Abstract
The present study aimed to investigate the genotoxic, cytotoxic and aneugenic effects of 1, 2, 3.75, 7.5, 15, 30 μM concentrations of the insecticides λ-cyhalothrin (LCT) and α-cypermethrin (CYP) on human peripheral blood lymphocyte culture using micronucleus (MN) and fluorescence in situ hybridisation (FISH) methods. All the concentrations were tested to assess the MN and apoptosis effects, and 1 and 2 μM LCT and 7.5 and 15 μM CYP concentrations were tested for FISH analysis. The cytotoxic effect was also observed using trypan blue and the acridine orange/ethidium bromide fluorescence staining method to measure the apoptotic effect. It was observed that both of the insecticides had a cytotoxic effect at all the concentrations (p ≤ 0.001) and apoptotic effect for LCT at 15–30 μM (p ≤ 0.05; p ≤ 0.01) for CYP between 2 and 30 μM concentrations (p ≤ 0.05; p ≤ 0.01). The micronuclei that developed after exposure were induced because of an aneugenic effect (p ≤ 0.001). LCT and CYP might be spindle poisons or caused damaged to centromere/kinetochore function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pyrethroids represent a class of chemical pesticides widely used in recent years. They are a synthetic form of pyrethrins, which are widely used for the control of various insect pests. α-cypermethrin (CYP) and λ-cyhalothrin (LCT) are synthetic type II pyrethroid insecticides that have been studied in cytogenetic investigations. However, the results of the studies have varied according to the different assays and materials used in the test systems, and thus, contradictory results have been observed. LCT is highly toxic to fish, aquatic arthropods and honeybees (WHO 1990). The World Health Organization (WHO 2004) declared that most genotoxicity tests (e.g. reverse mutations in bacteria, in vitro gene mutations, unscheduled DNA syntheses, cytogenetic effect tests in mammalian cells and in vivo micronucleus (MN) tests in mice exposed to LCT have yielded negative results. However, in recent studies, LCT has shown positive results in mammalian test systems (Celik et al. 2003, 2005). Moreover, it has been stated that although technical forms of λ-cyhalothrin show a weak toxic effect, formulations show high toxic activity (James 1991). Although the toxicity of LCT has been extensively investigated in insect and animal models, there are few reports of cytotoxicity and genotoxicity in humans in the in vitro models (Rupa et al. 1989; Amer and Aly 1992; Muranli 2009). Due to the lack of in vitro data on the effect of LCT on human peripheral blood lymphocytes and the conflicting results obtained in mammalian test systems, it is necessary to evaluate the effects of a commercial formula of LCT at the cytotoxic and genotoxic levels in vitro on peripheral blood samples of healthy human volunteers.
α-Cypermethrin is another highly active pyrethroid insecticide that is effective in public health, animal husbandry, and agriculture. It has been indicated that CYP does not induce genotoxic damage in human lymphocyte culture (Puig et al. 1989) in mouse bone marrow (Chauhan et al. 2005). It was also stated that CYP led to an increase in the frequency of CA and single strand-breaks in human lymphocytes (Suman and Jamil 2006), DNA damage and MN in vitro in human lymphocytes (Undeger and Basaran 2005; Kocaman and Topaktas 2009). These contradictory results necessitate genotoxicity studies of pesticides using different assays with different test materials.
The MN assay is a useful method for evaluating the genotoxic effect of chemicals and assessing DNA damage at the chromosome level. The cytokinesis-block MN (CBMN) assay has been widely used in different cell types including human lymphocytes for the evaluation of the clastogenic and aneugenic potential of various agents; that is, their potential to induce chromosome breakage and maldistribution. The combination of the cytokinesis-block method with hybridisation with general or chromosome-specific centromeric/telomeric probes allowed the identification of the major mechanisms responsible for MN induction: double DNA strand breaks leading to micronuclei with acentric fragments and failure of the mitotic apparatus resulting in micronuclei with entire chromosomes (Kirsch-Volders et al. 2003). In order to discriminate MN produced by agents causing chromosome breakage (clastogens) from those arising following treatment with agents causing spindle malfunctioning (aneugens), the fluorescent in situ hybridisation (FISH) technique with a centromere-specific alpha-satellite DNA probe was used (Farooqi et al. 1993).
Apoptosis, or programmed cell death, is a process in which cells play an active role in their own death. Apoptosis can be induced by a wide range of biological stimuli as well as by chemical, physical and genetic factors. It has been demonstrated that pyrethroid insecticides have an apoptotic effect on different test organisms (Casco et al. 2006; Fu et al. 2011b).
The present study aims to evaluate the genotoxic, cytotoxic, clastogenic/aneugenic and apoptotic effects of λ-cyhalothrin and α-cypermethrin on human peripheral blood lymphocyte culture using CBMN, FISH and Ethidium Bromide/Acridine Orange (EB/AO) fluorescence staining methods.
Materials and Methods
The following commercial formulations of synthetic pyrethroid insecticides were used as test substances: λ-cyhalothrin (commercial name: Tekvando 5 EC with 5 % active substance; CAS No: 91465-08-6; chemical name: (RS)-α-cyano-3-phenoxybenzyl 3-(2-chloro-3,3,3-trifluoropropenyl)-2,2,-dimethylcyclopropanecarboxylate) and α-cypermethrin (commercial name: Super Takimethrin 100 EC with 10 % active substance; CAS No: 67375-30-8; chemical name: (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2, 2-dimethylcyclopopanecarboxylate and (R)-a-cyano-3-phenoxybenzyl(1S)-cis-3-(2,2-ichlorovinyl)-2,2-dimethylcyclopopanecarboxylate). The test substances were prepared in sterile bidistilled water.
The concentrations (1, 2, 3.75, 7.5, 15, 30 μM) were chosen based on a previous study (Muranli 2009). Mitomycin C (MMC; CAS No: 50-07-7; SIGMA; 0.1 μg/mL) was used as a positive control for the MN assay, and 0.1 μM vinblastine sulphate (VBL; CAS No: 143-67-9; MP Biomedicals) was used as an aneugenic agent for the FISH assay. All the concentrations were tested to assess the MN and apoptosis effects, and 1 and 2 μM LCT and 7.5 and 15 μM CYP concentrations were tested for the FISH analysis.
The culture medium included 10 % foetal calf serum (Sigma), 1 % phytohaemagglutinin (GIBCO), 89 % Ham’s F-10 (PAN Biotech) and antibiotics (100 IU penicillin/mL and 100 μg streptomycin/mL) for the MN assay. For the MN test, whole-blood cultures were prepared according to Fenech and Morley (Fenech and Morley 1985). The whole-blood samples used in the cultures came from healthy, 30-year-old, non-smoking volunteers (two females and two males) with no history of pesticide exposure. Cytochalasin-B (final concentration 6 μg/mL) was added after 44 h of incubation. The cells were exposed to LCT and CYP concentrations for the last 24 and 48 h of the 68 h culture period. Whole-blood cultures were used for the MN and FISH analyses. The number of MN in 2,000 binucleated (BN) cells per donor (a total of 8,000 binucleated cells per concentration and treatment period) was scored. Slides were stored at −20°C for the FISH analysis. A total of 2,000 cells (500 cells per donor for each concentration and treatment period) were scored for the determination of the proliferation index. Nuclear Division Index (NDI) was calculated according to the following formula: NDI = (M1 + 2(M2) + 3(M3) + 4(M4)) / N, where M1–M4 represent the number of cells with one to four nuclei, respectively, and N is the total number of cells scored (Eastmond and Tucker 1989).
FISH was performed using an alpha-satellite probe for all human centromeres (prime FISH pancentromere; DIAGEN 1002-CR) according to the manufacturers instructions. The bandpass filters used were 510, 490 and 360 nm for green, blue and ultraviolet light. At least 50 MN were analysed for the presence of a centromere-positive signal for each experimental point.
For the EB/AO assay, heparinised blood (20 mL) was obtained from four healthy donors (two males and two females). Lymphocytes were separated using Ficoll’s single-step continuous density-gradient centrifugation technique. The separated lymphocytes were washed three times in Ham’s F-10 culture medium and counted under a microscope. The lymphocytes were suspended in the complete culture medium (Ham’s F-10, 10 % foetal calf serum and antibiotics) to a density of 3 × 106 cells/mL and incubated with LCT and CYP (1, 2, 3.75, 7.5, 15 and 30 μM concentrations) for 48 h at 37°C in an incubator. The control cultures were incubated without genotoxic agents. At the end of the incubation period, for the estimation of the dead cell fraction, cultures were stained with a 0.4 % solution of trypan blue in PBS. The number of blue-stained (dead) cells within 2,000 cells was counted. The staining of the cells with a mixture of fluorescent dyes (EB/AO) followed the procedure described in Current Protocols in Immunology (Martin 1998). The cell suspension (25 μL) was mixed with 1 μL of dye mix (100 μg/mL AO + 100 μg/mL EB). To determine the percentage of viable versus dead cells that had undergone apoptosis or necrosis, 300 cells for each concentration per donor were examined.
Significances of frequencies of binucleated micronucleus (MNBN) cells in the MN assay and centromere-positive MN (C+MN) cells in the FISH assay were evaluated using Fisher’s exact test compared to the control group. The student t test was used to determine apoptotic effect results. NDI was analysed using the χ2 test (p ≤ 0.05).
Results and Discussion
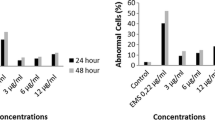
Total MN frequencies and proliferation index values obtained from four donors are shown in Table 1. Both of the pesticides decreased NDI significantly (p ≤ 0.001), indicating their cytotoxic effect. Concentrations of LCT of 3.75 μM and higher completely inhibited cell division and showed a cytotoxic effect. For CYP, a concentration of 30 μM inhibited cell division completely. NDI results showed that the cytotoxic effect of LCT was greater than that of CYP. The 48-h exposure period caused a greater genotoxic effect than the 24-h exposure period. Thus, the results of the MN assay reveal that the two insecticides have a high cytotoxic and a probable genotoxic effect on human peripheral blood lymphocyte culture.
The clastogenic or aneugenic reason for the probable genotoxic effect was investigated on the slides using FISH assays that were prepared after 1 and 2 μM LCT and 7.5 and 15 μM CYP for 48-h exposure on human lymphocyte culture with the MN assays of two donors. Table 2 shows the frequencies of centromere-positive (C+) and centromere-negative (C−) micronucleated cells induced by LCT, CYP and positive control vinblastine sulphate. The percentage of positive signals was statistically significant in treated cultures when compared with that of the control (p ≤ 0.001). Results obtained with the FISH assays demonstrated the aneugenic effect of LCT and CYP.
Lymphocyte viability and mode of cell death was studied in LCT- and CYP-exposed lymphocyte cells using a membrane-excluded dye (trypan blue) visible by light microscopy and a combination of membrane-excluded and non-excluded DNA-binding fluorescent dyes (AO/EB) visible by fluorescence microscopy. Apoptotic lymphocytes were observed depending on the deformation occurring in the cell membrane. During the trypan blue staining, healthy and living cells appeared white, while apoptotic cells appeared blue. As a result of AO/EB staining, four types of cells were observed: (1) viable cells with normal nuclei (bright green chromatin with organised structure), (2) viable cells with apoptotic nuclei (condensed or fragmented bright green chromatin), (3) nonviable cells with normal nuclei (bright orange chromatin with organised structure) and (4) nonviable cells with apoptotic nuclei (condensed or fragmented bright red chromatin (Fig. 1). Normal and apoptotic nuclei in live cells produce a bright fluorescent green, while normal and apoptotic nuclei in dead cells produce a bright orange or red (Fig. 1).
Apoptosis stages of lymphocytes. 1a Viable cell with normal nucleus- Bright green chromatin with organized structure. 1b Viable cell with apoptotic nucleus- Condensed bright green chromatin 1c Viable cell with apoptotic nucleus-Fragmented bright green chromatin. 2a Nonviable cell with normal nucleus-Bright orange chromatin with organized structure. 2b, 2c Nonviable cell with apoptotic nucleus- Condensed yellow-orange chromatin. 3a Nonviable cell with normal nucleus – Bright red chromatin with organized structure. 3b Nonviable cell with apoptotic nucleus – Condensed red chromatin. 3c Nonviable cell with apoptotic nucleus- Fragmented red chromatin (Color figure online)
Cypermethrin, one of the two insecticides tested in the same concentrations, has a greater apoptotic effect than LCT. Although LCT induced apoptosis at significantly higher concentrations (15 and 30 μM), CYP induced apoptosis even at significantly lower concentrations (2, 3.75, 7.5, 15 and 30 μM) (Table 3).
In the present study, the genotoxic, cytotoxic, clastogenic/aneugenic and apoptotic effects of λ-cyhalothrin and α-cypermethrin on human peripheral blood lymphocyte culture were investigated using MN and FISH assays and AO/EB staining methods. The MN assay results showed that two of the pesticides induced MN induction in some donors. The frequency of MN cells was not seen due to the cytotoxic effect that decreased the number of binucleated cells. NDI frequencies were significantly decreased, indicating the cytotoxic activity of insecticides.The results of the MN assay of the present study showed that the cytotoxic effect of LCT and CYP is much greater than the genotoxic effect. Similar results have been obtained in other investigations. For example, the pyrethroid insecticide supermethrin has been characterised as more toxic than clastogenic or genotoxic (Dianovský 1995). Supermethrin has not been shown to induce a significant increase in chromosome damage after in vivo and in vitro studies in sheep peripheral tissues (Dianovský 1992).
In the present study, although a significant cytotoxic effect was observed after LCT and CYP exposure, the insecticides showed a weak genotoxic effect on human peripheral blood lymphocytes. Similarly, Surralles et al. (1995) indicated that pyrethroid insecticides (including cypermethrin, deltamethrin and fenpropathrin) exhibit weak genotoxic activity in vitro in human lymphocyte culture (Surralles et al. 1995). In another study, independent exposure of a commercial formulation of cypermethrin (Cyperkill 25 % EC) showed significant inhibition of MI but did not show CA or micronucleated polychromatic erythrocytes (PCE) in mouse bone marrow (Chauhan et al. 2005). Permethrin (pyrethroid insecticide) showed a cytotoxic but not genotoxic effect in cultured human lymphocytes in an in vitro MN test (Djelic and Djelic 2000).
FISH experiments were carried out using an alpha-satellite probe, complementary to the centromeres of all human chromosomes. Two concentrations of both pesticides (1 and 2 μM LCT and 7.5 and 15 μM CYP) were studied in lymphocyte cultures. Frequencies of micronuclei exhibiting (C+MN) or not exhibiting (C−MN) centromeric signals, thus containing whole chromosomes or acentric chromosome fragments, were determined. C−MN is the result of breakage events, while C+MN is the result of chromosome loss. The frequency of C+MN, which contains whole chromosomes, was significantly different from the control, indicating an aneugenic effect. The result of the FISH assay demonstrated that MNi induction is due to the aneugenic effect. The aneugenic effect causes spindle disturbance, which leads to aneuploidy in dividing cells. These findings suggested that CYP and LCT might be spindle poisons or caused damaged to centromere/kinetochore function (Fenech and Morley 1989). Similarly, the same results were obtained in other investigations. Institoris et al. (1999) indicated that CYP and permethrin (pyrethroid insecticide) increased the number of numerical chromosome aberrations in bone marrow cells but did not change the number of structural aberrations in Wistar rats (Institoris et al. 1999). It has also been indicated that some commonly used pesticides are capable of inducing aneuploidy in human sperm (Harkonen 2005).
In our previous study (Muranli 2009), although LCT was not a clastogenic agent on human peripheral blood culture by CA assay, the insecticide induced aneuploidy in the early S phase of the cell cycle. This previous result also supports the present study’s finding that LCT and CYP has an aneugenic effect. Similarly, Hadnagy et al. (1999) indicated that pyrethroids inhibit cell cycle progression during mitosis by interfering with the mitotic spindle apparatus and reported that, depending on partial spindle disturbance, aneuploidy may occur in the subsequent cell division.
The results of the present study demonstrated that the apoptotic effect of CYP was greater than that of LCT. Even at lower concentrations, CYP has an apoptotic effect on isolated human peripheral lymphocytes. The results showed that LCT has a greater cytostatic effect than CYP and CYP has a greater apoptotic effect than LCT. Similarly, in previous studies, it has been demonstrated that pyrethroids induce apoptosis in the testicular tissues of rats (El-Gohary et al. 1999) and have an apoptotic effect on fish (Fu et al. 2011a). In the present study, both insecticide-inhibited cell division (in the MN assay) and induced cell death (in the AO/EB cell viability assay) were demonstrated. Aberrations might not be detected with cytogenetic methods due to the inhibition of cell division or cell death. A comparison of the two insecticides reveals that the apoptotic effect of CYP was greater than that of LCT.
The results of the MN assay revealed that LCT and CYP showed a weak genotoxic effect on human peripheral blood lymphocyte culture. LCT and CYP have aneugenic effect shown by the FISH assay. CYP and LCT might be spindle poisons or caused damaged to centromere/kinetochore function. The results of the cell viability method indicated that both of the insecticides induced cell death. CYP induced an apoptotic effect even at lower concentrations, and LCT affected the cell cycle even at lower concentrations. Different results may be obtained using pure or commercial formulations of pesticides on different test systems. Commercial formulations are commonly used forms in agriculture, and the genotoxic activity of these pesticides needs to be investigated using different test systems.
References
Amer SM, Aly FAE (1992) Cytogenetic effects of pesticides. IV. Cytogenetic effects of the insecticides Gardona and Dursban. Mutat Res Genet Toxicol 279:165–170
Casco VH, Izaguirre MF, Marin L, Vergara MN, Lajmanovich RC, Peltzer P, Soler AP (2006) Apoptotic cell death in the central nervous system of Bufo arenarum tadpoles induced by cypermethrin. Cell Biol Toxicol 22:199–211
Celik A, Mazmanci B, Camlica Y, Askin A, Comelekoglu U (2003) Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat Res Genet Toxicol Environ Mutagen 539:91–97
Celik A, Mazmanci B, Camlica Y, Askin A, Comelekoglu U (2005) Induction of micronuclei by lambda-cyhalothrin in Wistar rat bone marrow and gut epithelial cells. Mutagenesis 20:125–129
Chauhan LKS, Chandra S, Saxena PN, Gupta SK (2005) In vivo cytogenetic effects of a commercially formulated mixture of cypermethrin and quinalphos in mice. Mutat Res Genet Toxicol Environ Mutagen 587:120–125
Dianovský JSK (1992) Chromosome-aberrations induced after supermethrin in vivo and in vitro administration. Zivocisna Vyroba 37:1017–1022
Dianovský JSK (1995) In vivo and in vitro cytogenetic effect of supermethrin. Biomed Environ Sci 8:359–366
Djelic N, Djelic D (2000) Evaluation of cytotoxic and genotoxic effects of permethrin using in vitro micronucleus test. Acta Vet-Beograd 50:263–269
Eastmond DA, Tucker TJ (1989) Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environ Mol Mutagen 13:34–43
El-Gohary M, Awara WM, Nassar S, Hawas S (1999) Deltamethrin-induced testicular apoptosis in rats: the protective effect of nitric oxide synthase inhibitor. Toxicology 132:1–8
Farooqi Z, Darroudi F, Natarajan AT (1993) The Use of Fluorescence in-Situ Hybridization for the Detection of Aneugens in Cytokinesis-Blocked Mouse Splenocytes. Mutagenesis 8:329–334
Fenech M, Morley AA (1985) Measurement of micronuclei in lymphocytes. Mutat Res 147:29–36
Fenech M, Morley AA (1989) Kinetochore detection in micronuclei—an alternative method for measuring chromosome loss. Mutagenesis 4:98–104
Fu ZW, Jin YX, Zheng SS (2011a) Embryonic exposure to cypermethrin induces apoptosis and immunotoxicity in zebrafish (Danio rerio). Fish Shellfish Immunol 30:1049–1054
Fu ZW, Jin YX, Zheng SS, Pu Y, Shu LJ, Sun LW, Liu WP (2011b) Cypermethrin has the potential to induce hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish (Danio rerio). Chemosphere 82:398–404
Hadnagy W, Seemayer NH, Kuhn KH, Leng G, Idel H (1999) Induction of mitotic cell division disturbances and mitotic arrest by pyrethroids in V79 cell cultures. Toxicol Lett 107:81–87
Harkonen K (2005) Pesticides and the induction of aneuploidy in human sperm. Cytogenet Genome Res 111:378–383
Institoris L, Undeger U, Siroki O, Nehez M, Desi I (1999) Comparison of detection sensitivity of immuno- and genotoxicological effects of subacute cypermethrin and permethrin exposure in rats. Toxicology 137:47–55
James HKDR (1991) Agrochemicals handbook. Royal Society of Chemistry, Cambridge
Kirsch-Volders M, Sofuni T, Aardema C, Albertini S, Eastmond D, Fenech M, Ishidate M, Kirchner S, Lorge E, Morita T, Norppa H, Surralles J, Vanhauwaert A, Wakata A (2003) Report from the in vitro micronucleus assay working group. Mutat Res Genet Toxicol Environ Mutagen 540:153–163
Kocaman AY, Topaktas M (2009) The in vitro genotoxic effects of a commercial formulation of alpha-cypermethrin in human peripheral blood lymphocytes. Environ Mol Mutagen 50:27–36
Martin D, Lenardo M (1998) Morphological, biochemical, and flow cytometric assays of apoptosis. Curr Protoc Immunol 3(17):1–39
Muranli FDG (2009) Genotoxic and cytotoxic effects of a pyrethroid insecticide lambda-cyhalothrin on human peripheral blood lymphocytes investigated by chromosome aberration and flow cytometry assays. Fresen Environ Bull 18:1758–1763
Puig M, Carbonell E, Xamena N, Creus A, Marcos R (1989) Analysis of cytogenetic damage induced in cultured human-lymphocytes by the pyrethroid insecticides cypermethrin and fenvalerate. Mutagenesis 4:72–74
Rupa DS, Reddy PP, Reddi OS (1989) Analysis of sister-chromatid exchanges, cell-kinetics and mitotic index in lymphocytes of smoking pesticide sprayers. Mutat Res 223:253–258
Suman GNR, Jamil K (2006) In vitro cytogenetic studies of cypermethrin on human lymphocytes. Indian J Exp Biol 44:233–239
Surralles J, Xamena N, Creus A, Catalan J, Norppa H, Marcos R (1995) Induction of Micronuclei by 5 Pyrethroid Insecticides in whole-blood and isolated human lymphocyte-cultures. Mutat Res Genet Toxicol 341:169–184
Undeger U, Basaran N (2005) Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch Toxicol 79:169–176
WHO (1990) Environmental health criteria 99: cyhalothrin. WHO, p 13–21
WHO (2004) Evaluation of certain veterinary drug residues in food. Sizxty-second report of the Joint FAO/WHO Expert Committee on food additives. Technical Report Series Geneva:11
Acknowledgments
The work was supported by Trakya University [TUBAP 2008/35 Project].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muranli, F.D.G. Genotoxic and Cytotoxic Evaluation of Pyrethroid Insecticides λ-Cyhalothrin and α-Cypermethrin on Human Blood Lymphocyte Culture. Bull Environ Contam Toxicol 90, 357–363 (2013). https://doi.org/10.1007/s00128-012-0909-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0909-z