Abstract
Objective
To determine the impacts of 12 weeks of aerobic, resistance, or combined training on FAM19A5, glucose homeostasis, body composition, and physical performance in obese men.
Methods
Fifty-eight obese men [age = 49 ± 6 years; body mass index (BMI) = 29 ± 2 kg m−2] were randomly assigned to aerobic training (n = 14), resistance training (n = 15), combined training (n = 15), and control (n = 14) groups. Interventions were three sessions per week for 12 weeks.
Results
FAM19A5 levels remained unaltered in all three intervention groups. However, insulin concentrations, triglyceride, HOMA-IR, VAI, TyG, and fat% declined in all three interventions, whereas VO2max increased. In addition, glucose levels, LAP, body weight, and BMI were reduced in aerobic and combined groups, while systolic blood pressure was reduced in resistance and combined programs. We also observed a significant reduction in low-density lipoprotein following only combined exercises and a significant increase in high-density lipoprotein after only aerobic exercises. There was a significant negative relationship between serum levels of FAM19A5 and HOMA-IR.
Conclusion
This is the first report to assess the influence of exercise interventions on circulating FAM19A5 levels in obese adults. Although FAM19A5 levels remained unchanged in all three interventions, our work provides information to support that aerobic, resistance, and combined regimens can be effective in improving HOMA-IR, triglyceride, systolic blood pressure, BMI, and aerobic performance in obese men. Additional studies with large sample size should be conducted to further clarify the underlying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity globally has been acknowledged as a significant health concern, growing in prevalence worldwide [1] and strongly influencing mortality and morbidity [2]. The prevalence of obesity has doubled in the last 30 years; presently, approximately 15% of the population is obese [1]. There is growing evidence that adipose tissue acts as an endocrine organ by secreting numerous bioactive substances called adipokines, and these adipokines have a critical role in regulating cardiovascular and metabolic homeostasis [3,4,5].
The family with sequence similarity 19 [chemokine (C–C motif)-like] member A5 (FAM19A5), as well known as TAFA5, is a recently discovered adipokine that protects against CMD [5]. FAM19A5 has been reported to impede vascular smooth muscle cell proliferation, migration, and post-injury neointima formation in an animal model [5]. Little and inconsistent evidence is available regarding measuring serum FAM19A5 levels in human subjects. For example, Lee et al. reported that the serum FAM19A5 concentration was higher in people with type 2 diabetes than in individuals with standard glucose tolerance. Additionally, FAM19A5 levels were positively associated with abdominal obesity, fasting glucose, and hemoglobin A1C [6]. In contrast, Ali Yari et al. discovered that the level of FAM19A5 in subjects with non-alcoholic fatty liver disease was considerably lower than in controls [7]. Likewise, the authors reported significant inverse relationships between plasma FAM19A5 concentration and body mass index (BMI), visceral fat, alanine aminotransferase, aspartate aminotransferase, and liver stiffness. In light of conflicting research findings, there is evidence suggesting that FAM19A5 may play a crucial role in obesity-associated cardiometabolic disorders such as type 2 diabetes, atherosclerosis, and non-alcoholic fatty liver disease [7]. It has been reported that FAM19 members, including FAM19A5, may exert control over the activity of peripheral immune cells, particularly macrophages [6, 8]. Moreover, there have been observations indicating a positive correlation between circulating FAM19A5 concentration and hsCRP levels. Considering that chronic inflammation caused by obesity is responsible for insulin resistance [9, 10], it is plausible that FAM19A5 could impact this process [9, 10]. However, it is important to acknowledge the potential influence of race-ethnic differences between study populations. Previous research has highlighted significant race-ethnic disparities in circulating levels of various adipokines [11].
In recent years, numerous applicable risk prediction equations have been developed that are remarkable in the initial prevention of cardiovascular disease at both clinical and individual levels. In this context, lipid accumulation product (LAP), atherogenic index of plasma (AIP) [12], visceral adipose index (VAI) [13], and triglyceride-glucose index (TyG index) [14] have been evidenced as robust markers indicating coronary heart diseases’ risk. VAI is a marker of visceral adipose function [15], and LAP indicates the anthropometric and physiological alterations linked to excess lipid accumulation [16]. Several studies have revealed that these indices, additionally homeostasis model assessment-insulin resistance (HOMA-IR) and McAuley’s index, have high validity and reproducibility for evaluating insulin resistance in most conditions and diseases [17,18,19].
Exercise, dietary, and behavioral interventions are recommended by numerous guidelines to assist obese people [20,21,22]. Concerning exercise programs, aerobic exercise is advised as the major exercise component for additional weight loss, while resistance training, because of inadequate evidence on the impacts on decreasing body weight or BMI, has been deemed less crucial [20,21,22,23]. Nevertheless, it has been reported that resistance training can afford valuable benefits, including ameliorating glucose tolerance and insulin sensitivity in type 2 diabetes patients [24] and enhancing fitness in sedentary individuals [25] when correctly performed. Interestingly, other researchers discovered that combined (aerobic + resistance) exercise induces larger improvements in circulating glucose and HOMA-IR in obese adolescents than isolated aerobic exercise [26, 27].
Taking into account that not only the expression and role of FAM19A5 are still not understood, but there is also a lack of evidence about the efficacy of exercise interventions on this adipokine, we investigated the effect of various modes of exercise training on FAM19A5 serum concentrations in obese volunteers. Moreover, as the LAP and VAI as novel insulin resistance indexes, identifying exercise advantages in obese people may be more necessary than common ones; thus, we analyzed the effects of these interventions on LAP, VAI, McAuley, TyG index, and physical fitness in obese people.
Methods and Materials
Study design
The study was designed as a prospective randomized controlled trial and adhered to the standards laid down in the Declaration of Helsinki. Moreover, the present study was registered at the Iranian Registry of Clinical Trials (IRCT20151026024717N4).
Participants
Fifty-eight obese men participated in the current investigation. They were aged 49 ± 6 years and were 90 ± 8 kg in weight (BMI = 29 ± 2 kg m−2). We recruited volunteers from the community through advertisements. Enrolment and interventions were conducted from July 5, 2021, to March 10, 2022, in Tehran, Iran. The inclusion criteria were as follows: overweight and/or obese men (defined as a BMI ≥ 25 kg m−2) aged 18–60 years, inactive lifestyle, and stable weight in the 6 months prior to starting the study. The exclusion criteria included cardiopulmonary disease, infection, physical restriction or neuromuscular disability, consumption of weight loss medications, and excessively obese participants (BMI > 40 kg m−2).
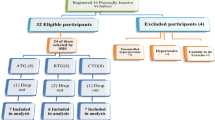
We randomized eligible participants, with an allocation ratio of 1:1:1:1, for a 12-week period to one of the following four groups: (1) control group, (2) aerobic exercise training, (3) resistance exercise training, and (4) combined (aerobic + resistance) exercise training. Randomization was conducted via random number generation after ending all initial assessments. Investigators and outcome assessors were blinded to allocations until all assessments were completed.
Participants allocated to the control group were instructed to maintain their usual lifestyle during the study period. Participants allocated to the aerobic training group participated in an aerobic training program supervised by an exercise physiologist. The aerobic training program was carried out on Monday, Wednesday, and Saturday, and consisted of a 10-min warm-up at 10% of maximum heart rate followed by 8 × 3-min intervals at 80% of maximum heart rate cycling, with 2-min exercise at 50% maximum heart rate between each interval. Subjects ended the session with a 10-min cooldown at the same intensity as the warm-up. Subjects allocated to the resistance training group participated in a supervised resistance training program, performing on Tuesday, Thursday, and Sunday. It included seven exercise weight machines, including lateral pull-down, chest press, seated row, triceps push-down, knee flexion, knee extension, and leg press. In the first week, subjects did two sets of 12–15 repetitions at an intensity of 40% one-repetition maximum (1RM). During the second week, the sets and intensity were increased to three and 50% 1RM, respectively while the repetitions were constant. During the third to fifth week, the number of repetitions decreased to 8–12 while the intensity progressed to 55–60% 1RM. Between weeks 6 to 12, the intensity increased to 65–80% 1RM. Participants allocated to the combined training group in the weeks of 1, 3, 5, 7, 9, and 11 conducted aerobic training twice a week and resistance training once a week, while in the weeks of 2, 4, 6, 8, 10, and 12, they conducted resistance training twice a week and aerobic training once a week. In each session of training, their workouts were similar to the workouts of the other two groups. The exercise training protocols were modified from those used by Mohammad Rahimi et al. in 2020 [27].
All training sessions were arranged by appointment. Subjects were excluded from the study if they missed more than 20% of the exercise sessions. Subjects in the aerobic training and combined training groups were required to achieve target heart rates throughout aerobic interval sessions; heart rate was verified by a heart rate monitor (Polar Electro, Finland).
All participants were instructed not to alter their dietary patterns. The training sessions in all three intervention groups were held in the afternoon. Moreover, the sessions were performed after lunch (approximately 3 h later; at 4 pm), and 1 h thereafter, participants had dinner.
Measurements
Body composition
BMI and waist circumference were calculated by the study administrator, blinded to the group allocation, at baseline and 12 weeks. Height (cm) and weight (kg) of all subjects were assessed applying a digital stadiometer (Seca, Germany) and a digital scale (Beurer PS07, Germany) to the nearest 0.1 cm and 0.1 kg, respectively. BMI was measured by [body mass (kilogram)]/[height (meter) × height (meter)]. Waist circumference (cm) was estimated by a calculating tape at the middle distance between the last free rib and the iliac crest (Seca 201, USA). An Inbody 720 Body Composition Analyzer (Biospace, Dogok-dong, South Korea) was utilized to calculate bioimpedance parameters such as fat mass, %fat, muscle mass, and body water.
Resting blood pressure
Resting blood pressure was evaluated via a digital blood pressure monitor (Model 705IT, Omron Corporation, Kyoto, Japan) following a 5-min rest period in the supine position. This measurement was completed in accordance with the European Society of Hypertension recommendation [28].
Energy intake
Energy intake was assessed using a 3-day food diary before and after the intervention. A dietician described diet adequacy and explained energy-dense foods, as well as those of lower nutritional value. Participants were instructed to record and analyze food diaries 3 days prior to initial testing by applying a food composition analysis software (Food 324 Processor SQL 2006; ESHA Research) and to repeat this dietary program prior to the follow-up test session (at 12 weeks).
Maximum oxygen uptake
VO2max (ml/kg/min) was measured before and after 12 weeks on a calibrated treadmill (Cosmos, Hp, Germany) using a Bruce walking test protocol [29]. It was measured by breath-by-breath technology (Cortex, MetaLyzer 3B-R2) continuously during exercise testing.
One repetition maximum test
In the first week prior to the start of the resistance training protocol, all subjects were familiarized with the program. Each subject was educated on the appropriate technique of the workouts prior to participating in the resistance training regimen. According to the instructions by Balady (2000), 2–3 days before 1RM calculation testing, each subject was presented to the exercise equipment [30]. We chose a weight that the subject considered as very low intensity, which was instructed and practiced with appropriate lifting and breathing techniques. In the next session, the 1RM of each workout was determined for each person. Each person was taught to complete a 3-min warm-up by walking on a treadmill and the whole-body stretching workouts during this session. Next, participants were requested to perform 10-reps of each exercise utilizing the amount of resistance applied for familiarization sessions. The resistance was then gradually increased until the individuals could only complete 9-reps of each workout. The aim of the resistance increase was to achieve the desired repetitions in 3–6 trials. Throughout each trial and between the two specific workouts, a 2- and 3-min rest period was allowed, respectively. The resistance and repetitions recorded were then measured as the 1RM by the Brzycki equation [31]. The 1RM of the bench press and leg press was considered as strength indices of the upper and lower extremities, respectively. The same instructors managed all tests according to individual adjustments.
Biochemical markers
At baseline and 12 weeks, we collected venous blood samples in the morning after participants had fasted for at least 12 h. Participants were taught to avoid physical activity or eating for 12 h before blood sampling. Blood sample for FAM19A5, glucose, insulin, triglyceride, total cholesterol, and high-density lipoprotein was sampled between 8:00 a.m. and 10:00 a.m. following a 5-min rest in the supine position. We separated the samples from blood cells by centrifugation at 3000 × g for 10 min. Serum was transferred with sterile pipettes and aliquotted into 0.5-ml serum tubes and immediately stored at − 80 °C refrigerator until assay. We used an ELISA Kit (ZellBio GmbH, Germany) to measure serum FAM19A5 in accordance with the manufacturer guidelines. We also used Pars-Azmun kits with an enzymatic method to measure serum glucose, insulin, triglyceride, total cholesterol, and high-density lipoprotein. However, low-density lipoprotein was calculated using the Friedewald equation [32].
Measurement of VAI and LAP
We used anthropometric variables and laboratory parameters to measure VAI and LAP according to the following formula in men [33,34,35]. The value of waist circumference (cm) and the values of triglyceride and high-density lipoprotein in mmol/l were used in this formula.
Measurement of homeostasis model assessment and McAuley and triglyceride-glucose (TyG) indexes
We utilized the following formula [18, 36,37,38] to calculate homeostasis model assessment (HOMA-IR), McAuley, and TyG indexes:
Statistical analysis
Data were tested using SPSS software (version 26, IBM, Armonk, NY), and data are illustrated as mean ± standard deviation for normally distributed data and median (interquartile range) for non-normally distributed data, yet in the figures, data are presented as mean ± standard error. We used the Shapiro–Wilk test to assess the data’s normal distribution. We compared baseline characteristics between groups using one-way analysis of variance (ANOVA). The effects of the interventions on outcomes were estimated by a 2 (time: baseline vs. 12 weeks) × 4 (group: control, aerobic training, resistance training, mixed training) repeated-measures ANOVA (or Kruskal–Wallis test for non-normally distributed data). When the main effect or the interaction was significant, a paired t test (or Wilcoxon signed rank tests for no normally distributed data) was applied for the analysis of differences between time points in the same group, and post hoc pair-wise comparisons were completed using Bonferroni’s test for the analysis of differences in time points between groups (or the Mann–Whitney test for no normally distributed data). Furthermore, Cohen’s D effect sizes were calculated for each group (post-test − pre-test/pooled SD). For better interpretation of the ES, the following criteria were used: < 0.2 = trivial, 0.2 to 0.6 = small effect, 0.6 to 1.2 = moderate effect, 1.2 to 2.0 = large effect, and > 2.0 = very large [39]. All statistical tests were two-tailed, and a p < 0.05 was considered statistically significant. Besides, figures were drawn in Graphpad Prism software (Version 8.4.3, Graphpad Software).
Results
Ten participants (two from the aerobic training group, two from the control group, three from the resistance training group, and three from the combined training group) were omitted from the study due to personal or medical reasons (Fig. 1). During the intervention, there were no adverse events reported. The four groups did not significantly differ in baseline characteristics, including age, height, weight, BMI, waist circumference, waist-to-hip ratio, fat percentage, and VO2max (Table 1).
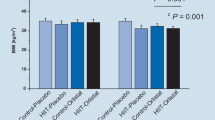
Changes in serum FAM19A5 levels are presented in Fig. 2. There were no significant changes in serum FAM19A5 concentrations between baseline and week 12 in any of the exercise training groups (aerobic training: 39.42 ± 67.80 pg/ml, p = 0.069, d = 0.77; combined training: 18.58 ± 46.80 pg/ml, p = 0.209, d = 0.33; and resistance training: 9.25 ± 46.80 pg/ml, p = 0.433, d = 0.21). However, it significantly decreased in the control group (− 14.08 ± 19.0, p = 0.026, d = 0.26). When changes in serum FAM19A5 concentrations for the four groups were examined, statistical analysis revealed a non-significant difference among groups.
In response to the exercise training, fasting glucose decreased in the aerobic (3.25 ± 1.71 mg/dl, d = 0.36, p < 0.001) and combined training (2.58 ± 2.02 mg/dl, d = 0.45, p = 0.001) groups but not in the control (0.17 ± 1.03 mg/dl, d = 0.02, p = 0.586) or resistance training (0.92 ± 2.57 mg/dl, d = 0.1, p = 0.243) groups (p > 0.05 for the between-group differences). The serum level of insulin in all three training groups was significantly lowered compared with pre-training. Insulin was significantly different in the resistance and combined training groups compared to the control group (− 3.6% and − 9% vs. 0.0%). Nevertheless, the alteration in the level of insulin was not different among training groups (Table 2).
The impact of exercise training programs on lipid parameters is shown in Table 2. Serum triglyceride was significantly reduced by 9.6%, 10.9%, and 14% following 12 weeks of aerobic (p < 0.001, d = 0.25), resistance (p = 0.002, d = 0.23), and combined training (p = 0.006, d = 0.33), respectively, with no significant changes in the control group. When changes in serum triglyceride for the four groups were tested, statistical analysis revealed a significant (p < 0.05) difference among all three training groups and the control group. However, triglyceride changes were not different among training groups. After the 12-week intervention, combined training significantly reduced low-density lipoprotein levels (p = 0.019, d = 0.37), while no effects of any other training programs were observed in this variable. There was a significant increase in serum high-density lipoprotein concentrations in the aerobic training group (5.8%, p = 0.007, d = 0.5), but not in the resistance or combined training groups.
Systolic blood pressure was significantly decreased in the combined (5.0 ± 3.67 mmHg, d = 0.46) and resistance (2.58 ± 2.43 mmHg, d = 0.17) groups, but not in the aerobic and control groups (Table 2). Systolic blood pressure was significantly different in the combined group compared to the control and aerobic groups, as well as in the resistance group compared to the aerobic group.
All three intervention groups significantly reduced HOMA-IR (aerobic training = 0.24, p = 0.001; combined training = 0.22, p = 0.005; resistance training = 0.21, p = 0.002). When changes in HOMA-IR for the four groups were assessed, statistical analysis revealed a significant difference among all three training groups compared to the control group. Moreover, all three training groups significantly decreased VAI (aerobic training = 0.18, p = 0.016; combined training = 0.15, p = 0.004; resistance training = 0.08, p = 0.030) and TyG index (aerobic training = 0.4, p = 0.001; combined training = 0.34, p = 0.030; resistance training = 0.24, p = 0.003) over time. Also, during the 12-week intervention, LAP was significantly reduced following the aerobic (1.5 ± 0.67, p < 0.001) and combined training (1.25 ± 0.87, p < 0.001) groups but not the resistance training group. Yet, no effects of any exercise training programs were observed in the McAuley index (Table 2).
The effect of exercise training programs on body composition and maximal oxygen consumption is illustrated in Fig. 3. Body weight decreased significantly in the combined group (5.17 ± 2.29 kg) and the aerobic group (3.33 ± 2.90 kg) but not in the resistance group (0.83 ± 2.21 kg); however, it significantly increased in the control group (1.42 ± 2.15 kg) (p > 0.05 for the between-group differences) (Fig. 3a). Compared with control, BMI was significantly lowered in the aerobic and combined groups (0.69 and 1.72 kg/m2, respectively), but was not in the resistance group (0.26 kg/m2). In addition, the reduction of BMI in the combined group was significantly greater than that of the aerobic and resistance groups, as well as in the aerobic group, which was greater than that in the resistance group (Fig. 3b). Besides, as presented in Fig. 3c, all three training groups significantly decreased percent body fat (aerobic training = 0.9; combined training = 2.08; resistance training = 0.87), with no differences among groups (p > 0.05).
VO2max increased significantly in the all three training groups (aerobic training = 2.33 ml/kg/min; combined training = 3.17 ml/kg/min; resistance training = 1.0 ml/kg/min) following the 12-week intervention. Furthermore, the increase of VO2max in the combined group was significantly different compared with the control group (~ + 10% vs. − 1.1%) (Fig. 3d).
Discussion
To our knowledge, this study represents the first randomized controlled trial investigating the effects of aerobic, resistance, and combined training on serum levels of FAM19A5, glucose homeostasis, and lipid profiles among obese adults. Our main finding was that none of the exercise training interventions had a significant effect on serum levels of FAM19A5. However, it was observed that serum levels of FAM19A5 decreased significantly in the control group. Conversely, significant reductions in fasting glucose, insulin, HOMA-IR, and triglyceride levels were observed after 12 weeks of aerobic and combined training in obese adults. Resistance training also led to significant decreases in insulin, HOMA-IR, and triglyceride levels. Systolic blood pressure showed a significant decrease following resistance and combined training. Additionally, high-density lipoprotein levels significantly increased after aerobic exercise. Importantly, there were no significant differences in the effects of the three exercise training interventions on any of the measured outcomes.
The FAM19A5, a newly identified adipokine [5], has a protective effect from cardio metabolic disorders. It is revealed that FAM19A5 inhibits vascular smooth muscle cell proliferation and inflammation linked to cardiovascular disease through obesity [40]. Based on the current study’s findings, there were no significant increases in serum FAM19A5 following 12 weeks of aerobic, resistance, or combined training. Thus, it is important to keep in mind that our study population was composed of obese adults who did not have type 2 diabetes or non-alcoholic fatty liver disease, and therefore may have different baseline levels of FAM19A5. However, our result reveals a significant negative correlation between serum levels of FAM19A5 and HOMA-IR. To the best of our knowledge, no study has been conducted to investigate the effects of exercise interventions on serum concentrations of FAM19A5. It has been reported that inflammation status in adipocytes induced by TNF-α reduced FAM19A5 expression proposing that a pro-inflammatory condition, as discovered in obesity [41], may cause downregulation of FAM19A5 [42, 43]. Because it was previously stated that FAM19A5 acts as a protective factor in atherosclerosis, obesity, and inflammation, we assumed that the lack of decrease in FAM19A5 in our exercise groups and the increased levels of it in the control group over time might indicate its protective role in obesity and some diseases’ pathogenesis [5, 42, 43]. Nevertheless, we are unable to rule out the likely impact of the race-ethnic difference among study populations. In this context, significant race-ethnic differences have been suggested in the concentration levels of other adipokines [11]. Therefore, further investigation is warranted to explore the potential impact of this factor on the relationship between FAM19A5 and obesity.
LAP, VAI, and McAuley’s index were other variables examined in this study that are robust and new indices for identifying insulin resistance, metabolic syndrome, and type 2 diabetes [16, 18, 35, 44]. We observed no declines in LAP, VAI, or McAuley’s index in any of the training groups compared with the control condition; nevertheless, VAI significantly decreased in all three training groups over time. Moreover, LAP was significantly reduced following 12 weeks of aerobic and combined training. On the other hand, HOMA-IR significantly decreased in all training groups compared with the control group. In line with this, Mohammadi et al. noted a significant reduction in VAI, LAP, and HOMA-IR after a 12-week aerobic and resistance training program in patients with type 2 diabetes [45]. Another randomized controlled trial reported the improvement of LAP and VAI after two exercise interventions (sprint interval training and combined aerobic + resistance training) in inactive obese women with type 2 diabetes [46]. The improvement of the LAP index reflected the possible decline of fat deposition, which could be responsible for the amelioration of HOMA-IR in patients with type 2 diabetes [47], as observed in the current study. Furthermore, related to the improvement of VAI, it gives the impression that an exercise regimen could change adipose tissue metabolism and regional visceral adipose tissue depot loss, probably by mobilizing free fatty acids from visceral adipose tissue in diverse abdominal regions [48]. Notwithstanding that the exact mechanism responsible for the alterations demonstrated in the current study is not obvious, a possible reason could be that the noticed improvements in some other outcomes, such as BMI, fasting glucose, triglyceride, high-density lipoprotein, and insulin concentration, were the result of the exercise training interventions. As the literature has noted, VAI and LAP are gender-specific indexes based on anthropometric measures like BMI and waist circumference and a number of laboratory tests such as triglyceride and high-density lipoprotein [34, 35, 49]. In the present study also, we observed a significant correlation between LAP and high-density lipoprotein and waist circumference, as well as between LAP with fasting glucose and insulin levels.
Another index investigated in our study was TyG, which is also employed as a useful biomarker for the development of diabetes, as it has established an association with incident diabetes risk [50]. Interestingly, we discovered that the TyG index significantly decreased after 12 weeks of aerobic, resistance, and combined training. Moreover, there was a positive relationship between serum levels of insulin and TyG in our population. Although it has been acknowledged the relationship between TyG index and cardiovascular disease risk [51, 52], to date, little is known regarding the effect of exercise training on TyG in obese people. It has been presented that the TyG index is a reliable predictor of detecting insulin resistance and type 2 diabetes [14, 53]. The utilization of fasting glucose and triglyceride parameters is one main benefit of the TyG index. It looks like the TyG index, for the most part, displays muscle insulin resistance, whereas HOMA-IR generally reveals liver insulin resistance [54]. A number of candidate mechanisms have been proposed to elucidate the influence of exercise training on the TyG index in the obese population. As observed in this study, improvements in insulin resistance may be associated with improvements in TyG index in obese people or people with type 2 diabetes [14, 46].
Compared with the control condition, we found decreases in serum triglyceride levels in all three training groups. Moreover, systolic blood pressure was significantly reduced in all three training groups compared with the control group. Our results are consistent with the findings of Mohammad Rahimi et al., who reported a significant reduction in triglyceride and total cholesterol concentrations after the aerobic interval and combined exercise training among obese adults with metabolic syndrome [27]. Due to the nature of exercise training, which enhances the aerobic capacity [27], as seen in the current study, these types of interventions are likely to cause compatibility with the oxidative pathway, thereby increasing lipid oxidation and decreasing lipid profiles. Although the mechanisms of the effect of exercise training on the lipid profile remain ambiguous, exercise training seems to boost the ability of muscles to oxidize lipids, resulting in a decline in plasma lipid levels. These mechanisms may include rises in lecithin–cholesterol acyltransferase due to exercise and increases in lipoprotein lipase activity involved in lipid metabolism, which increases the ability of muscle fibers in the oxidation of plasma lipids such as triglycerides [55]. In relation to the changes in blood pressure, a meta-analysis of studies on the impacts of progressive resistance training on resting systolic and diastolic blood pressure in adults has discovered an improvement in blood pressure [56]. Besides, significant improvement in blood pressure with aerobic training was also revealed [57]. Whereas an increase in sympathetic nervous system activity occurs throughout physical exercise, reduced BMI because of long-term exercise training may have an influence on blood pressure.
Maximum VO2 was significantly enhanced in all three aerobic, resistance, and combined exercise programs after 12 weeks. We could not distinguish which of these methods was superior, as there was no statistically noteworthy difference between the exercise interventions. The alteration in maximum VO2 in the combined group (3.2 ml/kg/min) was nearly 1 MET (3.5 ml/kg/min), which is undeniably of clinical significance [58]. Inadequate age-predicted VO2max has been linked to an increased risk of death in the general population [59] as well as in a number of chronic diseases [60, 61]. The mechanism responsible for the increase in VO2max alterations in our study is unclear; however, a possible explanation is increased mitochondrial content or function, which is frequently displayed in response to physical exercise in non-obese populations [62, 63]. Cardiovascular adaptations linked to obesity comprise raised blood volume, stroke volume, and cardiac output, leading to left ventricular hypertrophy [64, 65]. Furthermore, systolic and diastolic functions decline, which may elicit myocardial dysfunction linked to obesity [66].
This randomized controlled trial faces some limitations that should be considered. First of all, this study has a relatively small sample size, which our power of analysis may have been affected. Hence, the link between exercise interventions and FAM19A5 and other variables related to glucose homeostasis undoubtedly requires further studies. Secondly, since the participants of this study were limited to Iranians, the findings of the current study should be cautiously extrapolated to populations of different ethnicities. Thirdly, dietary intake was assessed using self-reported data; therefore, dietary intake was not accurately evaluated. Finally, our study population comprised only men; previous research noted gender differences in circulating levels of adipokines [67, 68].
Conclusion
To the best of our knowledge, this is the first study examining the impact of a 12-week aerobic, resistance, and combined exercise on serum FAM19A5 concentrations and glucose homeostasis among adults with obesity. Although FAM19A5 levels remained unaltered in all three modes of exercise training, our work provides information to support that aerobic, resistance, and combined regimens can be effective in improving HOMA-IR, triglyceride, systolic blood pressure, BMI, and aerobic performance in our population. Additional studies with large sample sizes should be conducted to further clarify the underlying mechanisms.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
References
World Health Organization. Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed on 25 January 2022.
Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7):161.
Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63(4):250–9.
Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3(1):35–42.
Wang Y, Chen D, Zhang Y, Wang P, Zheng C, et al. Novel adipokine, FAM19A5, inhibits neointima formation after injury through sphingosine-1-phosphate receptor 2. Circulation. 2018;138(1):48–63.
Lee Y-B, Hwang H-J, Kim JA, Hwang SY, Roh E, et al. Association of serum FAM19A5 with metabolic and vascular risk factors in human subjects with or without type 2 diabetes. Diab Vasc Dis Res. 2019;16(6):530–8.
Ali Yari F, Shabani P, Karami S, Sarmadi N, Poustchi H, et al. Circulating levels of FAM19A5 are inversely associated with subclinical atherosclerosis in non-alcoholic fatty liver disease. BMC Endocr Disord. 2021;21(1):153.
Wang W, Li T, Wang X, Yuan W, Cheng Y, et al. FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cell Mol Immunol. 2015;12(5):615–24.
Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409.
Cefalu WT. Inflammation, insulin resistance, and type 2 diabetes: back to the future? Diabetes. 2009;58(2):307–8.
Khan UI, Wang D, Sowers MR, Mancuso P, Everson-Rose SA, et al. Race–ethnic differences in adipokine levels: the Study of Women’s Health Across the Nation (SWAN). Metabolism. 2012;61(9):1261–9.
Hosseinpanah F, Barzin M, Mirbolouk M, Abtahi H, Cheraghi L, et al. Lipid accumulation product and incident cardiovascular events in a normal weight population: Tehran Lipid and Glucose Study. Eur J Prev Cardiol. 2016;23(2):187–93.
Han L, Fu K-L, Zhao J, Wang Z-H, Tang M-X, et al. Visceral adiposity index score indicated the severity of coronary heart disease in Chinese adults. Diabetol Metab Syndr. 2014;6(1):1–6.
Lee EY, Yang HK, Lee J, Kang B, Yang Y, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):1–7.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Xia C, Li R, Zhang S, Gong L, Ren W, et al. Lipid accumulation product is a powerful index for recognizing insulin resistance in non-diabetic individuals. Eur J Clin Nutr. 2012;66(9):1035–8.
Kim TJ, Kim HJ, Kim YB, Lee JY, Lee HS, et al. Comparison of surrogate markers as measures of uncomplicated insulin resistance in Korean adults. Korean J Fam Med. 2016;37(3):188–96.
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24(3):460–4.
Straczkowski M, Stepien A, Kowalska I, Kinalska I. Comparison of simple indices of insulin sensitivity using the euglycemic hyperinsulinemic clamp technique. Medical science monitor. Int Med J Exp Clin Res. 2004;10(8):CR480-4.
Semlitsch T, Stigler FL, Jeitler K, Horvath K, Siebenhofer A. Management of overweight and obesity in primary care—a systematic overview of international evidence-based guidelines. Obes Rev. 2019;20(9):1218–30.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, American College of Sports Medicine Position Stand, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71.
Oppert JM, Bellicha A, van Baak MA, Battista F, Beaulieu K, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes Rev. 2021;22:e13273.
Morze J, Rücker G, Danielewicz A, Przybyłowicz K, Neuenschwander M, et al. Impact of different training modalities on anthropometric outcomes in patients with obesity: a systematic review and network meta-analysis. Obes Rev. 2021;22(7): e13218.
Codella R, Ialacqua M, Terruzzi I, Luzi L. May the force be with you: why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine. 2018;62(1):14–25.
Shaw BS, Shaw I. Effect of resistance training on cardiorespiratory endurance and coronary artery disease risk: cardiovascular topics. Cardiovasc J S Afr. 2005;16(5):256–9.
Racil G, Zouhal H, Elmontassar W, Abderrahmane AB, De Sousa MV, et al. Plyometric exercise combined with high-intensity interval training improves metabolic abnormalities in young obese females more so than interval training alone. Appl Physiol Nutr Metab. 2016;41(1):103–9.
Mohammad Rahimi GR, Bijeh N, Rashidlamir A. Effects of exercise training on serum preptin, undercarboxylated osteocalcin and high molecular weight adiponectin in adults with metabolic syndrome. Exp Physiol. 2020;105(3):449–59.
Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press. 2009;18(6):308–47.
Bruce R. Exercise testing of patients with coronary artery disease. Ann Clin Res. 1971;3:323–32.
Balady GJ. ACSM’s guidelines for exercise testing and prescription. Am College Sports Med. 2000.
Brzycki M. Strength testing—predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Dance. 1993;64(1):88–90.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Mirmiran P, Bahadoran Z, Azizi F. Lipid accumulation product is associated with insulin resistance, lipid peroxidation, and systemic inflammation in type 2 diabetic patients. Endocrinol Metab (Seoul, Korea). 2014;29(4):443–9.
Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in Japanese women and men. J Atheroscler Thromb. 2014;21(3):282–8.
Nascimento-Ferreira MV, Rendo-Urteaga T, Vilanova-Campelo RC, Carvalho HB, de Paz Oliveira G, et al. The lipid accumulation product is a powerful tool to predict metabolic syndrome in undiagnosed Brazilian adults. Clin Nutr. 2017;36(6):1693–700.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Lopes WA, Oliveira GHD, Locateli JC, Simões CF. TyG in insulin resistance prediction. Jornal de Pediatria. 2020;96(1):132–3.
Hopkins W, Marshall S, Batterham A, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3.
Zarzour A, Kim HW, Weintraub NL. Understanding obesity-related cardiovascular disease: it’s all about balance. Am Heart Assoc. 2018;138:64–6.
Mohammad Rahimi GR, AlizaeiYousefabadi H, Niyazi A, Mohammad Rahimi N, Alikhajeh Y. Effects of lifestyle intervention on inflammatory markers and waist circumference in overweight/obese adults with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Biol Res Nurs. 2022;24(1):94–105.
Recinella L, Orlando G, Ferrante C, Chiavaroli A, Brunetti L, et al. Adipokines: new potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol. 2020;11: 578966.
Tourniaire F, Romier-Crouzet B, Lee JH, Marcotorchino J, Gouranton E, et al. Chemokine expression in inflamed adipose tissue is mainly mediated by NF-κB. PLoS ONE. 2013;8(6): e66515.
Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9(1):9693.
Mohammadi A, Bijeh N, Moazzami M, Khodaei K, Rahimi N. Effect of exercise training on spexin level, appetite, lipid accumulation product, visceral adiposity index, and body composition in adults with type 2 diabetes. Biol Res Nurs. 2022;24(2):152–62.
Banitalebi E, Faramarzi M, Ghahfarrokhi MM, Nasiri S. The effects of two exercise modalities on novel cardiovascular risk factors in overweight women with type 2 diabetes: a randomized controlled trial. 2019. https://doi.org/10.21203/rs.2.13913/v1.
Sambataro M, Perseghin G, Lattuada G, Beltramello G, Luzi L, et al. Lipid accumulation in overweight type 2 diabetic subjects: relationships with insulin sensitivity and adipokines. Acta Diabetol. 2013;50(3):301–7.
Idoate F, Ibañez J, Gorostiaga E, García-Unciti M, Martínez-Labari C, et al. Weight-loss diet alone or combined with resistance training induces different regional visceral fat changes in obese women. Int J Obes. 2011;35(5):700–13.
Misra A, Alappan NK, Vikram NK, Goel K, Gupta N, et al. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care. 2008;31(7):1282–7.
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7.
Zheng S, Shi S, Ren X, Han T, Li Y, et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14(1):1–10.
Kim J-H, Lee D-Y, Park SE, Park C-Y, Lee W-Y, et al. Triglyceride glucose index predicts coronary artery calcification better than other indices of insulin resistance in Korean adults: the Kangbuk Samsung Health Study. Precis Futur Med. 2017;1(1):43–51.
Lee DY, Lee ES, Kim JH, Park SE, Park C-Y, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS ONE. 2016;11(9): e0163465.
Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27(9):2204–10.
Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44(2):211–21.
Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35(3):838–43.
Pescatello LS, Miller B, Danias PG, Werner M, Hess M, et al. Dynamic exercise normalizes resting blood pressure in mildly hypertensive premenopausal women. Am Heart J. 1999;138(5):916–21.
Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60(19):1899–905.
Lee D-C, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4_suppl):27–35.
Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int. 2004;65(2):719–24.
Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14(1):77–86.
Bishop DJ, Granata C, Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta (BBA)-Gen Subj. 2014;1840(4):1266–75.
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(6):1011–22.
de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, et al. Obesity and cardiac function. Circulation. 1981;64(3):477–82.
Gaasch WH, Quinones MA, Waisser E, Thiel HG, Alexander JK. Diastolic compliance of the left ventricle in man. Am J Cardiol. 1975;36(2):193–201.
Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43(8):1399–404.
Bonakdari H, Tardif G, Abram F, Pelletier J-P, Martel-Pelletier J. Serum adipokines/related inflammatory factors and ratios as predictors of infrapatellar fat pad volume in osteoarthritis: applying comprehensive machine learning approaches. Sci Rep. 2020;10(1):1–12.
Eglit T, Lember M, Ringmets I, Rajasalu T. Gender differences in serum high-molecular-weight adiponectin levels in metabolic syndrome. Eur J Endocrinol. 2013;168(3):385–91.
Acknowledgment
The authors thank all of the participants for their time and effort in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent of patient
The institutional review board at the Baqiyatallah University of Medical Sciences (Project No. IR.BMSU.BAQ.REC.1400.023) approved the study research protocol, and written informed consent was provided by all participants.
Trial registration
ClinicalTrials.gov Identifier: IRCT20151026024717N4.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mir, E., Shamseddini, A., Rahimi, N. et al. Impacts of a 12-week aerobic, resistance, and combined exercise training on serum FAM19A5, glucose homeostasis, and novel cardiovascular risk factors among adults with obesity. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-024-01315-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-024-01315-7