2791
Romero A et al (2017) Oxidative stress and gene expression profiling of cell death pathways in alpha-cypermethrin-treated SH-SY5Y cells. Arch Toxicol 91:2151–2164
Martínez MA et al (2020) Toxicologic evidence of developmental neurotoxicity of Type II pyrethroids cyfluthrin and alpha-cypermethrin in SH-SY5Y cells. Food Chem Toxicol 137:111173
Saleh M, Ezz-din D. & Al-Masri A (2021) In vitro genotoxicity study of the lambda-cyhalothrin insecticide on Sf9 insect cells line using Comet assay. Jordan Journal of Biological Sciences 14.
Organization, W. H. (2020) The WHO recommended classification of pesticides by hazard and guidelines to classification 2019.World Health Organization.<https://www.who.int/publications/i/item/9789240005662>.
Zhou L, Zhou M, Tan H, Xiao M (2020) Cypermethrin-induced cortical neurons apoptosis via the Nrf2/ARE signaling pathway. Pestic Biochem Physiol 165:104547
Li H et al (2018) Lambda-cyhalothrin delays pubertal Leydig cell development in rats. Environ Pollut 242:709–717
El-Demerdash FM (2012) Cytotoxic effect of fenitrothion and lambda-cyhalothrin mixture on lipid peroxidation and antioxidant defense system in rat kidney. J Environ Sci Health B 47:262–268
Luan S et al (2017) Emamectin benzoate induces ROS-mediated DNA damage and apoptosis in Trichoplusia Tn5B1-4 cells. Chem Biol Interact 273:90–98
Abou-Zeid SM, AbuBakr HO, Mohamed MA, El-Bahrawy A (2018) Ameliorative effect of pumpkin seed oil against emamectin induced toxicity in mice. Biomed Pharmacother 98:242–251
Wu X et al (2016) Detection on emamectin benzoate-induced apoptosis and DNA damage in Spodoptera frugiperda Sf-9 cell line. Pestic Biochem Physiol 126:6–12
Zhang Z, Zhao X, Qin X (2017) Potential genotoxic and cytotoxicity of emamectin benzoate in human normal liver cells. Oncotarget 8:82185–82195
Niu C et al (2020) Toxic effects of the Emamectin Benzoate exposure on cultured human bronchial epithelial (16HBE) cells. Environ Pollut 257:113618
Çelik A, Mazmanci B, Çamlica Y, Aşkin A, Çömelekoǧlu Ü (2003) Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat Res/Genet Toxicol Environ Mutagen 539:91–97
Muranli FDG (2013) Genotoxic and Cytotoxic Evaluation of Pyrethroid Insecticides λ-Cyhalothrin and α-Cypermethrin on Human Blood Lymphocyte Culture. Bull Environ Contam Toxicol 90:357–363
Naravaneni R, Jamil K (2005) Evaluation of cytogenetic effects of lambda-cyhalothrin on human lymphocytes. J Biochem Mol Toxicol 19:304–310
Zhang Q, Wang C, Sun L, Li L, Zhao M (2010) Cytotoxicity of lambda-cyhalothrin on the macrophage cell line RAW 264.7. J Environ Sci 22:428–432
Kaisarevic S et al (2019) Comparative analyses of cellular physiological responses of non-target species to cypermethrin and its formulated product: Contribution to mode of action research. Environ Toxicol Pharmacol 65:31–39
Kmetič I et al (2008) Atrazine exposure decreases cell proliferation in Chinese hamster ovary (CHO-K1) cell line. Bull Environ Contam Toxicol 81:205–209
Yun X et al (2017) A comparative assessment of cytotoxicity of commonly used agricultural insecticides to human and insect cells. Ecotoxicol Environ Saf 137:179–185
Raszewski G, Lemieszek MK, Łukawski K, Juszczak M, Rzeski W (2015) Chlorpyrifos and cypermethrin induce apoptosis in human neuroblastoma cell line SH-SY5Y. Basic Clin Pharmacol Toxicol 116:158–167
Yun X, Rao W, Xiao C, Huang Q (2017) Apoptosis of leukemia K562 and Molt-4 cells induced by emamectin benzoate involving mitochondrial membrane potential loss and intracellular Ca2+ modulation. Environ Toxicol Pharmacol 52:280–287
Hussien HM, Abdou HM, Yousef MI (2013) Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: the protective effect of sesame oil. Brain Res Bull 92:76–83
Kalita MK, Haloi K, Devi D (2017) Cypermethrin formulation (Ustad-10 EC) Induces genotoxicity via apoptosis, affects nutritional physiology, and modulates immune response in silkworm philosamia ricini (Lepidoptera: Saturniidae). J Econ Entomol 110:1010–1024
Mukhopadhyay I, Chowdhuri DK, Bajpayee M, Dhawan A (2004) Evaluation of in vivo genotoxicity of cypermethrin in Drosophila melanogaster using the alkaline Comet assay. Mutagenesis 19:85–90
Patel S, Pandey AK, Bajpayee M, Parmar D, Dhawan A (2006) Cypermethrin-induced DNA damage in organs and tissues of the mouse: Evidence from the comet assay. Mutat Res/Genet Toxicol Environ Mutagenesis 607:176–183
Gökalp Muranli FD, Güner U (2011) Induction of micronuclei and nuclear abnormalities in erythrocytes of mosquito fish (Gambusia affinis) following exposure to the pyrethroid insecticide lambda-cyhalothrin. Mutat Res/Genet Toxicol Environ Mutagenesis 726:104–108
Aghmasheh S, Ostad SN, Abedi A (2020) Pharmacological Properties of Pt(II) and Pt(IV) Complexes with 2,2′-Dipyridylamine; the Comparative In Vitro Thereof. Cell Biochem Biophys 78:521–529
Acknowledgements
This research was supported by Tehran University of Medical Sciences (TUMS) under Grant Number 50894.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Habibeh sadat Mohseni, Roxana Sahebnasagh, Shohreh Tavajohi, Mohammad Hossein Ghahremani, Abbas Kebriaeezadeh, Shima Aliebrahimi, and Seyed Nasser Ostad declare that they have no conflict of interest.
Ethical approval
This study was performed as standard procedure based on ethical policy of Tehran University of Medical Sciences in the cell culture laboratory of Faculty of Pharmacy under supervision me (S N Ostad) Professor of Toxicology and Pharmacology.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohseni, H.s., Sahebnasagh, R., Tavajohi, S. et al. Evaluation of genotoxic and cytotoxic effects of some insecticides used in Iran on murine fibroblast cells (L-929). Toxicol. Environ. Health Sci. 14, 301–306 (2022). https://doi.org/10.1007/s13530-022-00143-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-022-00143-8